

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thioredoxin reductase (Escherichia coli (strain K12)) | BDBM50385299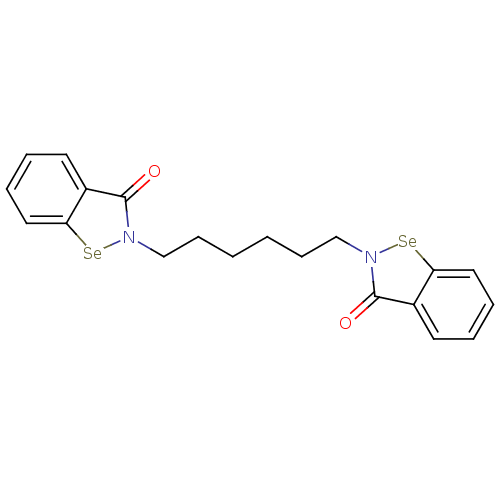 (CHEMBL2035464 | US8592468, EbSe16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 10 | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB US Patent | Assay Description All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one derivatives were tested as potential E. coli TrxR inhibitors by standard DTNB ... | US Patent US8592468 (2013) BindingDB Entry DOI: 10.7270/Q29P3081 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Escherichia coli (strain K12)) | BDBM50385302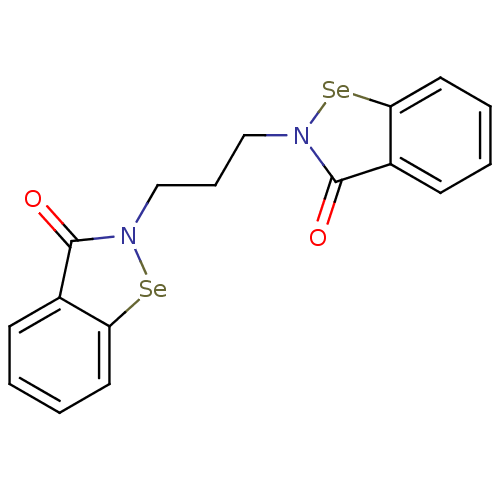 (CHEMBL2035461 | US8592468, EbSe15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 40 | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB US Patent | Assay Description All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one derivatives were tested as potential E. coli TrxR inhibitors by standard DTNB ... | US Patent US8592468 (2013) BindingDB Entry DOI: 10.7270/Q29P3081 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Escherichia coli (strain K12)) | BDBM50385303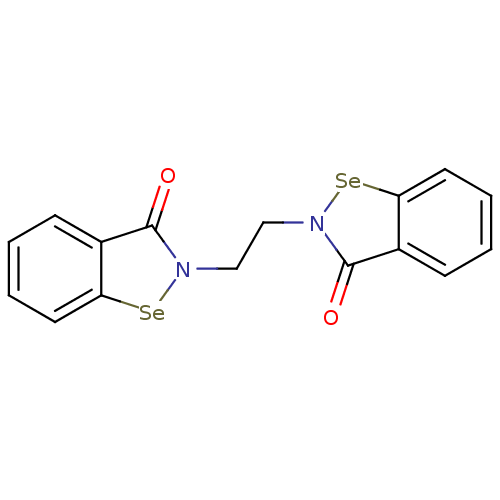 (CHEMBL2035460 | US8592468, EbSe14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | 50 | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB US Patent | Assay Description All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one derivatives were tested as potential E. coli TrxR inhibitors by standard DTNB ... | US Patent US8592468 (2013) BindingDB Entry DOI: 10.7270/Q29P3081 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Escherichia coli (strain K12)) | BDBM106948 (US8592468, EbSe12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 250 | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB US Patent | Assay Description All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one derivatives were tested as potential E. coli TrxR inhibitors by standard DTNB ... | US Patent US8592468 (2013) BindingDB Entry DOI: 10.7270/Q29P3081 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Escherichia coli (strain K12)) | BDBM106941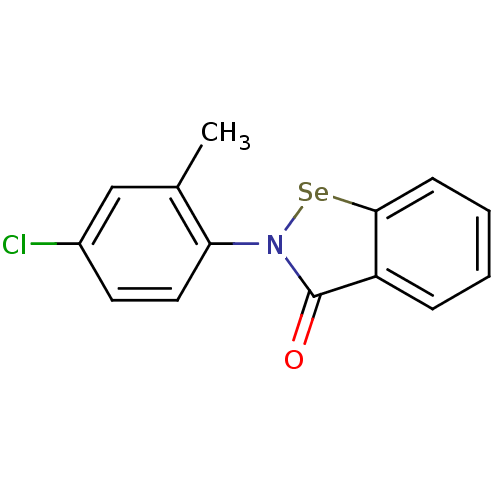 (US8592468, EbSe8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 250 | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB US Patent | Assay Description All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one derivatives were tested as potential E. coli TrxR inhibitors by standard DTNB ... | US Patent US8592468 (2013) BindingDB Entry DOI: 10.7270/Q29P3081 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Escherichia coli (strain K12)) | BDBM34233 (2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | 300 | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB US Patent | Assay Description All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one derivatives were tested as potential E. coli TrxR inhibitors by standard DTNB ... | US Patent US8592468 (2013) BindingDB Entry DOI: 10.7270/Q29P3081 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Escherichia coli (strain K12)) | BDBM106940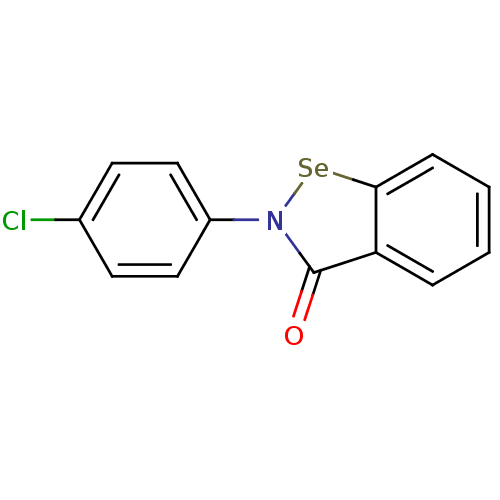 (US8592468, EbSe7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 550 | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB US Patent | Assay Description All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one derivatives were tested as potential E. coli TrxR inhibitors by standard DTNB ... | US Patent US8592468 (2013) BindingDB Entry DOI: 10.7270/Q29P3081 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Escherichia coli (strain K12)) | BDBM106944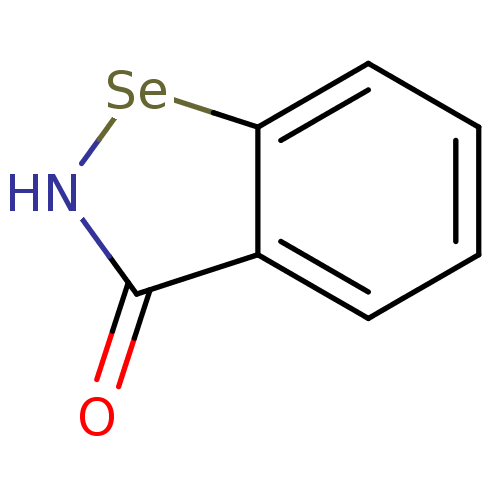 (US8592468, EbSe2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.00E+3 | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB US Patent | Assay Description All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one derivatives were tested as potential E. coli TrxR inhibitors by standard DTNB ... | US Patent US8592468 (2013) BindingDB Entry DOI: 10.7270/Q29P3081 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Escherichia coli (strain K12)) | BDBM106942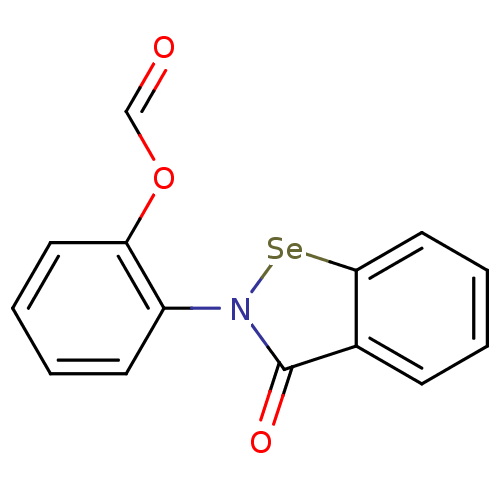 (US8592468, EbSe9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20E+3 | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB US Patent | Assay Description All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one derivatives were tested as potential E. coli TrxR inhibitors by standard DTNB ... | US Patent US8592468 (2013) BindingDB Entry DOI: 10.7270/Q29P3081 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Escherichia coli (strain K12)) | BDBM106949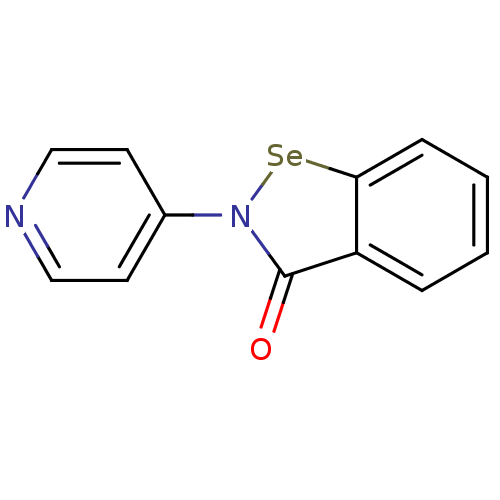 (US8592468, EbSe13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.50E+3 | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB US Patent | Assay Description All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one derivatives were tested as potential E. coli TrxR inhibitors by standard DTNB ... | US Patent US8592468 (2013) BindingDB Entry DOI: 10.7270/Q29P3081 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MHC class II antigen (Homo sapiens (Human)) | BDBM50464582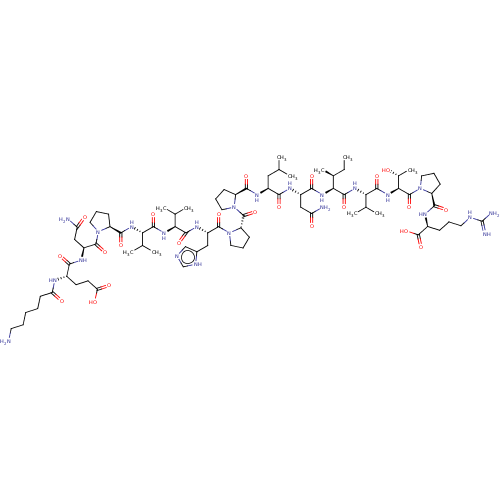 (CHEMBL4284706) | PDB MMDB NCI pathway Reactome pathway UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Patras Curated by ChEMBL | Assay Description Inhibition of biotin-labeled MBP (85 to 99 residues) binding to HLA class 2 DRB1*1501 mutant allele after 2 hrs by TMB + substrate-chromogen based as... | Eur J Med Chem 143: 621-631 (2018) Article DOI: 10.1016/j.ejmech.2017.11.063 BindingDB Entry DOI: 10.7270/Q2NP273D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MHC class II antigen (Homo sapiens (Human)) | BDBM50464580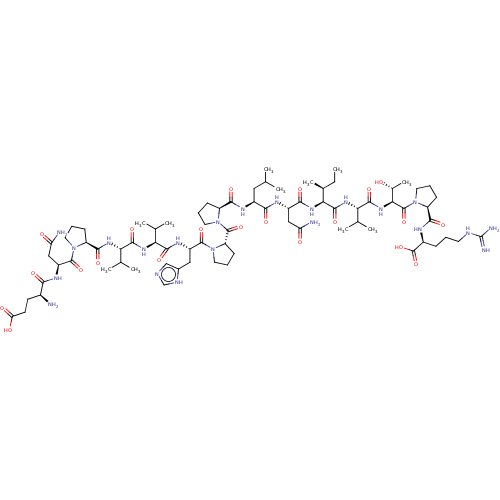 (CHEMBL4277461) | PDB MMDB NCI pathway Reactome pathway UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Patras Curated by ChEMBL | Assay Description Inhibition of biotin-labeled MBP (85 to 99 residues) binding to HLA class 2 DRB1*1501 mutant allele after 2 hrs by TMB + substrate-chromogen based as... | Eur J Med Chem 143: 621-631 (2018) Article DOI: 10.1016/j.ejmech.2017.11.063 BindingDB Entry DOI: 10.7270/Q2NP273D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MHC class II antigen (Homo sapiens (Human)) | BDBM50464581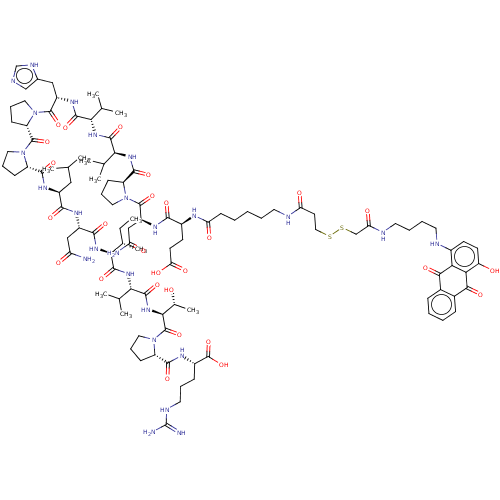 (CHEMBL4292594) | PDB MMDB NCI pathway Reactome pathway UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Patras Curated by ChEMBL | Assay Description Inhibition of biotin-labeled MBP (85 to 99 residues) binding to HLA class 2 DRB1*1501 mutant allele after 2 hrs by TMB + substrate-chromogen based as... | Eur J Med Chem 143: 621-631 (2018) Article DOI: 10.1016/j.ejmech.2017.11.063 BindingDB Entry DOI: 10.7270/Q2NP273D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MHC class II antigen (Homo sapiens (Human)) | BDBM50464580 (CHEMBL4277461) | PDB MMDB NCI pathway Reactome pathway UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Patras Curated by ChEMBL | Assay Description Inhibition of biotin-labeled MBP (85 to 99 residues) binding to HLA class 2 DRB1*0101 mutant allele after 2 hrs by TMB + substrate-chromogen based as... | Eur J Med Chem 143: 621-631 (2018) Article DOI: 10.1016/j.ejmech.2017.11.063 BindingDB Entry DOI: 10.7270/Q2NP273D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MHC class II antigen (Homo sapiens (Human)) | BDBM50464582 (CHEMBL4284706) | PDB MMDB NCI pathway Reactome pathway UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 355 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Patras Curated by ChEMBL | Assay Description Inhibition of biotin-labeled MBP (85 to 99 residues) binding to HLA class 2 DRB1*0101 mutant allele after 2 hrs by TMB + substrate-chromogen based as... | Eur J Med Chem 143: 621-631 (2018) Article DOI: 10.1016/j.ejmech.2017.11.063 BindingDB Entry DOI: 10.7270/Q2NP273D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Escherichia coli (strain K12)) | BDBM106943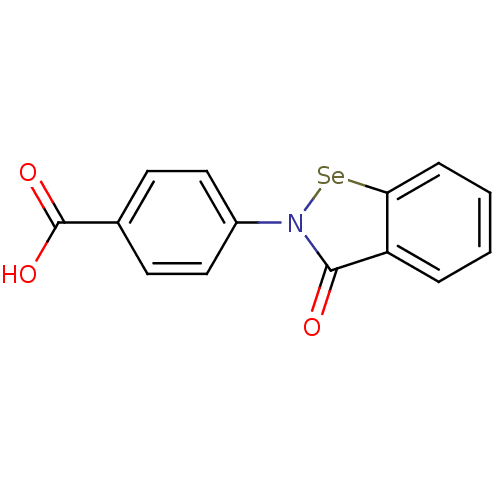 (US8592468, EbSe10 | acs.jmedchem.1c00409_ST.159) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB US Patent | Assay Description All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one derivatives were tested as potential E. coli TrxR inhibitors by standard DTNB ... | US Patent US8592468 (2013) BindingDB Entry DOI: 10.7270/Q29P3081 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MHC class II antigen (Homo sapiens (Human)) | BDBM50464581 (CHEMBL4292594) | PDB MMDB NCI pathway Reactome pathway UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Patras Curated by ChEMBL | Assay Description Inhibition of biotin-labeled MBP (85 to 99 residues) binding to HLA class 2 DRB1*0101 mutant allele after 2 hrs by TMB + substrate-chromogen based as... | Eur J Med Chem 143: 621-631 (2018) Article DOI: 10.1016/j.ejmech.2017.11.063 BindingDB Entry DOI: 10.7270/Q2NP273D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Escherichia coli (strain K12)) | BDBM106946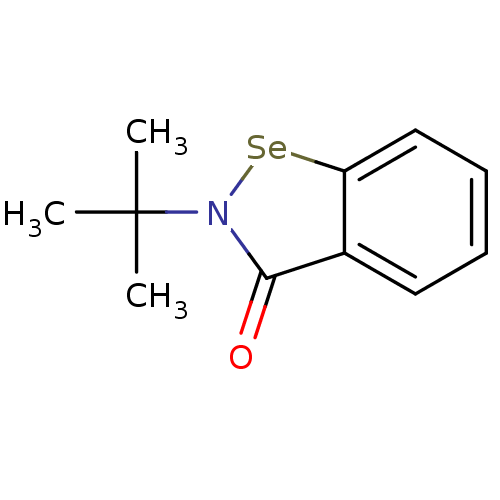 (US8592468, EbSe4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB US Patent | Assay Description All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one derivatives were tested as potential E. coli TrxR inhibitors by standard DTNB ... | US Patent US8592468 (2013) BindingDB Entry DOI: 10.7270/Q29P3081 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Escherichia coli (strain K12)) | BDBM106945 (US8592468, EbSe3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB US Patent | Assay Description All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one derivatives were tested as potential E. coli TrxR inhibitors by standard DTNB ... | US Patent US8592468 (2013) BindingDB Entry DOI: 10.7270/Q29P3081 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase (Escherichia coli (strain K12)) | BDBM106947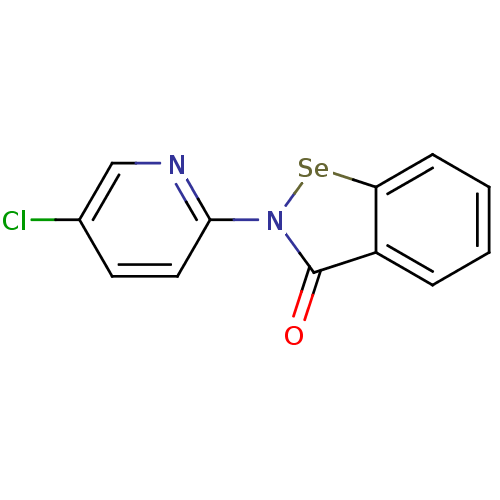 (US8592468, EbSe11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB US Patent | Assay Description All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one derivatives were tested as potential E. coli TrxR inhibitors by standard DTNB ... | US Patent US8592468 (2013) BindingDB Entry DOI: 10.7270/Q29P3081 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||