

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50412114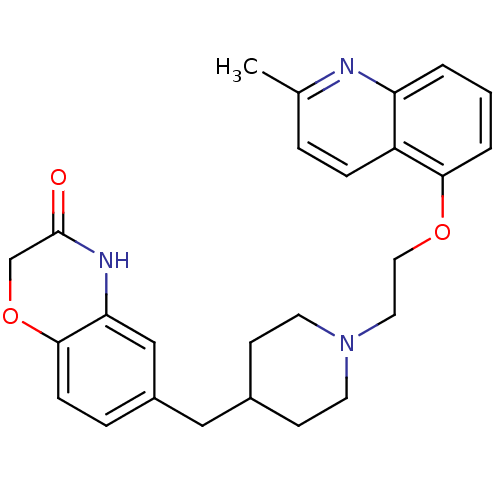 (CHEMBL183460 | SB-649915) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 | Bioorg Med Chem Lett 15: 737-41 (2005) Article DOI: 10.1016/j.bmcl.2004.11.030 BindingDB Entry DOI: 10.7270/Q2RR220M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50477395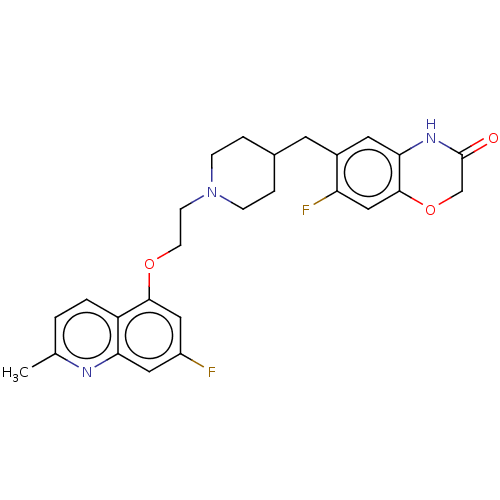 (CHEMBL238520) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]WAY-100635 from human cloned 5HT1A receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 1033-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.031 BindingDB Entry DOI: 10.7270/Q2348P5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50477399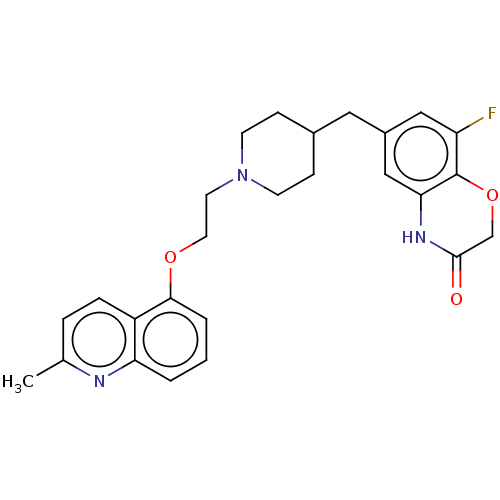 (CHEMBL241463) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human cloned 5HT1D receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 1033-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.031 BindingDB Entry DOI: 10.7270/Q2348P5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50477400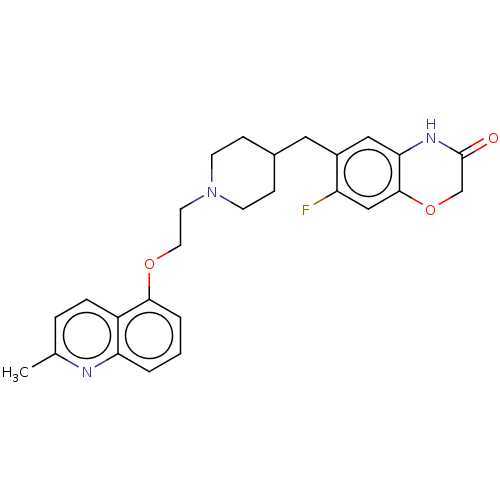 (CHEMBL239168) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]WAY-100635 from human cloned 5HT1A receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 1033-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.031 BindingDB Entry DOI: 10.7270/Q2348P5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50027185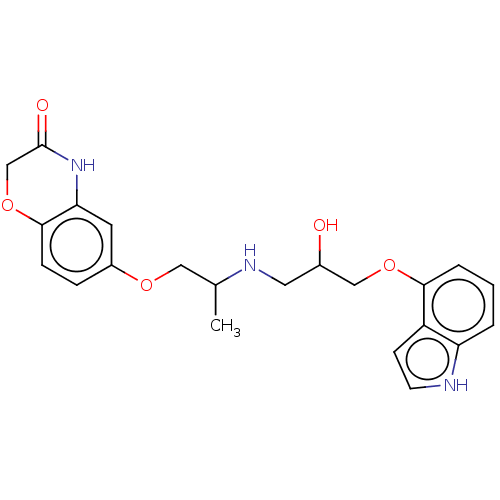 (CHEMBL183921) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity for human beta-2 adrenergic receptor by displacing [125I]iodocyanopindolol expressed in hamster CHO cells | Bioorg Med Chem Lett 15: 737-41 (2005) Article DOI: 10.1016/j.bmcl.2004.11.030 BindingDB Entry DOI: 10.7270/Q2RR220M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50027185 (CHEMBL183921) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity for human beta-2 adrenergic receptor by displacing [125I]iodocyanopindolol expressed in hamster CHO cells | Bioorg Med Chem Lett 15: 737-41 (2005) Article DOI: 10.1016/j.bmcl.2004.11.030 BindingDB Entry DOI: 10.7270/Q2RR220M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50027185 (CHEMBL183921) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity for human beta-2 adrenergic receptor by displacing [125I]iodocyanopindolol expressed in hamster CHO cells | Bioorg Med Chem Lett 15: 737-41 (2005) Article DOI: 10.1016/j.bmcl.2004.11.030 BindingDB Entry DOI: 10.7270/Q2RR220M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50027185 (CHEMBL183921) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 | Bioorg Med Chem Lett 15: 737-41 (2005) Article DOI: 10.1016/j.bmcl.2004.11.030 BindingDB Entry DOI: 10.7270/Q2RR220M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50477399 (CHEMBL241463) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human cloned 5HT1B receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 1033-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.031 BindingDB Entry DOI: 10.7270/Q2348P5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50477402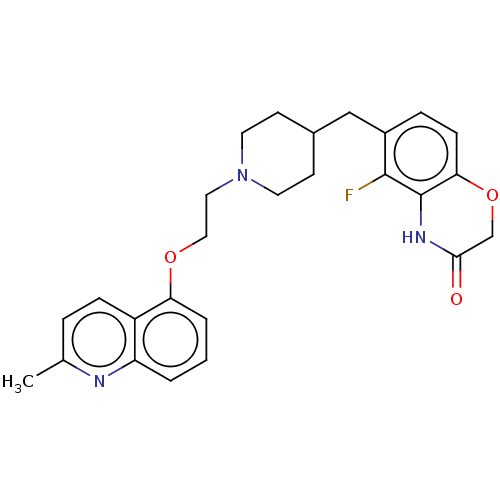 (CHEMBL241503) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human cloned 5HT1D receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 1033-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.031 BindingDB Entry DOI: 10.7270/Q2348P5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474384 (CHEMBL2113364) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50027185 (CHEMBL183921) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 | Bioorg Med Chem Lett 15: 737-41 (2005) Article DOI: 10.1016/j.bmcl.2004.11.030 BindingDB Entry DOI: 10.7270/Q2RR220M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50477404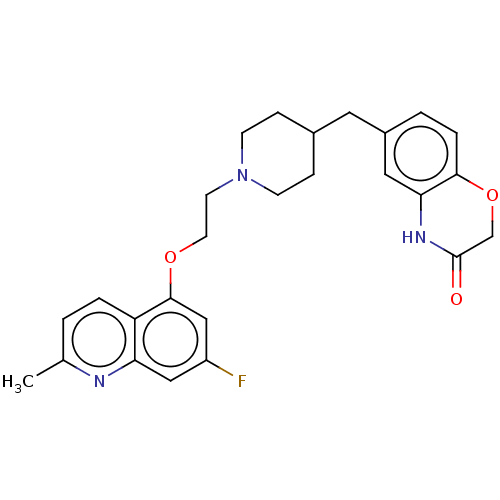 (CHEMBL442087) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]WAY-100635 from human cloned 5HT1A receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 1033-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.031 BindingDB Entry DOI: 10.7270/Q2348P5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50477397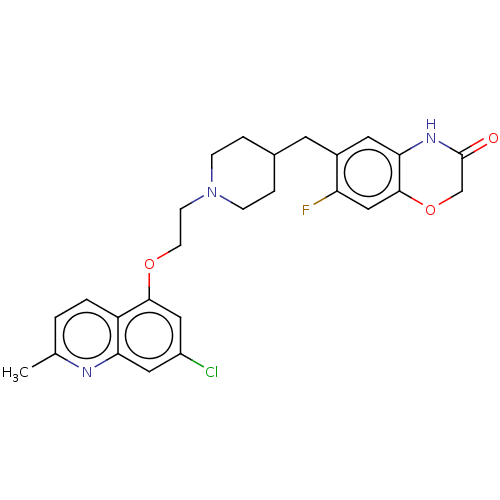 (CHEMBL391661) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at rat 5HT1A receptor | Bioorg Med Chem Lett 17: 1033-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.031 BindingDB Entry DOI: 10.7270/Q2348P5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474398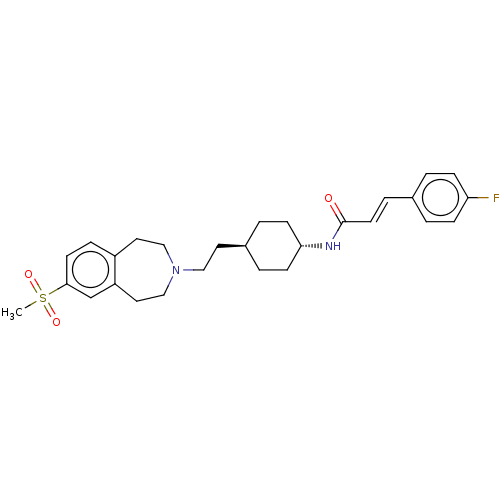 (CHEMBL2368629) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474396 (CHEMBL2113356) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50477395 (CHEMBL238520) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at rat 5HT1A receptor | Bioorg Med Chem Lett 17: 1033-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.031 BindingDB Entry DOI: 10.7270/Q2348P5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474403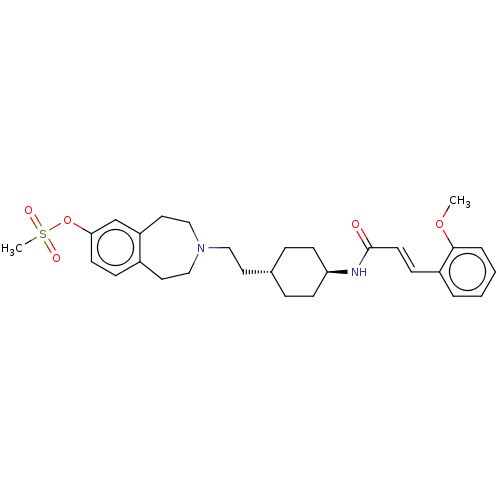 (CHEMBL2368633) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474395 (CHEMBL2368626) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474406 (CHEMBL3084598) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474399 (CHEMBL2368625) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474389 (CHEMBL3084597) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50474983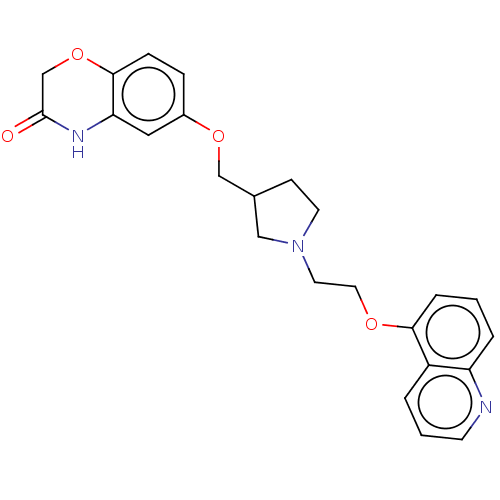 (CHEMBL183034) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 | Bioorg Med Chem Lett 15: 737-41 (2005) Article DOI: 10.1016/j.bmcl.2004.11.030 BindingDB Entry DOI: 10.7270/Q2RR220M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50474976 (CHEMBL180557) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 | Bioorg Med Chem Lett 15: 737-41 (2005) Article DOI: 10.1016/j.bmcl.2004.11.030 BindingDB Entry DOI: 10.7270/Q2RR220M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474412 (CHEMBL2368622) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50477393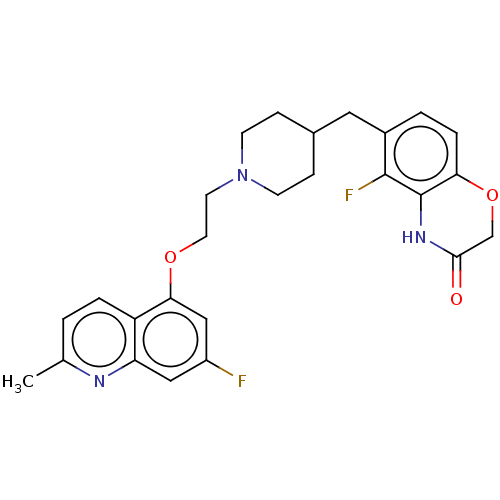 (CHEMBL393539) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]WAY-100635 from human cloned 5HT1A receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 1033-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.031 BindingDB Entry DOI: 10.7270/Q2348P5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474410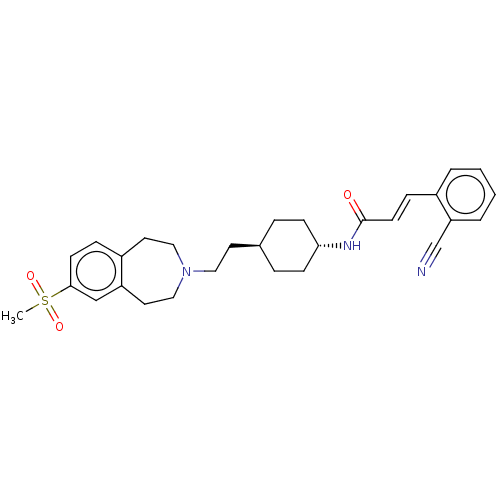 (CHEMBL2368620) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50027184 (CHEMBL180700) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 | Bioorg Med Chem Lett 15: 737-41 (2005) Article DOI: 10.1016/j.bmcl.2004.11.030 BindingDB Entry DOI: 10.7270/Q2RR220M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474393 (CHEMBL2368623) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50412114 (CHEMBL183460 | SB-649915) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human cloned 5HT1D receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 1033-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.031 BindingDB Entry DOI: 10.7270/Q2348P5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50474977 (CHEMBL183901) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 | Bioorg Med Chem Lett 15: 737-41 (2005) Article DOI: 10.1016/j.bmcl.2004.11.030 BindingDB Entry DOI: 10.7270/Q2RR220M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50477403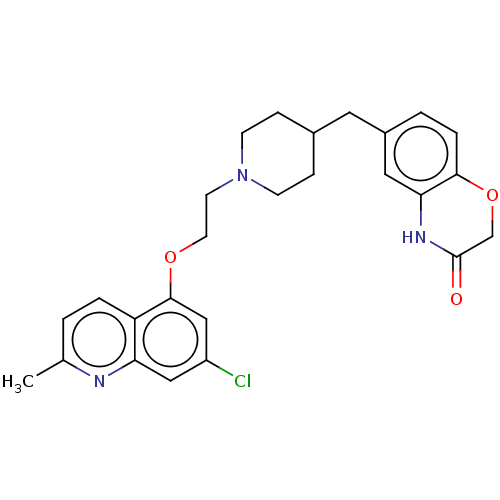 (CHEMBL239166) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]WAY-100635 from human cloned 5HT1A receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 1033-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.031 BindingDB Entry DOI: 10.7270/Q2348P5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50000027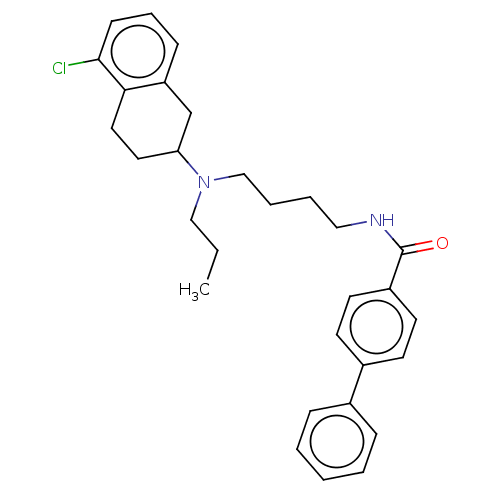 (CHEMBL51977) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]iodosulpiride from human Dopamine receptor D3 expressed in CHO cells | Bioorg Med Chem Lett 9: 179-84 (1999) BindingDB Entry DOI: 10.7270/Q2057J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50000022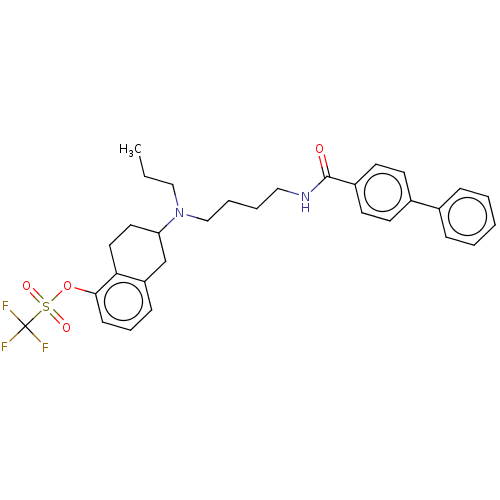 (CHEMBL57219) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]iodosulpiride from human Dopamine receptor D3 expressed in CHO cells | Bioorg Med Chem Lett 9: 179-84 (1999) BindingDB Entry DOI: 10.7270/Q2057J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50474975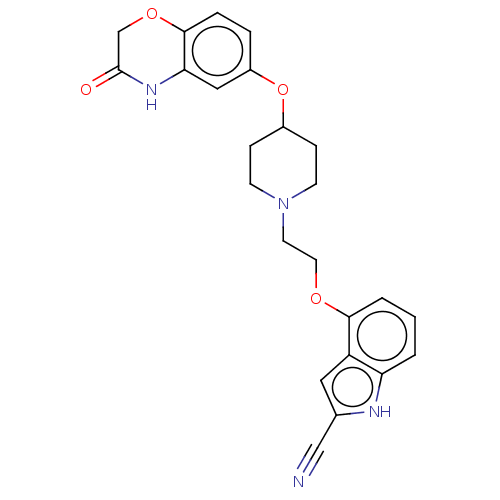 (CHEMBL3706797) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 | Bioorg Med Chem Lett 15: 737-41 (2005) Article DOI: 10.1016/j.bmcl.2004.11.030 BindingDB Entry DOI: 10.7270/Q2RR220M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474407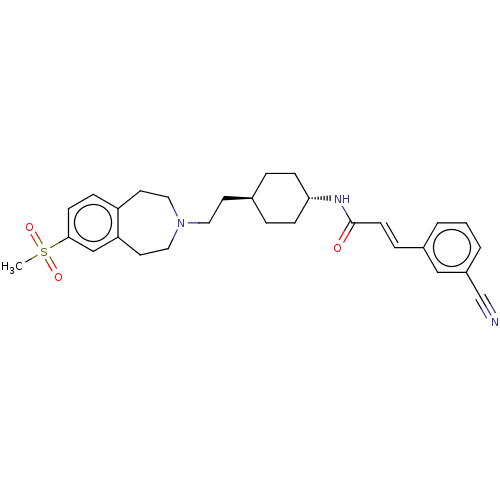 (CHEMBL2368624) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474392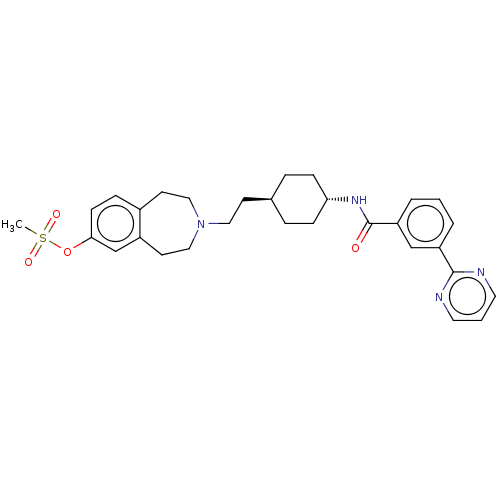 (CHEMBL3084601) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50474980 (CHEMBL180405) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 | Bioorg Med Chem Lett 15: 737-41 (2005) Article DOI: 10.1016/j.bmcl.2004.11.030 BindingDB Entry DOI: 10.7270/Q2RR220M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474413 (CHEMBL3084626) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474414 (CHEMBL2368617) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474388 (CHEMBL3084625) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474404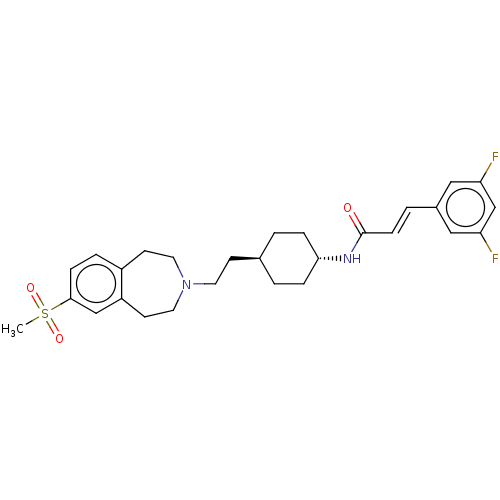 (CHEMBL2368627) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50474401 (CHEMBL2368618) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand | J Med Chem 46: 4952-64 (2003) Article DOI: 10.1021/jm030817d BindingDB Entry DOI: 10.7270/Q2TT4TPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50027185 (CHEMBL183921) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Mean negative logarithim of binding affinity was measured for the human 5-hydroxytryptamine 1A receptor; n>/=3 | Bioorg Med Chem Lett 15: 737-41 (2005) Article DOI: 10.1016/j.bmcl.2004.11.030 BindingDB Entry DOI: 10.7270/Q2RR220M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50217169 (CHEMBL279925) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]iodosulpiride from human Dopamine receptor D3 expressed in CHO cells | Bioorg Med Chem Lett 9: 179-84 (1999) BindingDB Entry DOI: 10.7270/Q2057J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50217155 (CHEMBL291545) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]iodosulpiride from human Dopamine receptor D3 expressed in CHO cells | Bioorg Med Chem Lett 9: 179-84 (1999) BindingDB Entry DOI: 10.7270/Q2057J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50217172 (CHEMBL52724) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]iodosulpiride from human Dopamine receptor D3 expressed in CHO cells | Bioorg Med Chem Lett 9: 179-84 (1999) BindingDB Entry DOI: 10.7270/Q2057J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50477402 (CHEMBL241503) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human cloned 5HT1B receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 1033-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.031 BindingDB Entry DOI: 10.7270/Q2348P5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50477398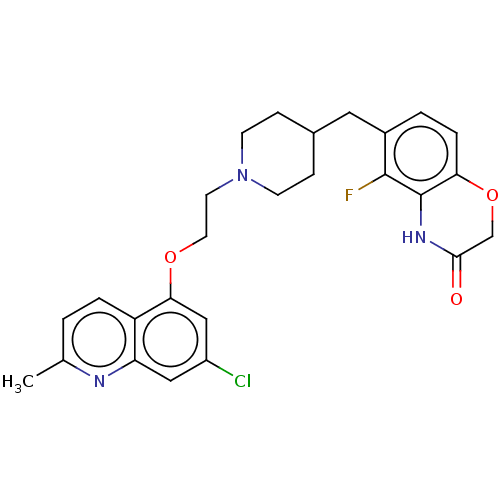 (CHEMBL238519) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]WAY-100635 from human cloned 5HT1A receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 1033-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.031 BindingDB Entry DOI: 10.7270/Q2348P5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50477407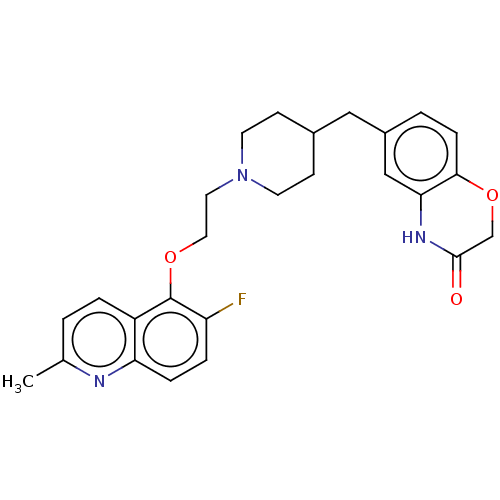 (CHEMBL391033) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from human cloned 5HT1D receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 1033-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.031 BindingDB Entry DOI: 10.7270/Q2348P5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 392 total ) | Next | Last >> |