

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502427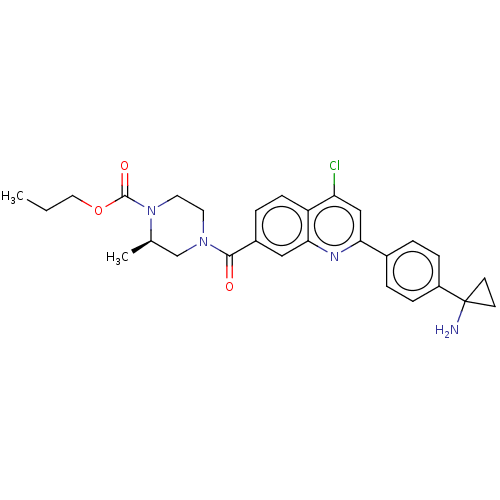 (CHEMBL4542517) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Irreversible inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 5 mins followed by substrate addition and measured... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502433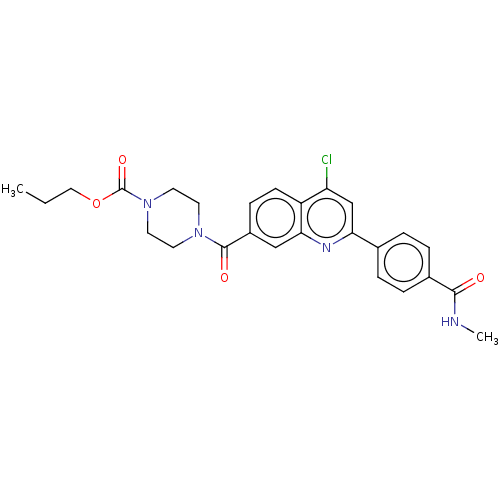 (CHEMBL4575866) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Irreversible inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 5 mins followed by substrate addition and measured... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502434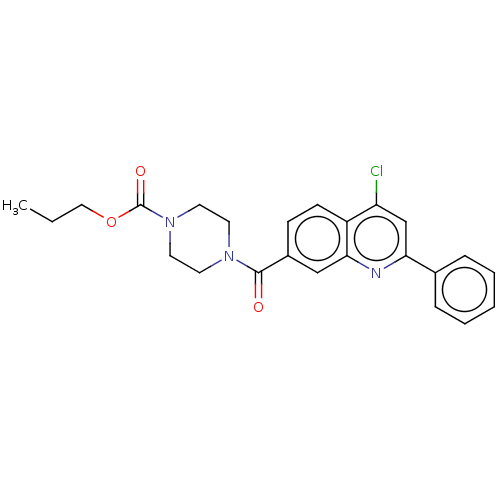 (CHEMBL4551647) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Irreversible inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 5 mins followed by substrate addition and measured... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375523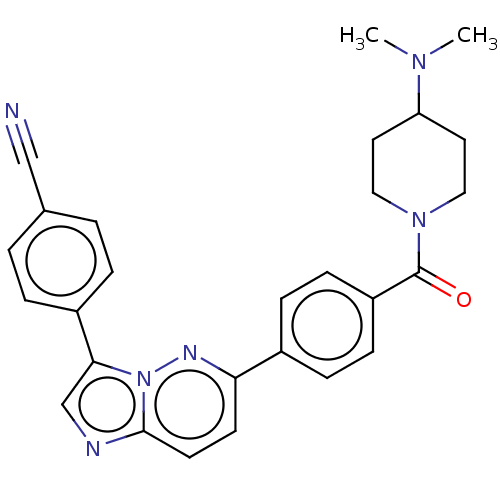 (4-(6-(4-(4-(dimethylamino)piperidine-1-carbonyl)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375523 (4-(6-(4-(4-(dimethylamino)piperidine-1-carbonyl)ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502427 (CHEMBL4542517) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375525 (4-(6-(4-(piperazine-1-carbonyl)phenyl)imidazo[1,2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375513 ((4-(3-(4-chlorophenyl)imidazo[1,2-b]pyridazin-6-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375522 (4-(6-(4-(morpholine-4-carbonyl)phenyl)imidazo[1,2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375513 ((4-(3-(4-chlorophenyl)imidazo[1,2-b]pyridazin-6-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375525 (4-(6-(4-(piperazine-1-carbonyl)phenyl)imidazo[1,2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50455538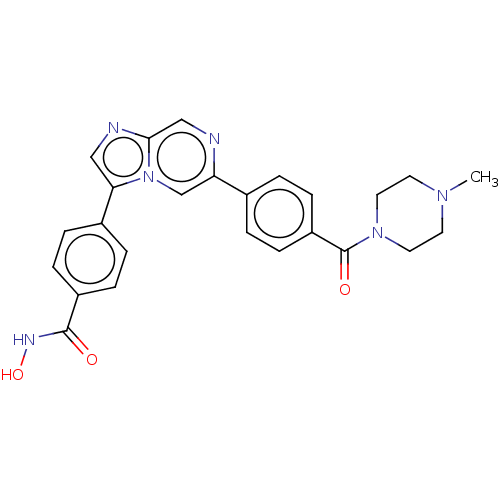 (CHEMBL4214092) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375522 (4-(6-(4-(morpholine-4-carbonyl)phenyl)imidazo[1,2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50455538 (CHEMBL4214092) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375544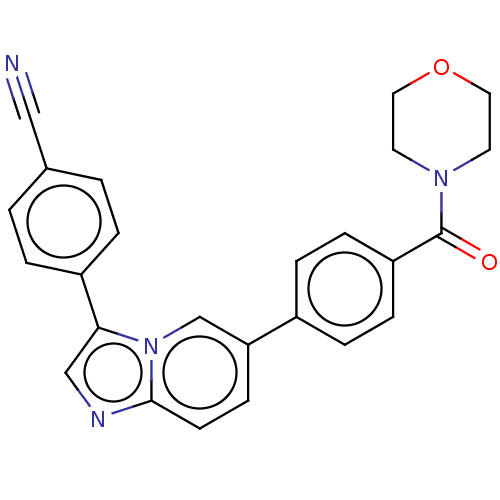 (4-(6-(4-(morpholine-4-carbonyl)phenyl)imidazo[1,2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502413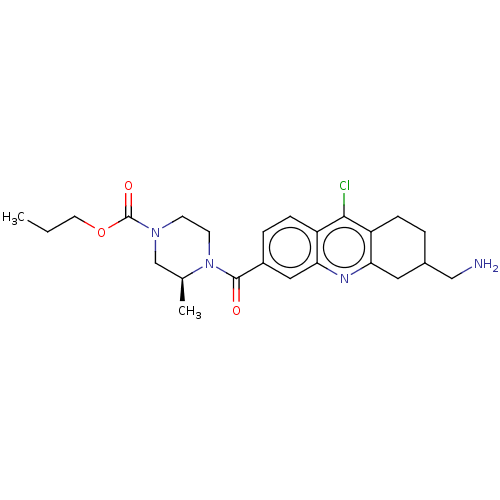 (CHEMBL4521324) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375543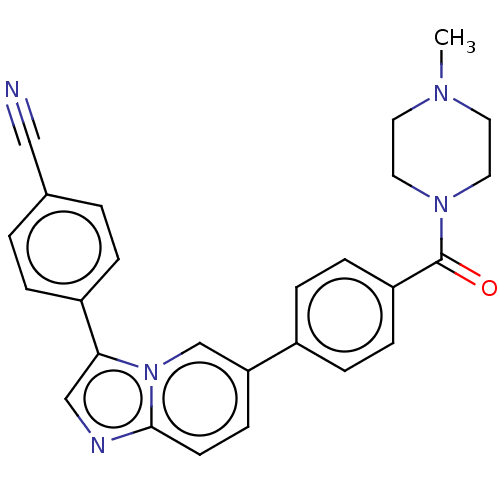 (4-(6-(4-(4-Methylpiperazine-1-carbonyl)phenyl)imid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375543 (4-(6-(4-(4-Methylpiperazine-1-carbonyl)phenyl)imid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375544 (4-(6-(4-(morpholine-4-carbonyl)phenyl)imidazo[1,2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375315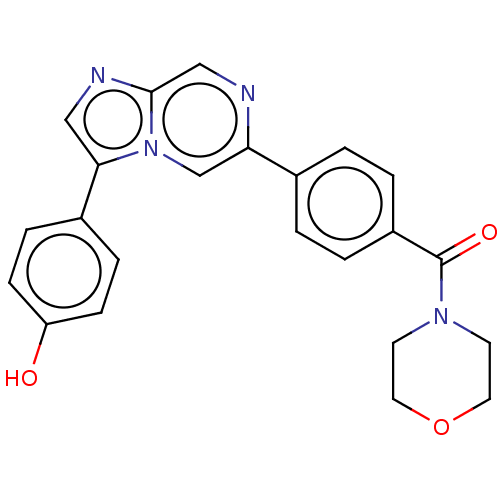 ((4-(3-(4-hydroxyphenyl)imidazo[1,2-a]pyrazin-6-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375315 ((4-(3-(4-hydroxyphenyl)imidazo[1,2-a]pyrazin-6-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1/2 (Homo sapiens (Human)) | BDBM375315 ((4-(3-(4-hydroxyphenyl)imidazo[1,2-a]pyrazin-6-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of MNK1/2 in human HeLa cells assessed as decrease in eIF4E phosphorylation at Ser209 after 2 hrs by AlphaScreen assay | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375313 ((4-(3-(4-hydroxyphenyl)imidazo[1,2-a]pyrazin-6-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502429 (CHEMBL4475972) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375353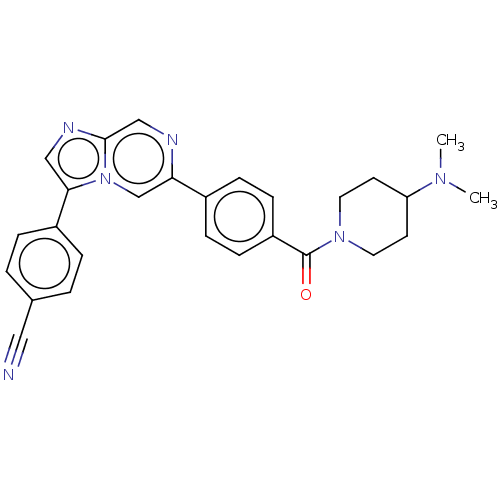 (4-(6-(4-(4-(dimethylamino)piperidine-1-carbonyl)ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50455541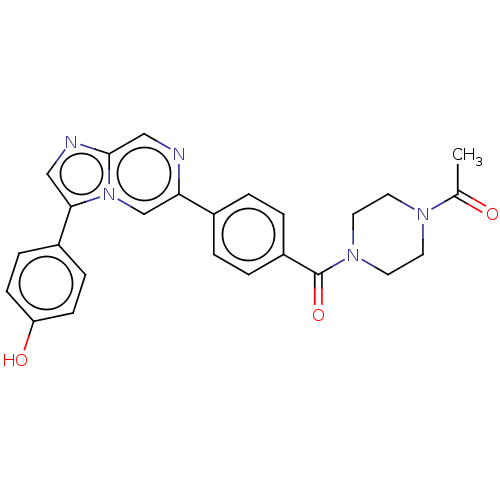 (CHEMBL4208890) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502416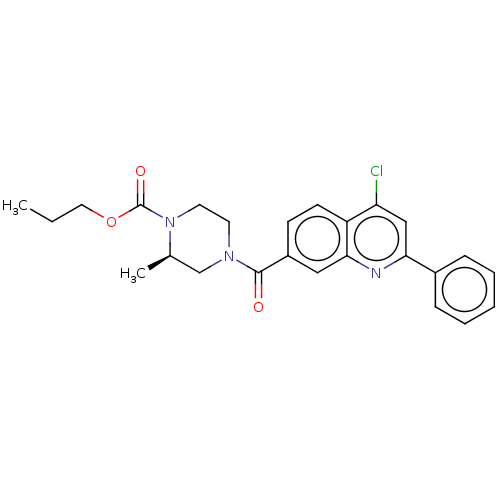 (CHEMBL4472455) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50455545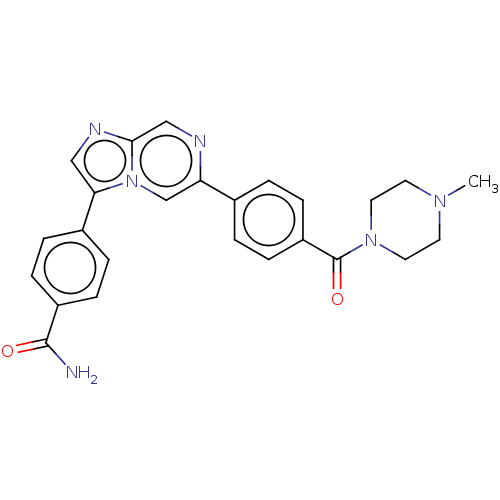 (CHEMBL4204506) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 187 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1/2 (Homo sapiens (Human)) | BDBM375313 ((4-(3-(4-hydroxyphenyl)imidazo[1,2-a]pyrazin-6-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of MNK1/2 in human HeLa cells assessed as decrease in eIF4E phosphorylation at Ser209 after 2 hrs by AlphaScreen assay | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50455540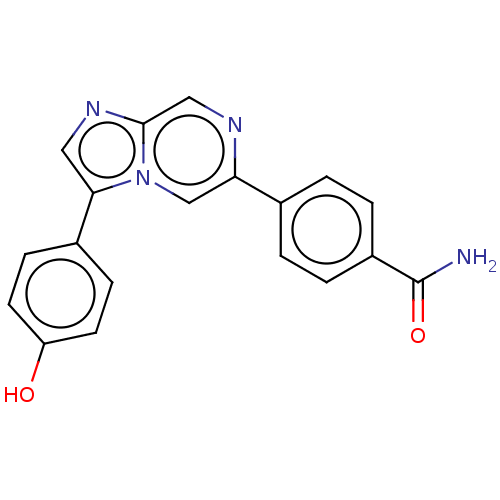 (CHEMBL4212480) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 193 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50455543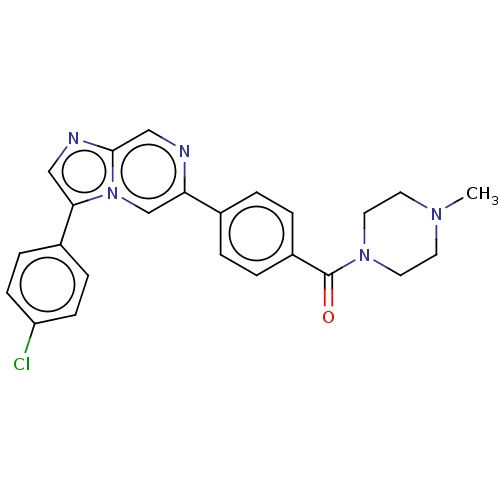 (CHEMBL4211757) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1/2 (Homo sapiens (Human)) | BDBM375525 (4-(6-(4-(piperazine-1-carbonyl)phenyl)imidazo[1,2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of MNK1/2 in human HeLa cells assessed as decrease in eIF4E phosphorylation at Ser209 after 2 hrs by AlphaScreen assay | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50455541 (CHEMBL4208890) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 224 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1/2 (Homo sapiens (Human)) | BDBM375543 (4-(6-(4-(4-Methylpiperazine-1-carbonyl)phenyl)imid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 229 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of MNK1/2 in human HeLa cells assessed as decrease in eIF4E phosphorylation at Ser209 after 2 hrs by AlphaScreen assay | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375353 (4-(6-(4-(4-(dimethylamino)piperidine-1-carbonyl)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 238 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502433 (CHEMBL4575866) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502436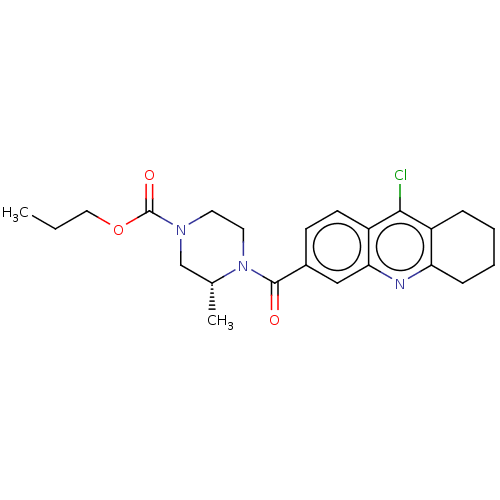 (CHEMBL4450314) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502414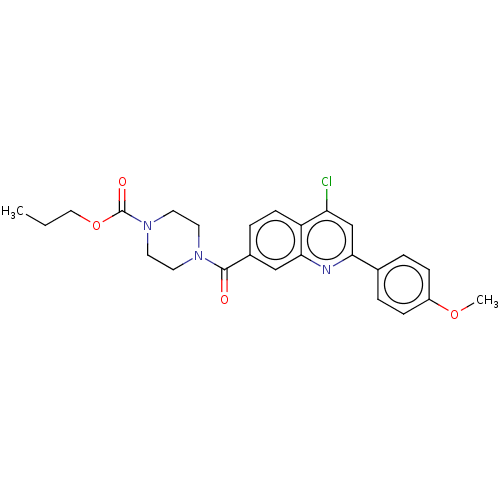 (CHEMBL4440399) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375313 ((4-(3-(4-hydroxyphenyl)imidazo[1,2-a]pyrazin-6-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 284 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50455545 (CHEMBL4204506) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 294 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502434 (CHEMBL4551647) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50578087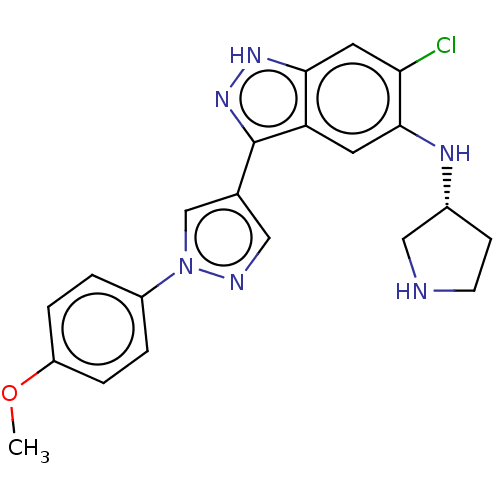 (CHEMBL4871523) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Axl (unknown origin) using 5'FAM labeled KKKKEEIYFFF-NH2 peptide as substrate incubated for 1.5 hrs | Citation and Details Article DOI: 10.1016/j.bmc.2021.116437 BindingDB Entry DOI: 10.7270/Q2H70KN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50455540 (CHEMBL4212480) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1/2 (Homo sapiens (Human)) | BDBM375544 (4-(6-(4-(morpholine-4-carbonyl)phenyl)imidazo[1,2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 321 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of MNK1/2 in human HeLa cells assessed as decrease in eIF4E phosphorylation at Ser209 after 2 hrs by AlphaScreen assay | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1/2 (Homo sapiens (Human)) | BDBM375513 ((4-(3-(4-chlorophenyl)imidazo[1,2-b]pyridazin-6-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 326 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of MNK1/2 in human HeLa cells assessed as decrease in eIF4E phosphorylation at Ser209 after 2 hrs by AlphaScreen assay | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50455543 (CHEMBL4211757) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 331 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50455548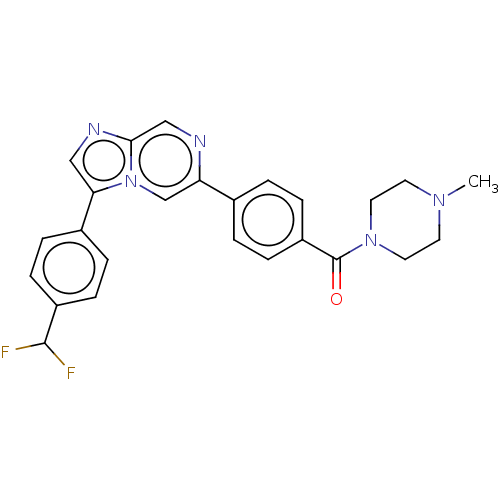 (CHEMBL4202593) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 336 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50455539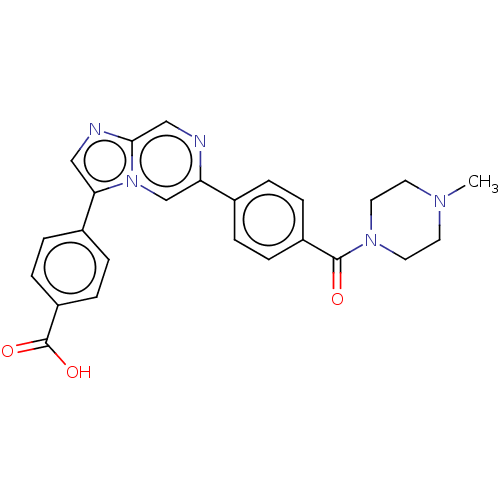 (CHEMBL4217366) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 355 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502426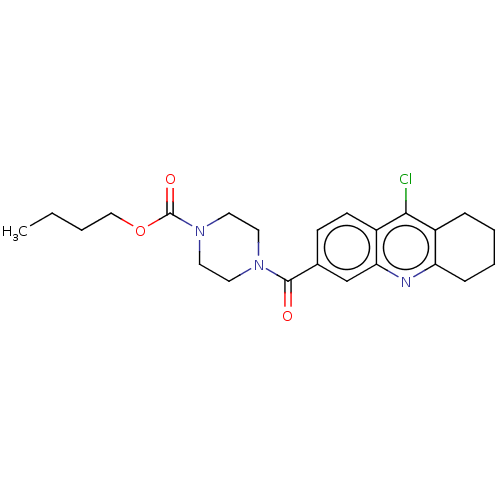 (CHEMBL4448008) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50455548 (CHEMBL4202593) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 369 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 192 total ) | Next | Last >> |