

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isoform 1 of Fibronectin (1) (Homo sapiens (Human)) | BDBM50375222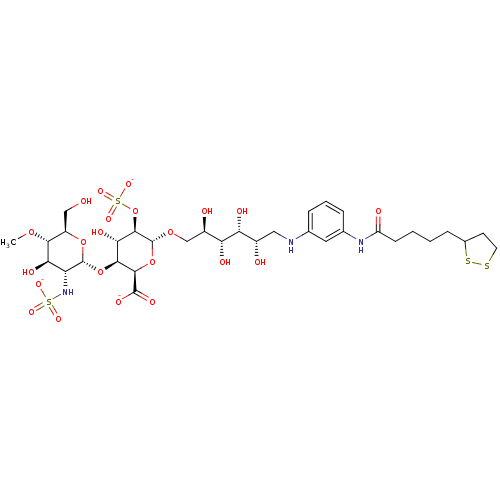 (CHEMBL256614) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a |
Kagoshima University Curated by ChEMBL | Assay Description Binding affinity to fibronectin using surface plasmon resonance imaging sensor method | Bioorg Med Chem Lett 18: 2499-504 (2008) Article DOI: 10.1016/j.bmcl.2008.01.069 BindingDB Entry DOI: 10.7270/Q2FF3T6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Fibronectin (1) (Homo sapiens (Human)) | BDBM50375224 (CHEMBL441895) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a |
Kagoshima University Curated by ChEMBL | Assay Description Binding affinity to fibronectin using surface plasmon resonance imaging sensor method | Bioorg Med Chem Lett 18: 2499-504 (2008) Article DOI: 10.1016/j.bmcl.2008.01.069 BindingDB Entry DOI: 10.7270/Q2FF3T6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Fibronectin (1) (Homo sapiens (Human)) | BDBM50375221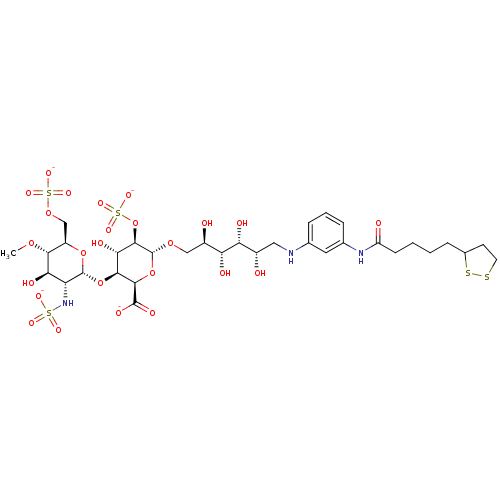 (CHEMBL404104) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a |
Kagoshima University Curated by ChEMBL | Assay Description Binding affinity to fibronectin using surface plasmon resonance imaging sensor method | Bioorg Med Chem Lett 18: 2499-504 (2008) Article DOI: 10.1016/j.bmcl.2008.01.069 BindingDB Entry DOI: 10.7270/Q2FF3T6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Fibronectin (1) (Homo sapiens (Human)) | BDBM50375223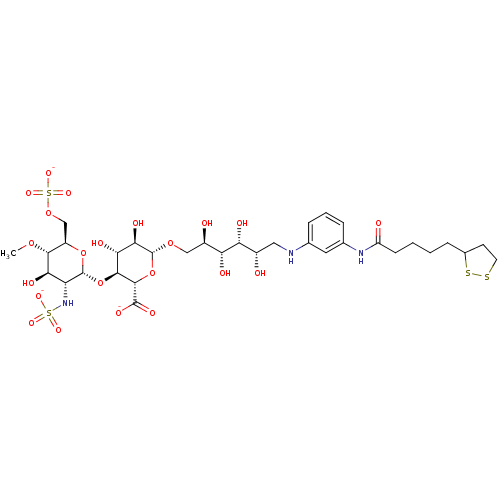 (CHEMBL404103) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a |
Kagoshima University Curated by ChEMBL | Assay Description Binding affinity to fibronectin using surface plasmon resonance imaging sensor method | Bioorg Med Chem Lett 18: 2499-504 (2008) Article DOI: 10.1016/j.bmcl.2008.01.069 BindingDB Entry DOI: 10.7270/Q2FF3T6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor 2 (Homo sapiens (Human)) | BDBM50063776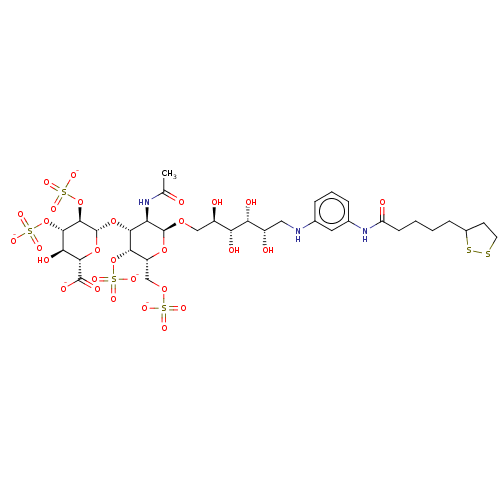 (CHEMBL3397498) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a |
Kagoshima University Curated by ChEMBL | Assay Description Binding affinity to basic fibroblast growth factor (unknown origin) using sugar chip immobilized compound by surface plasmon resonance method | Bioorg Med Chem Lett 25: 1407-11 (2015) Article DOI: 10.1016/j.bmcl.2015.02.054 BindingDB Entry DOI: 10.7270/Q2FB54N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor 2 (Homo sapiens (Human)) | BDBM50063808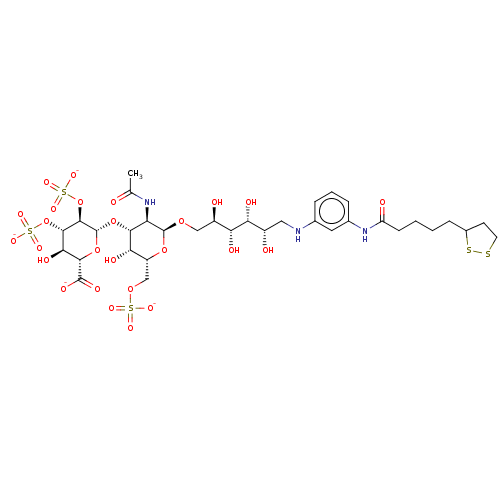 (CHEMBL3397497) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a |
Kagoshima University Curated by ChEMBL | Assay Description Binding affinity to basic fibroblast growth factor (unknown origin) using sugar chip immobilized compound by surface plasmon resonance method | Bioorg Med Chem Lett 25: 1407-11 (2015) Article DOI: 10.1016/j.bmcl.2015.02.054 BindingDB Entry DOI: 10.7270/Q2FB54N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor 2 (Homo sapiens (Human)) | BDBM50063810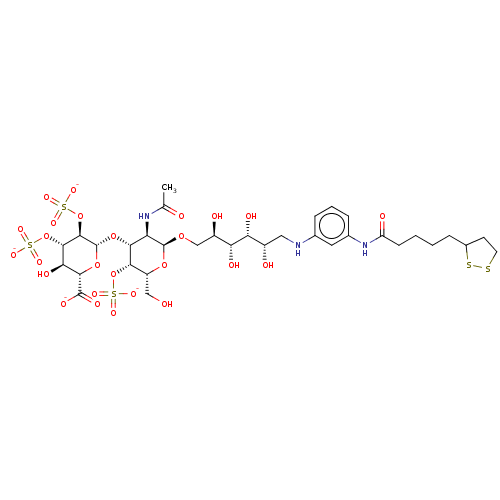 (CHEMBL3397496) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a |
Kagoshima University Curated by ChEMBL | Assay Description Binding affinity to basic fibroblast growth factor (unknown origin) using sugar chip immobilized compound by surface plasmon resonance method | Bioorg Med Chem Lett 25: 1407-11 (2015) Article DOI: 10.1016/j.bmcl.2015.02.054 BindingDB Entry DOI: 10.7270/Q2FB54N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor 2 (Homo sapiens (Human)) | BDBM50063812 (CHEMBL3397495) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a |
Kagoshima University Curated by ChEMBL | Assay Description Binding affinity to basic fibroblast growth factor (unknown origin) using sugar chip immobilized compound by surface plasmon resonance method | Bioorg Med Chem Lett 25: 1407-11 (2015) Article DOI: 10.1016/j.bmcl.2015.02.054 BindingDB Entry DOI: 10.7270/Q2FB54N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor 2 (Homo sapiens (Human)) | BDBM50063824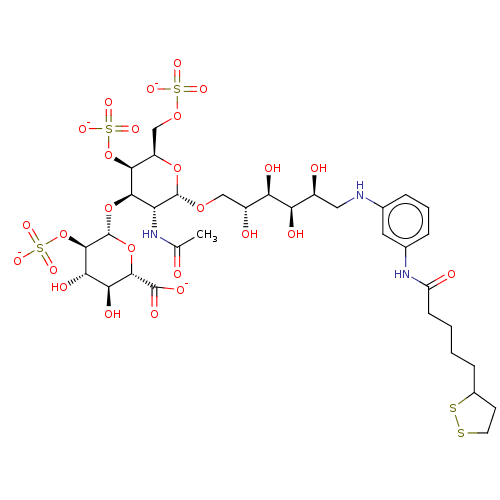 (CHEMBL3397494) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a |
Kagoshima University Curated by ChEMBL | Assay Description Binding affinity to basic fibroblast growth factor (unknown origin) using sugar chip immobilized compound by surface plasmon resonance method | Bioorg Med Chem Lett 25: 1407-11 (2015) Article DOI: 10.1016/j.bmcl.2015.02.054 BindingDB Entry DOI: 10.7270/Q2FB54N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor 2 (Homo sapiens (Human)) | BDBM50063835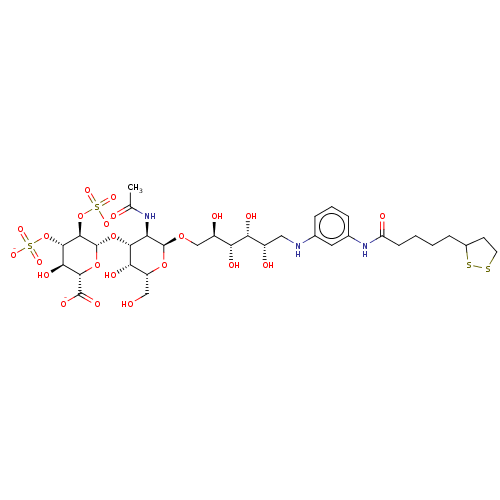 (CHEMBL3397493) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a |
Kagoshima University Curated by ChEMBL | Assay Description Binding affinity to basic fibroblast growth factor (unknown origin) using sugar chip immobilized compound by surface plasmon resonance method | Bioorg Med Chem Lett 25: 1407-11 (2015) Article DOI: 10.1016/j.bmcl.2015.02.054 BindingDB Entry DOI: 10.7270/Q2FB54N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor 2 (Homo sapiens (Human)) | BDBM50063837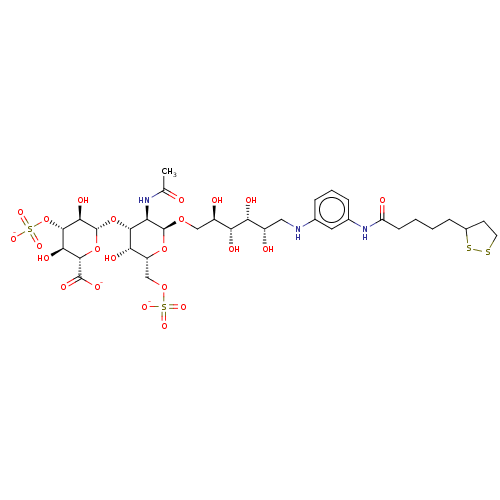 (CHEMBL3397492) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a |
Kagoshima University Curated by ChEMBL | Assay Description Binding affinity to basic fibroblast growth factor (unknown origin) using sugar chip immobilized compound by surface plasmon resonance method | Bioorg Med Chem Lett 25: 1407-11 (2015) Article DOI: 10.1016/j.bmcl.2015.02.054 BindingDB Entry DOI: 10.7270/Q2FB54N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor 2 (Homo sapiens (Human)) | BDBM50063843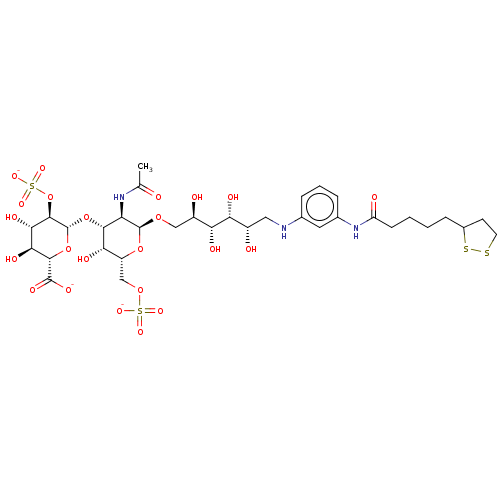 (CHEMBL3397491) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a |
Kagoshima University Curated by ChEMBL | Assay Description Binding affinity to basic fibroblast growth factor (unknown origin) using sugar chip immobilized compound by surface plasmon resonance method | Bioorg Med Chem Lett 25: 1407-11 (2015) Article DOI: 10.1016/j.bmcl.2015.02.054 BindingDB Entry DOI: 10.7270/Q2FB54N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor 2 (Homo sapiens (Human)) | BDBM50063844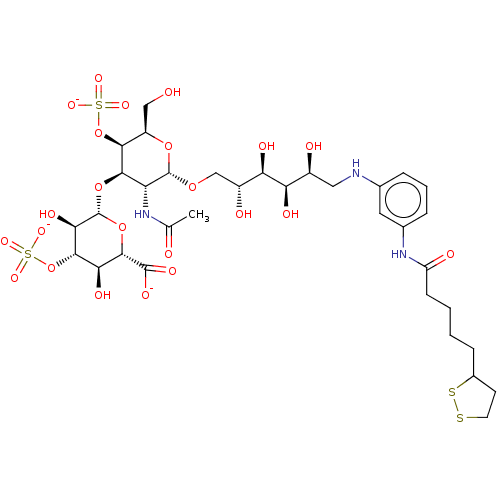 (CHEMBL3397490) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a |
Kagoshima University Curated by ChEMBL | Assay Description Binding affinity to basic fibroblast growth factor (unknown origin) using sugar chip immobilized compound by surface plasmon resonance method | Bioorg Med Chem Lett 25: 1407-11 (2015) Article DOI: 10.1016/j.bmcl.2015.02.054 BindingDB Entry DOI: 10.7270/Q2FB54N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor 2 (Homo sapiens (Human)) | BDBM50063846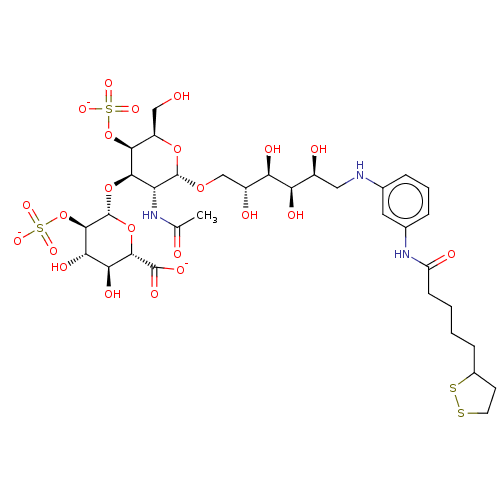 (CHEMBL3397489) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a |
Kagoshima University Curated by ChEMBL | Assay Description Binding affinity to basic fibroblast growth factor (unknown origin) using sugar chip immobilized compound by surface plasmon resonance method | Bioorg Med Chem Lett 25: 1407-11 (2015) Article DOI: 10.1016/j.bmcl.2015.02.054 BindingDB Entry DOI: 10.7270/Q2FB54N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor 2 (Homo sapiens (Human)) | BDBM50063847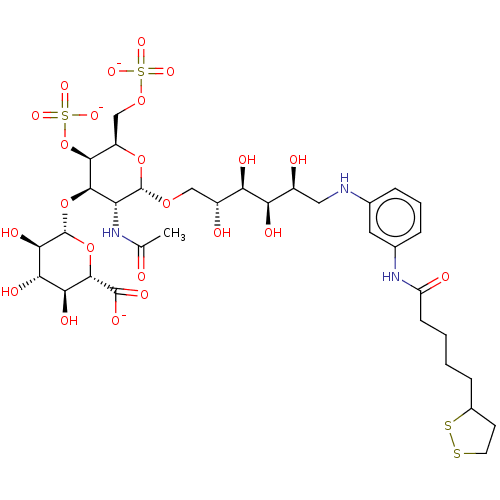 (CHEMBL3397488) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a |
Kagoshima University Curated by ChEMBL | Assay Description Binding affinity to basic fibroblast growth factor (unknown origin) using sugar chip immobilized compound by surface plasmon resonance method | Bioorg Med Chem Lett 25: 1407-11 (2015) Article DOI: 10.1016/j.bmcl.2015.02.054 BindingDB Entry DOI: 10.7270/Q2FB54N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor 2 (Homo sapiens (Human)) | BDBM50063848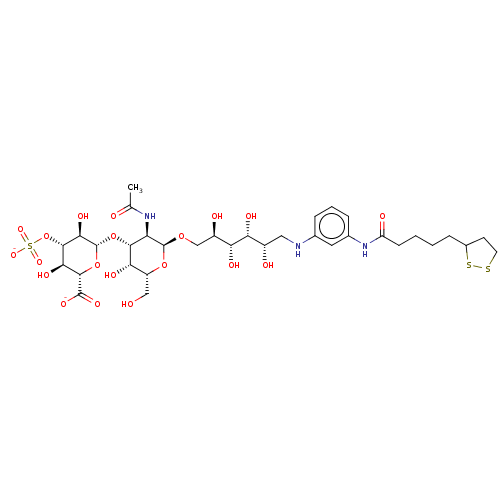 (CHEMBL3397487) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a |
Kagoshima University Curated by ChEMBL | Assay Description Binding affinity to basic fibroblast growth factor (unknown origin) using sugar chip immobilized compound by surface plasmon resonance method | Bioorg Med Chem Lett 25: 1407-11 (2015) Article DOI: 10.1016/j.bmcl.2015.02.054 BindingDB Entry DOI: 10.7270/Q2FB54N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor 2 (Homo sapiens (Human)) | BDBM50063849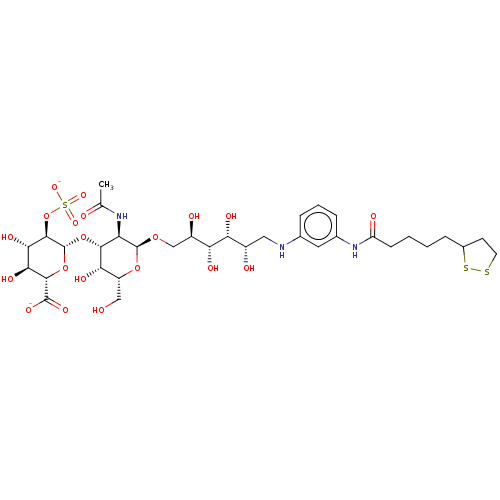 (CHEMBL3397486) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a |
Kagoshima University Curated by ChEMBL | Assay Description Binding affinity to basic fibroblast growth factor (unknown origin) using sugar chip immobilized compound by surface plasmon resonance method | Bioorg Med Chem Lett 25: 1407-11 (2015) Article DOI: 10.1016/j.bmcl.2015.02.054 BindingDB Entry DOI: 10.7270/Q2FB54N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor 2 (Homo sapiens (Human)) | BDBM50063850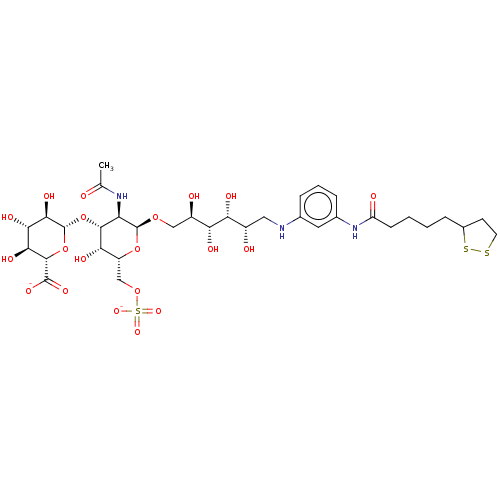 (CHEMBL3397485) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a |
Kagoshima University Curated by ChEMBL | Assay Description Binding affinity to basic fibroblast growth factor (unknown origin) using sugar chip immobilized compound by surface plasmon resonance method | Bioorg Med Chem Lett 25: 1407-11 (2015) Article DOI: 10.1016/j.bmcl.2015.02.054 BindingDB Entry DOI: 10.7270/Q2FB54N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor 2 (Homo sapiens (Human)) | BDBM50073747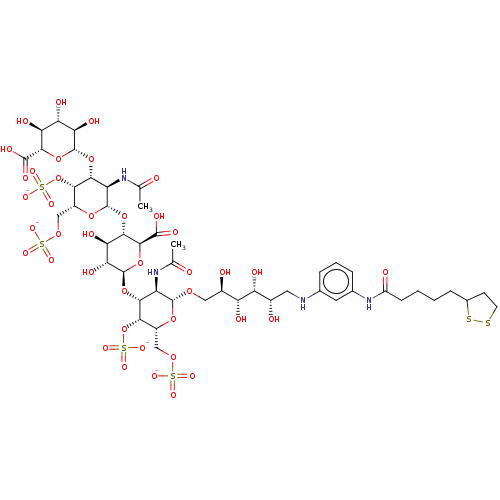 (CHEMBL3409457) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a |
Kagoshima University Curated by ChEMBL | Assay Description Binding affinity to FGF2 (unknown origin) assessed as change in luminance intensity at at 25 degC by SPR imaging sensor method | Bioorg Med Chem Lett 25: 1552-5 (2015) Article DOI: 10.1016/j.bmcl.2015.02.011 BindingDB Entry DOI: 10.7270/Q2Z89F4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor 2 (Homo sapiens (Human)) | BDBM50073748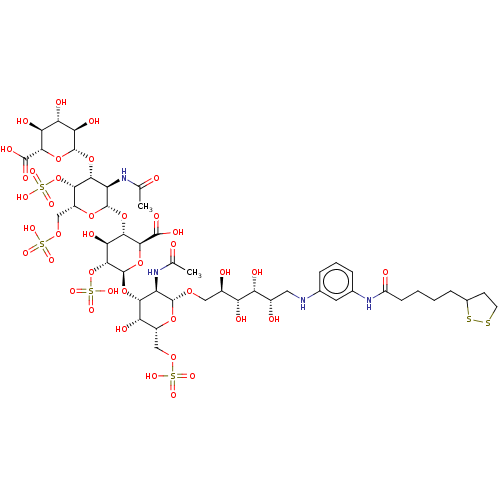 (CHEMBL3559508) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a |
Kagoshima University Curated by ChEMBL | Assay Description Binding affinity to FGF2 (unknown origin) assessed as change in luminance intensity at at 25 degC by SPR imaging sensor method | Bioorg Med Chem Lett 25: 1552-5 (2015) Article DOI: 10.1016/j.bmcl.2015.02.011 BindingDB Entry DOI: 10.7270/Q2Z89F4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor 2 (Homo sapiens (Human)) | BDBM50073749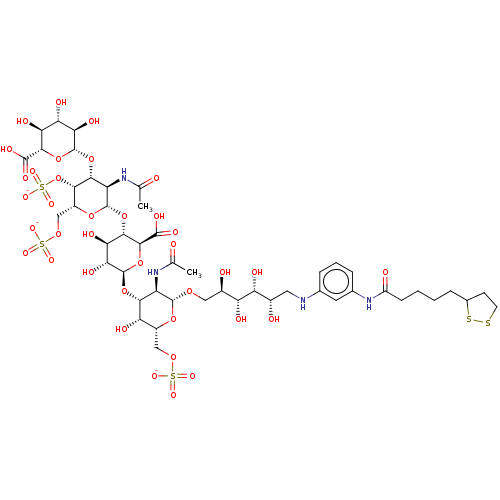 (CHEMBL3409455) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a |
Kagoshima University Curated by ChEMBL | Assay Description Binding affinity to FGF2 (unknown origin) assessed as change in luminance intensity at at 25 degC by SPR imaging sensor method | Bioorg Med Chem Lett 25: 1552-5 (2015) Article DOI: 10.1016/j.bmcl.2015.02.011 BindingDB Entry DOI: 10.7270/Q2Z89F4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor 2 (Homo sapiens (Human)) | BDBM50073750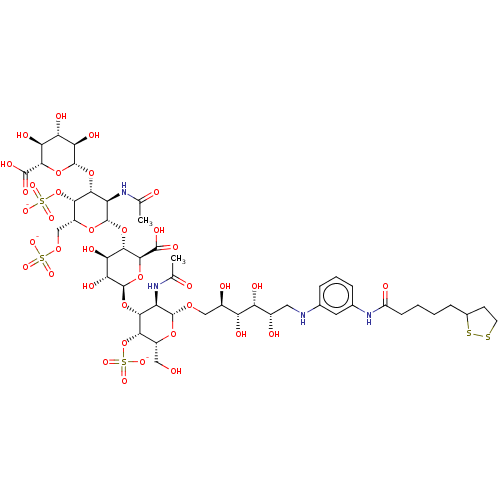 (CHEMBL3409454) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a |
Kagoshima University Curated by ChEMBL | Assay Description Binding affinity to FGF2 (unknown origin) assessed as change in luminance intensity at at 25 degC by SPR imaging sensor method | Bioorg Med Chem Lett 25: 1552-5 (2015) Article DOI: 10.1016/j.bmcl.2015.02.011 BindingDB Entry DOI: 10.7270/Q2Z89F4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor 2 (Homo sapiens (Human)) | BDBM50073751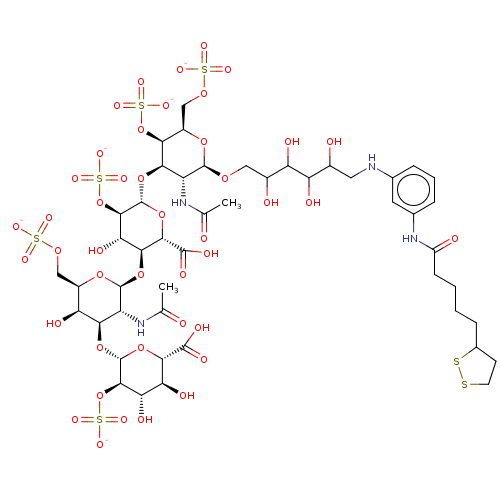 (CHEMBL3409453) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a |
Kagoshima University Curated by ChEMBL | Assay Description Binding affinity to FGF2 (unknown origin) assessed as change in luminance intensity at at 25 degC by SPR imaging sensor method | Bioorg Med Chem Lett 25: 1552-5 (2015) Article DOI: 10.1016/j.bmcl.2015.02.011 BindingDB Entry DOI: 10.7270/Q2Z89F4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor 2 (Homo sapiens (Human)) | BDBM50073810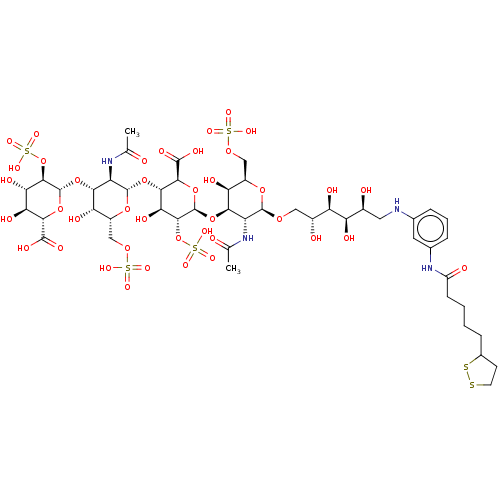 (CHEMBL3559507) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a |
Kagoshima University Curated by ChEMBL | Assay Description Binding affinity to FGF2 (unknown origin) assessed as change in luminance intensity at at 25 degC by SPR imaging sensor method | Bioorg Med Chem Lett 25: 1552-5 (2015) Article DOI: 10.1016/j.bmcl.2015.02.011 BindingDB Entry DOI: 10.7270/Q2Z89F4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50250403 (CHEMBL2386454) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.26E+3 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at human TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50250404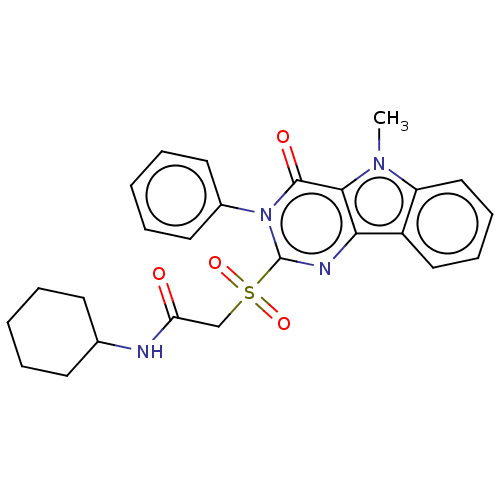 (CHEMBL4077391) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at human TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50250405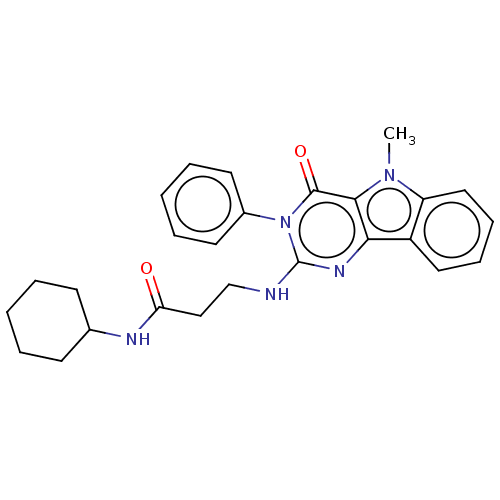 (CHEMBL4065144) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at human TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50250406 (CHEMBL4092815) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at human TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50250407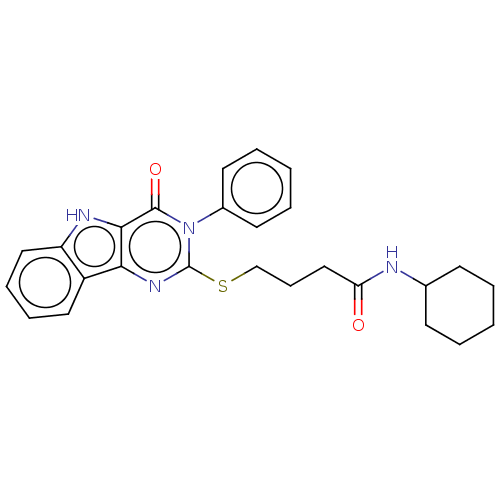 (CHEMBL4085061) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at human TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50250408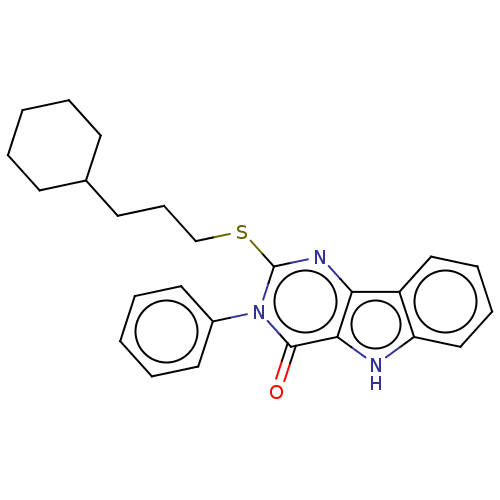 (CHEMBL4095263) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at human TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50250409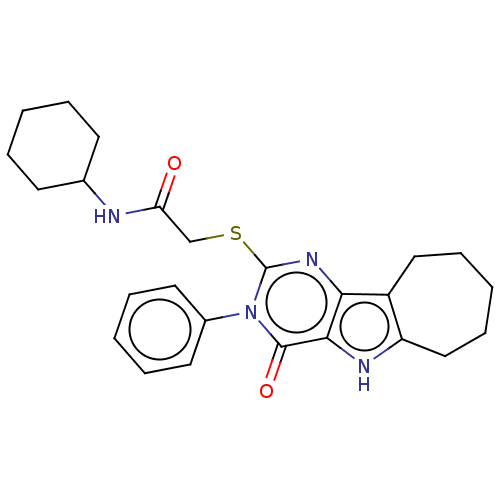 (CHEMBL4074812) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.42E+3 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at human TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50250410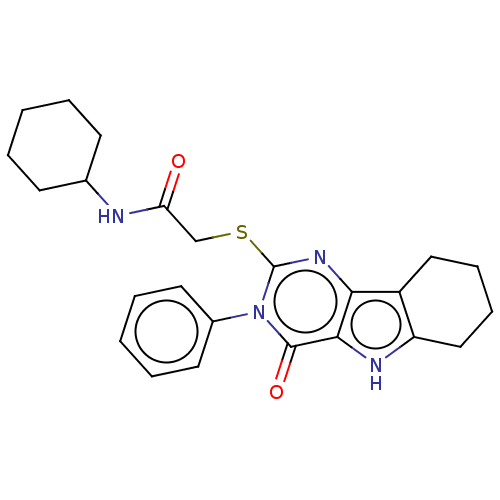 (CHEMBL4089458) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at human TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50250411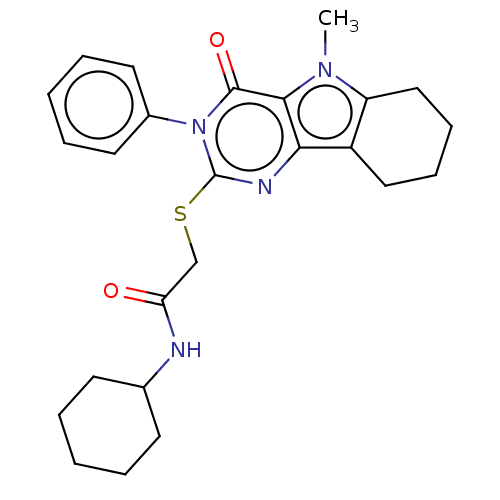 (CHEMBL4061465) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at human TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50250412 (CHEMBL4091234) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at human TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Mus musculus) | BDBM50250413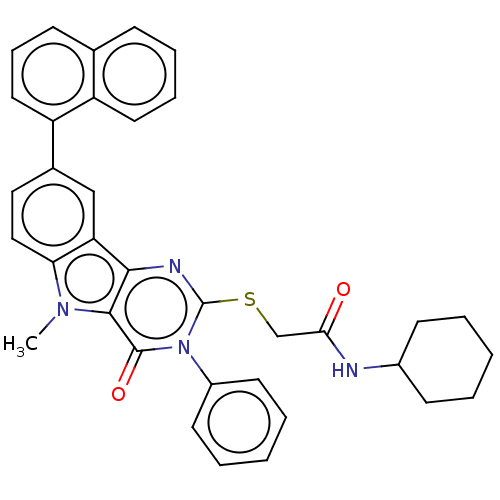 (CHEMBL4078865) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.58E+3 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at mouse TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Mus musculus) | BDBM50250414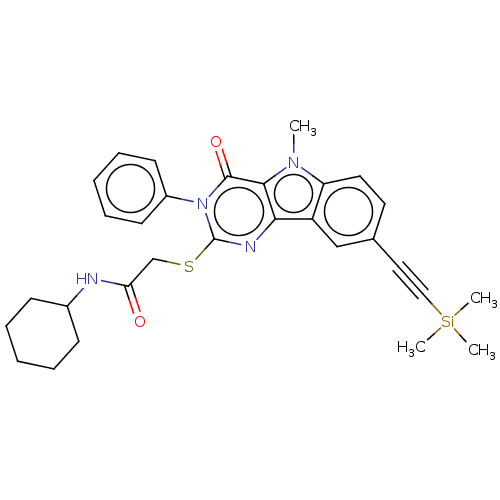 (CHEMBL4086723) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at mouse TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Mus musculus) | BDBM50250415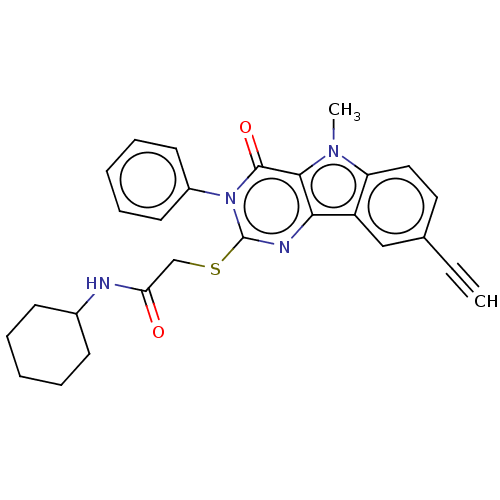 (CHEMBL4065290) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.74E+3 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at mouse TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50250416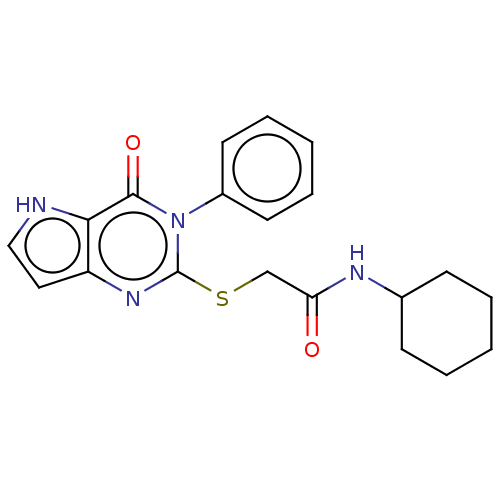 (CHEMBL4063373) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at human TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50250417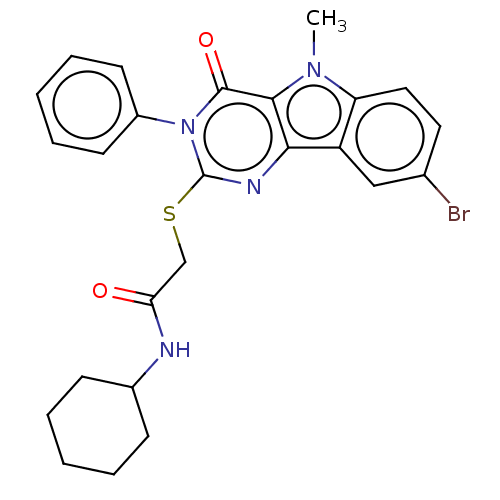 (CHEMBL4101433) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.58E+3 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at human TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50250418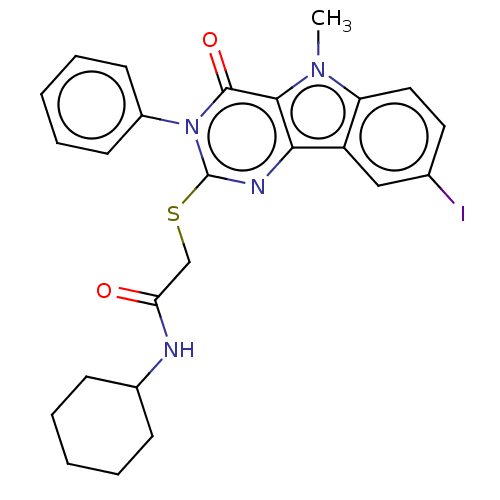 (CHEMBL4073017) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.75E+3 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at human TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50250419 (CHEMBL4064434) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at human TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50250420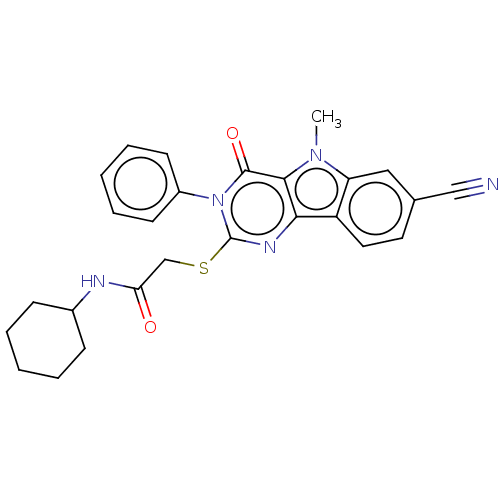 (CHEMBL4085969) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at human TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50250421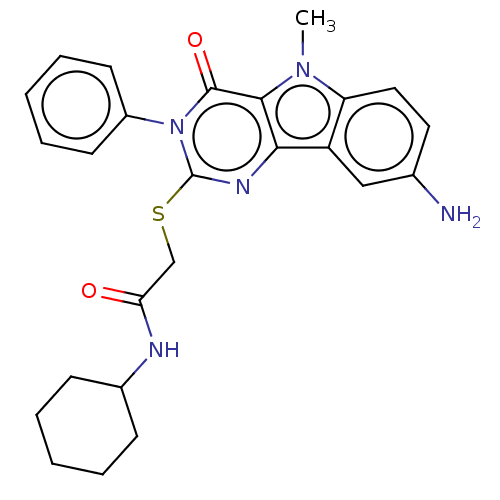 (CHEMBL4097843) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at human TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50250423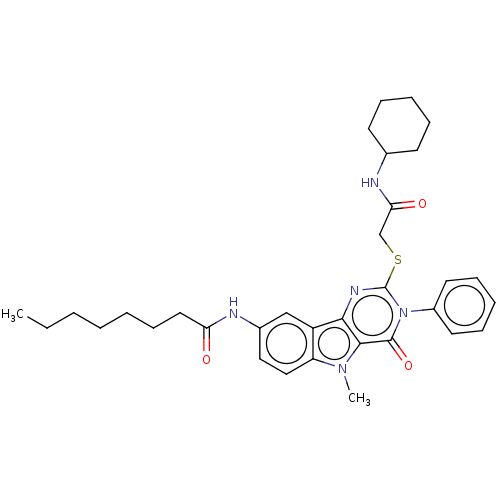 (CHEMBL4105577) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at human TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50250424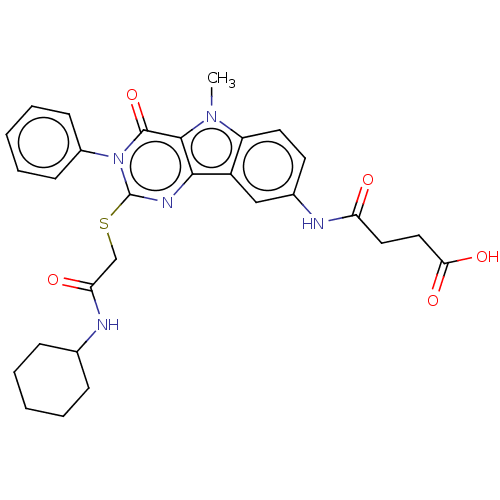 (CHEMBL4087701) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at human TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50250433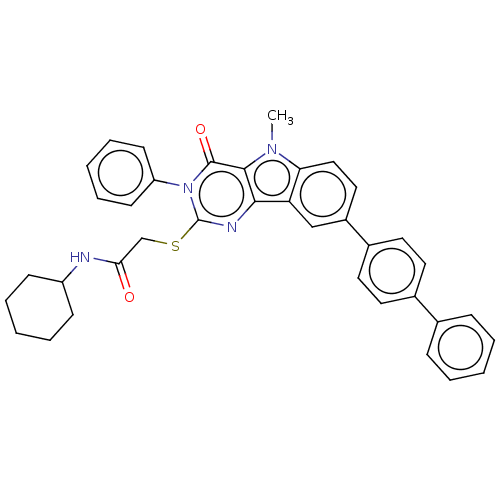 (CHEMBL4095394) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at human TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50250413 (CHEMBL4078865) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at human TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50250434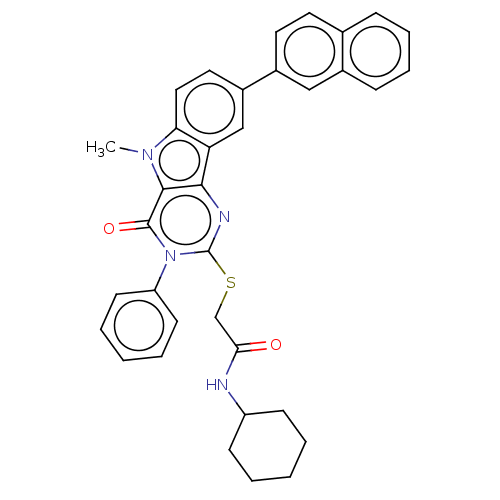 (CHEMBL4103142) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 920 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at human TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Homo sapiens (Human)) | BDBM50250415 (CHEMBL4065290) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.27E+3 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at human TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 4 (Mus musculus) | BDBM50250403 (CHEMBL2386454) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.17E+3 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at mouse TLR4 expressed in HEK293 blue cells assessed as induction of NF-kappaB activation-mediated SEAP production after 20 to 24 h... | J Med Chem 60: 9142-9161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00797 BindingDB Entry DOI: 10.7270/Q2TQ63ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 122 total ) | Next | Last >> |