

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50187684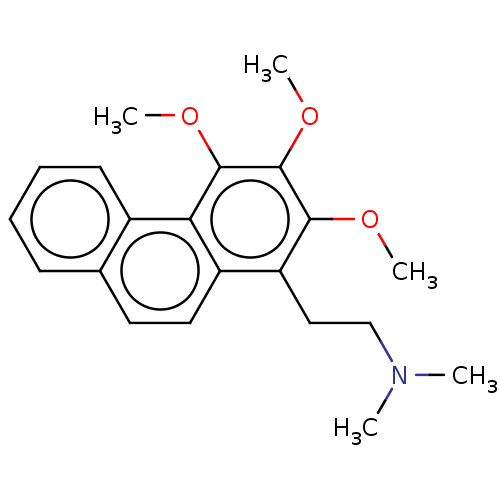 (CHEMBL3827910) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malaya Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BChE preincubated for 15 mins followed by addition of S-butyrylthiocholine chloride as substrate measured after... | Bioorg Med Chem 24: 4464-4469 (2016) Article DOI: 10.1016/j.bmc.2016.07.043 BindingDB Entry DOI: 10.7270/Q2MG7RFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 2.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malaya Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) pre-incubated for 15 mins before S-butyrylthiocholine chloride substrate addition and measured after 30 mins by c... | Bioorg Med Chem 24: 4464-4469 (2016) Article DOI: 10.1016/j.bmc.2016.07.043 BindingDB Entry DOI: 10.7270/Q2MG7RFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malaya Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 15 mins followed by addition of acetylthiocholine iodide as substrate measured after 30 mins by Ellm... | Bioorg Med Chem 24: 4464-4469 (2016) Article DOI: 10.1016/j.bmc.2016.07.043 BindingDB Entry DOI: 10.7270/Q2MG7RFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malaya Curated by ChEMBL | Assay Description Inhibition of equine serum BChE preincubated for 15 mins followed by addition of S-butyrylthiocholine chloride as substrate measured after 30 mins by... | Bioorg Med Chem 24: 4464-4469 (2016) Article DOI: 10.1016/j.bmc.2016.07.043 BindingDB Entry DOI: 10.7270/Q2MG7RFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50187684 (CHEMBL3827910) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malaya Curated by ChEMBL | Assay Description Inhibition of equine serum BChE preincubated for 15 mins followed by addition of S-butyrylthiocholine chloride as substrate measured after 30 mins by... | Bioorg Med Chem 24: 4464-4469 (2016) Article DOI: 10.1016/j.bmc.2016.07.043 BindingDB Entry DOI: 10.7270/Q2MG7RFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50187683 (CHEMBL1186488) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malaya Curated by ChEMBL | Assay Description Inhibition of equine serum BChE preincubated for 15 mins followed by addition of S-butyrylthiocholine chloride as substrate measured after 30 mins by... | Bioorg Med Chem 24: 4464-4469 (2016) Article DOI: 10.1016/j.bmc.2016.07.043 BindingDB Entry DOI: 10.7270/Q2MG7RFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50187681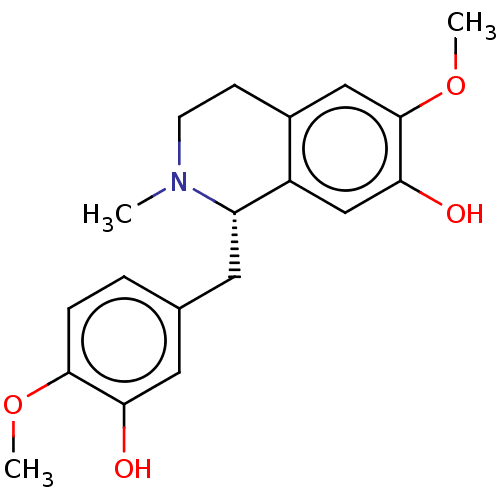 ((+)-Reticuline | (S)-Reticuline | CHEBI:16718 | L-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malaya Curated by ChEMBL | Assay Description Inhibition of equine serum BChE preincubated for 15 mins followed by addition of S-butyrylthiocholine chloride as substrate measured after 30 mins by... | Bioorg Med Chem 24: 4464-4469 (2016) Article DOI: 10.1016/j.bmc.2016.07.043 BindingDB Entry DOI: 10.7270/Q2MG7RFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50187679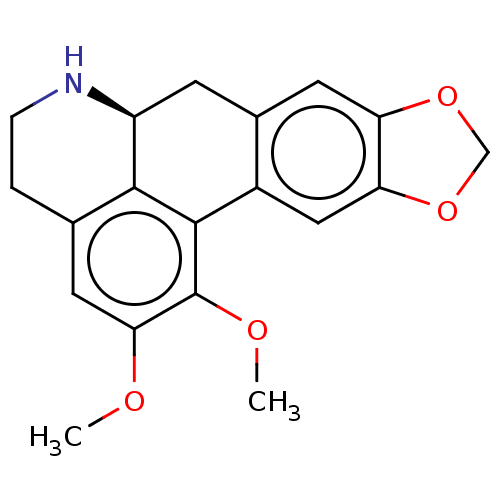 (CHEMBL3827872) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malaya Curated by ChEMBL | Assay Description Inhibition of equine serum BChE preincubated for 15 mins followed by addition of S-butyrylthiocholine chloride as substrate measured after 30 mins by... | Bioorg Med Chem 24: 4464-4469 (2016) Article DOI: 10.1016/j.bmc.2016.07.043 BindingDB Entry DOI: 10.7270/Q2MG7RFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50187680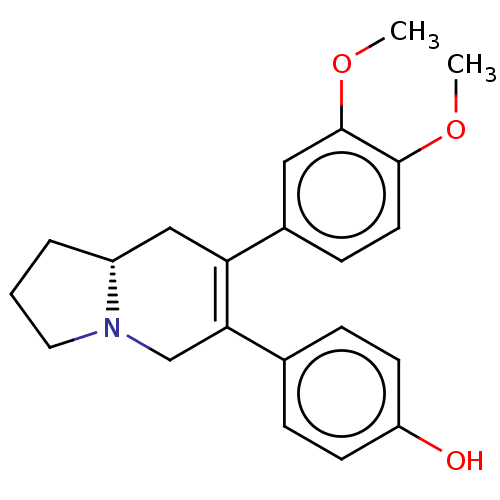 (CHEMBL483227) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malaya Curated by ChEMBL | Assay Description Inhibition of equine serum BChE preincubated for 15 mins followed by addition of S-butyrylthiocholine chloride as substrate measured after 30 mins by... | Bioorg Med Chem 24: 4464-4469 (2016) Article DOI: 10.1016/j.bmc.2016.07.043 BindingDB Entry DOI: 10.7270/Q2MG7RFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50187680 (CHEMBL483227) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malaya Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 15 mins followed by addition of acetylthiocholine iodide as substrate measured after 30 mins by Ellm... | Bioorg Med Chem 24: 4464-4469 (2016) Article DOI: 10.1016/j.bmc.2016.07.043 BindingDB Entry DOI: 10.7270/Q2MG7RFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50187679 (CHEMBL3827872) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malaya Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 15 mins followed by addition of acetylthiocholine iodide as substrate measured after 30 mins by Ellm... | Bioorg Med Chem 24: 4464-4469 (2016) Article DOI: 10.1016/j.bmc.2016.07.043 BindingDB Entry DOI: 10.7270/Q2MG7RFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50250422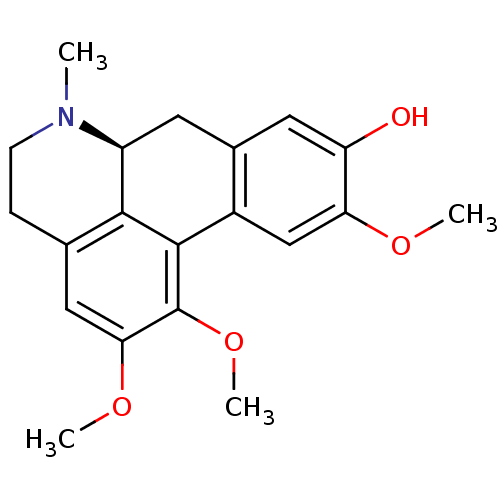 ((+)-N-methyl-laurotetanine | CHEMBL464099 | N-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malaya Curated by ChEMBL | Assay Description Inhibition of equine serum BChE preincubated for 15 mins followed by addition of S-butyrylthiocholine chloride as substrate measured after 30 mins by... | Bioorg Med Chem 24: 4464-4469 (2016) Article DOI: 10.1016/j.bmc.2016.07.043 BindingDB Entry DOI: 10.7270/Q2MG7RFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50187682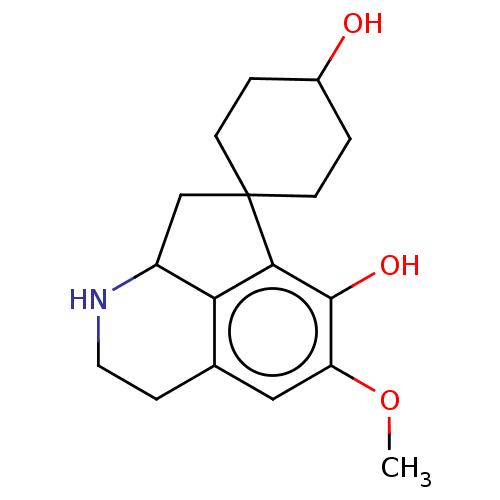 (CHEMBL3827670) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.88E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malaya Curated by ChEMBL | Assay Description Inhibition of equine serum BChE preincubated for 15 mins followed by addition of S-butyrylthiocholine chloride as substrate measured after 30 mins by... | Bioorg Med Chem 24: 4464-4469 (2016) Article DOI: 10.1016/j.bmc.2016.07.043 BindingDB Entry DOI: 10.7270/Q2MG7RFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50187681 ((+)-Reticuline | (S)-Reticuline | CHEBI:16718 | L-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malaya Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 15 mins followed by addition of acetylthiocholine iodide as substrate measured after 30 mins by Ellm... | Bioorg Med Chem 24: 4464-4469 (2016) Article DOI: 10.1016/j.bmc.2016.07.043 BindingDB Entry DOI: 10.7270/Q2MG7RFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||