 Found 156 hits with Last Name = 'wang' and Initial = 'zy'
Found 156 hits with Last Name = 'wang' and Initial = 'zy' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM370555
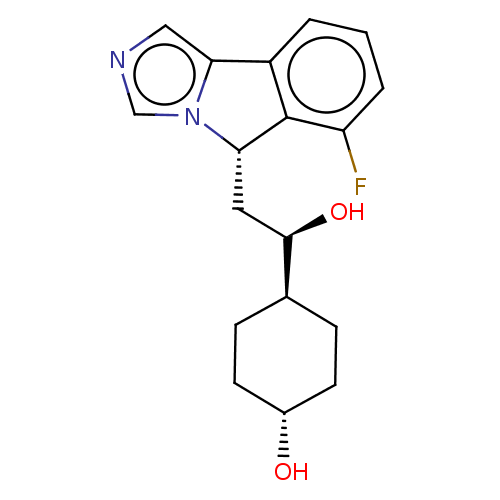
((1R,4r)-4-((R)-2-((S)-6-fluoro-5H-imidazo[5,1- a]i...)Show SMILES O[C@H](C[C@H]1c2c(cccc2F)-c2cncn12)[C@H]1CC[C@H](O)CC1 |r,wU:19.22,wD:3.2,1.0,16.19,(-.99,-3.03,;-.22,-1.7,;-.99,-.37,;-2.53,-.37,;-3.37,.92,;-4.85,.53,;-5.94,1.61,;-5.54,3.1,;-4.06,3.5,;-2.97,2.41,;-1.48,2.81,;-4.93,-1.01,;-5.9,-2.21,;-5.06,-3.5,;-3.58,-3.1,;-3.5,-1.56,;1.32,-1.7,;2.09,-3.03,;3.63,-3.03,;4.4,-1.7,;5.94,-1.7,;3.63,-.37,;2.09,-.37,)| Show InChI InChI=1S/C18H21FN2O2/c19-14-3-1-2-13-16-9-20-10-21(16)15(18(13)14)8-17(23)11-4-6-12(22)7-5-11/h1-3,9-12,15,17,22-23H,4-8H2/t11-,12-,15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50365773

(CHEMBL1956552)Show SMILES CCN(CC)CCCNc1ccc2C(=O)c3cccc4ccnc(-c2c1)c34 Show InChI InChI=1S/C23H25N3O/c1-3-26(4-2)14-6-12-24-17-9-10-18-20(15-17)22-21-16(11-13-25-22)7-5-8-19(21)23(18)27/h5,7-11,13,15,24H,3-4,6,12,14H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of Electrophorus electricus AChE assessed as hydrolysis of acetylthiocholineiodide after 15 mins incubation by spectrophot... |
Bioorg Med Chem Lett 22: 2257-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.090
BindingDB Entry DOI: 10.7270/Q2HH6KJX |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50606592

(CHEMBL5219838) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50200540

(CHEMBL3972619)Show InChI InChI=1S/C14H12BrN3O/c15-10-5-13(12-8-17-18-14(12)6-10)16-7-9-1-3-11(19)4-2-9/h1-6,8,16,19H,7H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50200540

(CHEMBL3972619)Show InChI InChI=1S/C14H12BrN3O/c15-10-5-13(12-8-17-18-14(12)6-10)16-7-9-1-3-11(19)4-2-9/h1-6,8,16,19H,7H2,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50606592

(CHEMBL5219838) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 4.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50207089
(D-1-Methyltryptophan | D-1MT | Indoximod)Show InChI InChI=1S/C12H14N2O2/c1-14-7-8(6-10(13)12(15)16)9-4-2-3-5-11(9)14/h2-5,7,10H,6,13H2,1H3,(H,15,16)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50285416
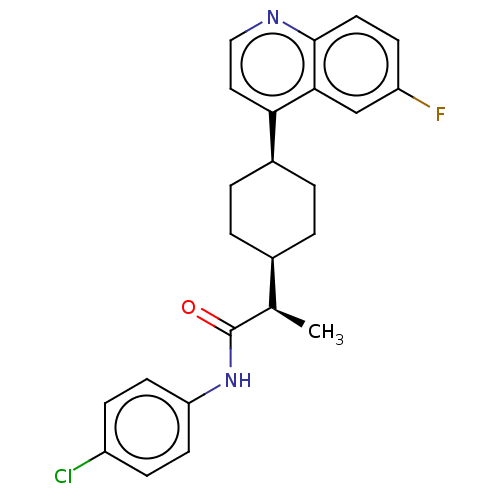
(CHEMBL4161733)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc2ccc(F)cc12)[C@@H](C)C(=O)Nc1ccc(Cl)cc1 |r,wU:18.21,1.0,wD:4.7,(66.16,-9.22,;66.17,-7.68,;66.16,-6.15,;67.5,-5.38,;68.84,-6.15,;68.83,-7.69,;67.5,-8.46,;70.18,-5.39,;71.5,-6.16,;72.84,-5.4,;72.84,-3.85,;71.5,-3.08,;71.5,-1.55,;70.17,-.79,;68.84,-1.56,;67.5,-.8,;68.85,-3.09,;70.18,-3.85,;64.83,-8.46,;64.84,-10,;63.5,-7.69,;63.49,-6.14,;62.16,-8.46,;60.83,-7.69,;59.49,-8.47,;58.15,-7.69,;58.16,-6.14,;56.82,-5.38,;59.49,-5.38,;60.83,-6.14,)| Show InChI InChI=1S/C24H24ClFN2O/c1-15(24(29)28-20-9-6-18(25)7-10-20)16-2-4-17(5-3-16)21-12-13-27-23-11-8-19(26)14-22(21)23/h6-17H,2-5H2,1H3,(H,28,29)/t15-,16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50562497
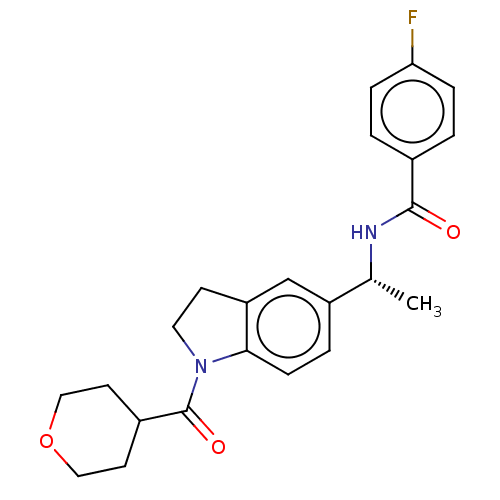
(CHEMBL4778760)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCc2c1)C(=O)C1CCOCC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50590774

(CHEMBL5203175) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126143

(Epacadostat | INCB-024360)Show SMILES NS(=O)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C11H13BrFN7O4S/c12-7-5-6(1-2-8(7)13)17-11(18-21)9-10(20-24-19-9)15-3-4-16-25(14,22)23/h1-2,5,16,21H,3-4H2,(H,15,20)(H,17,18)(H2,14,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BChE after 15 mins incubation by spectrophotometry based Ellman's method |
Bioorg Med Chem Lett 22: 2257-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.090
BindingDB Entry DOI: 10.7270/Q2HH6KJX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50590774

(CHEMBL5203175) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50606613

(CHEMBL5219865)Show SMILES O[C@@H]1CCCC[C@H]1CNc1cc(Br)cc2[nH]ncc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50590773
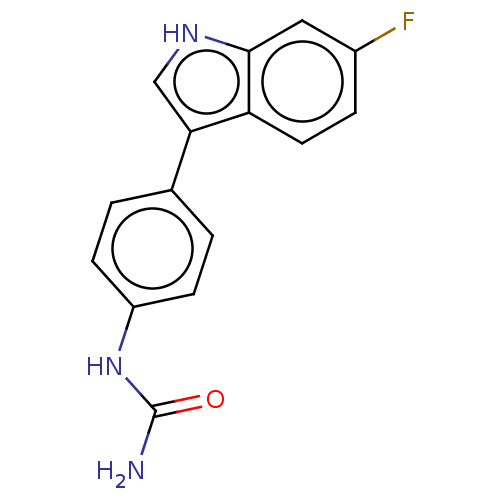
(CHEMBL5177466) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126143

(Epacadostat | INCB-024360)Show SMILES NS(=O)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C11H13BrFN7O4S/c12-7-5-6(1-2-8(7)13)17-11(18-21)9-10(20-24-19-9)15-3-4-16-25(14,22)23/h1-2,5,16,21H,3-4H2,(H,15,20)(H,17,18)(H2,14,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50590773

(CHEMBL5177466) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
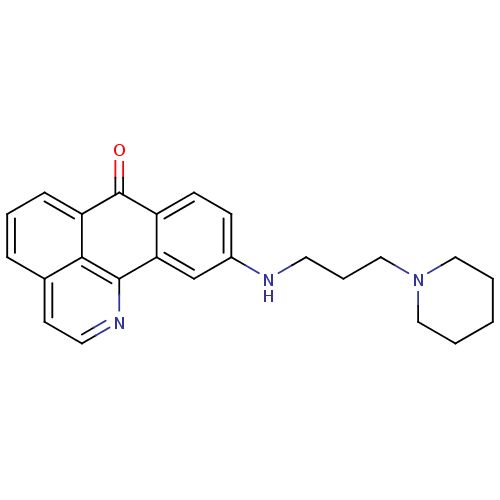
(Homo sapiens (Human)) | BDBM370555

((1R,4r)-4-((R)-2-((S)-6-fluoro-5H-imidazo[5,1- a]i...)Show SMILES O[C@H](C[C@H]1c2c(cccc2F)-c2cncn12)[C@H]1CC[C@H](O)CC1 |r,wU:19.22,wD:3.2,1.0,16.19,(-.99,-3.03,;-.22,-1.7,;-.99,-.37,;-2.53,-.37,;-3.37,.92,;-4.85,.53,;-5.94,1.61,;-5.54,3.1,;-4.06,3.5,;-2.97,2.41,;-1.48,2.81,;-4.93,-1.01,;-5.9,-2.21,;-5.06,-3.5,;-3.58,-3.1,;-3.5,-1.56,;1.32,-1.7,;2.09,-3.03,;3.63,-3.03,;4.4,-1.7,;5.94,-1.7,;3.63,-.37,;2.09,-.37,)| Show InChI InChI=1S/C18H21FN2O2/c19-14-3-1-2-13-16-9-20-10-21(16)15(18(13)14)8-17(23)11-4-6-12(22)7-5-11/h1-3,9-12,15,17,22-23H,4-8H2/t11-,12-,15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50606612
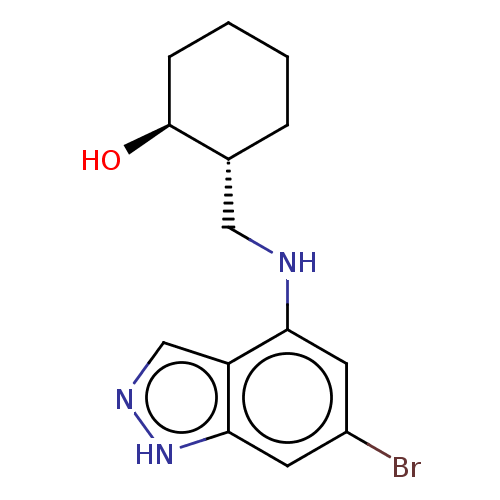
(CHEMBL5220396)Show SMILES O[C@H]1CCCC[C@@H]1CNc1cc(Br)cc2[nH]ncc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50606610
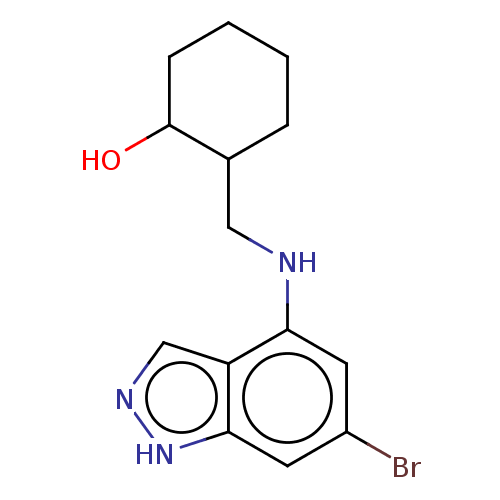
(CHEMBL5220677) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50606620

(CHEMBL5218595)Show SMILES O[C@@H]1CCCC[C@H]1CNc1cc(Cl)cc2[nH]ncc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method |
Bioorg Med Chem Lett 22: 2257-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.090
BindingDB Entry DOI: 10.7270/Q2HH6KJX |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM370555

((1R,4r)-4-((R)-2-((S)-6-fluoro-5H-imidazo[5,1- a]i...)Show SMILES O[C@H](C[C@H]1c2c(cccc2F)-c2cncn12)[C@H]1CC[C@H](O)CC1 |r,wU:19.22,wD:3.2,1.0,16.19,(-.99,-3.03,;-.22,-1.7,;-.99,-.37,;-2.53,-.37,;-3.37,.92,;-4.85,.53,;-5.94,1.61,;-5.54,3.1,;-4.06,3.5,;-2.97,2.41,;-1.48,2.81,;-4.93,-1.01,;-5.9,-2.21,;-5.06,-3.5,;-3.58,-3.1,;-3.5,-1.56,;1.32,-1.7,;2.09,-3.03,;3.63,-3.03,;4.4,-1.7,;5.94,-1.7,;3.63,-.37,;2.09,-.37,)| Show InChI InChI=1S/C18H21FN2O2/c19-14-3-1-2-13-16-9-20-10-21(16)15(18(13)14)8-17(23)11-4-6-12(22)7-5-11/h1-3,9-12,15,17,22-23H,4-8H2/t11-,12-,15-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50606628

(CHEMBL5220436)Show SMILES CC(=O)O[C@@H]1CCCC[C@H]1CNc1cc(Br)cc2[nH]ncc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50365773

(CHEMBL1956552)Show SMILES CCN(CC)CCCNc1ccc2C(=O)c3cccc4ccnc(-c2c1)c34 Show InChI InChI=1S/C23H25N3O/c1-3-26(4-2)14-6-12-24-17-9-10-18-20(15-17)22-21-16(11-13-25-22)7-5-8-19(21)23(18)27/h5,7-11,13,15,24H,3-4,6,12,14H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method |
Bioorg Med Chem Lett 22: 2257-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.090
BindingDB Entry DOI: 10.7270/Q2HH6KJX |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50606631
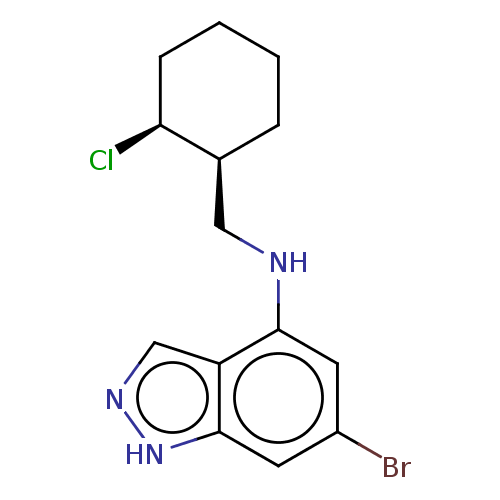
(CHEMBL5218978)Show SMILES Cl[C@H]1CCCC[C@H]1CNc1cc(Br)cc2[nH]ncc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50606591
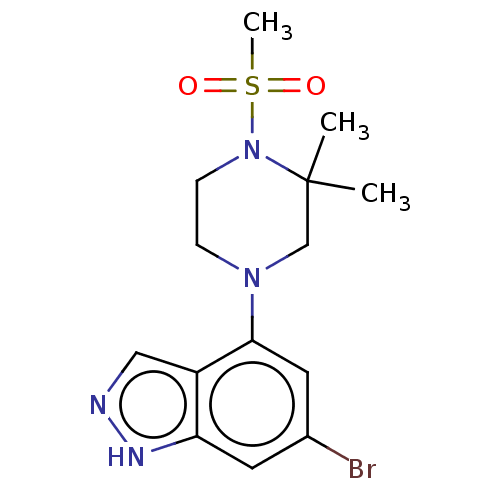
(CHEMBL5219380)Show SMILES CC1(C)CN(CCN1S(C)(=O)=O)c1cc(Br)cc2[nH]ncc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50606631

(CHEMBL5218978)Show SMILES Cl[C@H]1CCCC[C@H]1CNc1cc(Br)cc2[nH]ncc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50504430
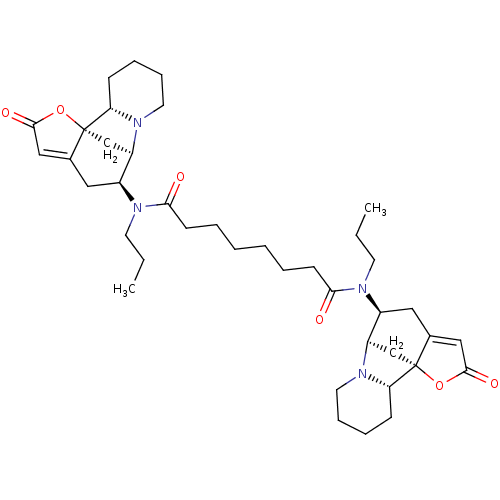
(CHEMBL4474330)Show SMILES [H][C@@]12C[C@]3(OC(=O)C=C3C[C@@H]1N(CCC)C(=O)CCCCCCC(=O)N(CCC)[C@H]1CC3=CC(=O)O[C@]33C[C@@]1([H])N1CCCC[C@@]31[H])[C@]1([H])CCCCN21 |r,c:7,t:33| Show InChI InChI=1S/C40H58N4O6/c1-3-17-43(29-21-27-23-37(47)49-39(27)25-31(29)41-19-11-9-13-33(39)41)35(45)15-7-5-6-8-16-36(46)44(18-4-2)30-22-28-24-38(48)50-40(28)26-32(30)42-20-12-10-14-34(40)42/h23-24,29-34H,3-22,25-26H2,1-2H3/t29-,30-,31+,32+,33-,34-,39+,40+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Jinan University
Curated by ChEMBL
| Assay Description
Reversal of P-gp mediated multidrug resistance in human HepG2/DOX cells assessed as doxorubicin IC50 at 10 uM measured within 48 hrs by MTT assay (Rv... |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111726
BindingDB Entry DOI: 10.7270/Q20R9SNN |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50606593
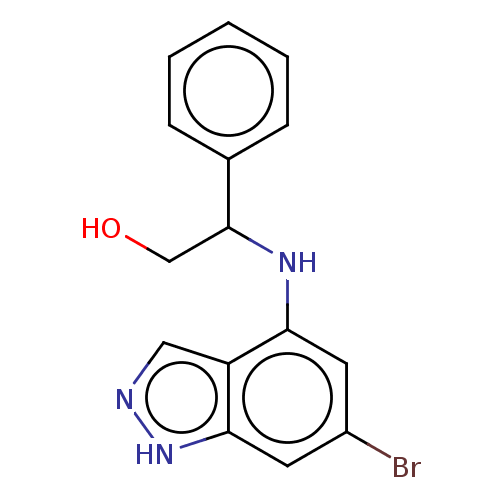
(CHEMBL5219702) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50606629
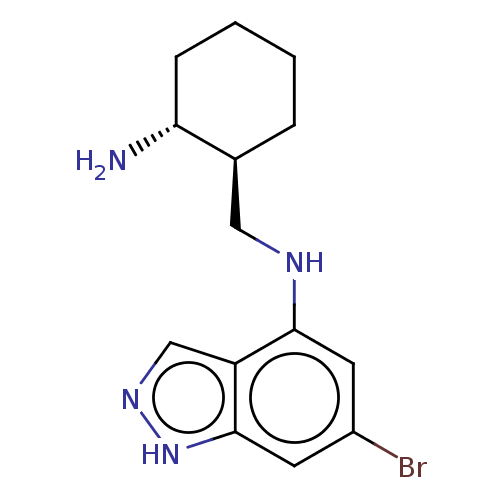
(CHEMBL5219595)Show SMILES N[C@@H]1CCCC[C@H]1CNc1cc(Br)cc2[nH]ncc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50606622
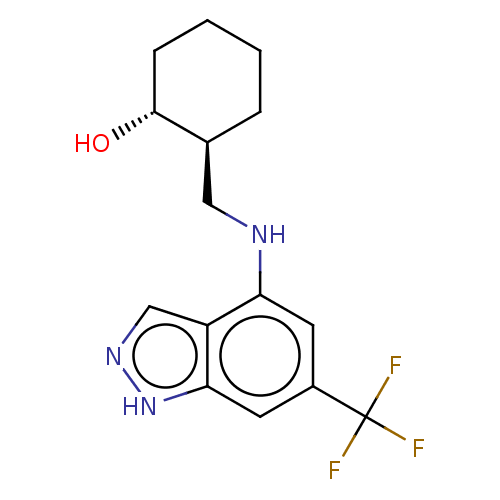
(CHEMBL5219170)Show SMILES O[C@@H]1CCCC[C@H]1CNc1cc(cc2[nH]ncc12)C(F)(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50606621
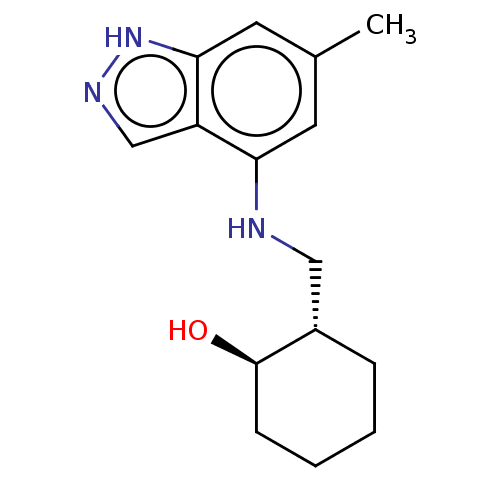
(CHEMBL5220129)Show SMILES Cc1cc(NC[C@@H]2CCCC[C@H]2O)c2cn[nH]c2c1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50606591

(CHEMBL5219380)Show SMILES CC1(C)CN(CCN1S(C)(=O)=O)c1cc(Br)cc2[nH]ncc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50606609
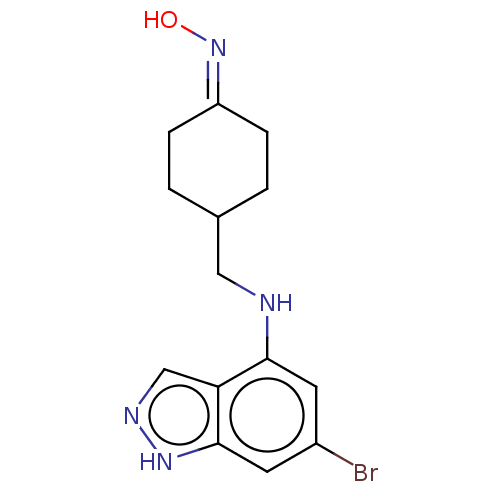
(CHEMBL5218511)Show SMILES ON=C1CCC(CNc2cc(Br)cc3[nH]ncc23)CC1 |(6.16,-2.13,;5.39,-.8,;3.85,-.8,;3.09,-2.14,;1.55,-2.14,;.78,-.8,;-.76,-.8,;-1.53,.54,;-3.07,.54,;-3.85,1.87,;-5.39,1.87,;-6.16,3.2,;-6.16,.53,;-5.39,-.81,;-5.85,-2.28,;-4.6,-3.2,;-3.38,-2.32,;-3.85,-.81,;1.55,.53,;3.08,.53,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50365771
(CHEMBL1956550)Show InChI InChI=1S/C21H21N3O/c1-24(2)12-4-10-22-15-7-8-16-18(13-15)20-19-14(9-11-23-20)5-3-6-17(19)21(16)25/h3,5-9,11,13,22H,4,10,12H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method |
Bioorg Med Chem Lett 22: 2257-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.090
BindingDB Entry DOI: 10.7270/Q2HH6KJX |
More data for this
Ligand-Target Pair | |
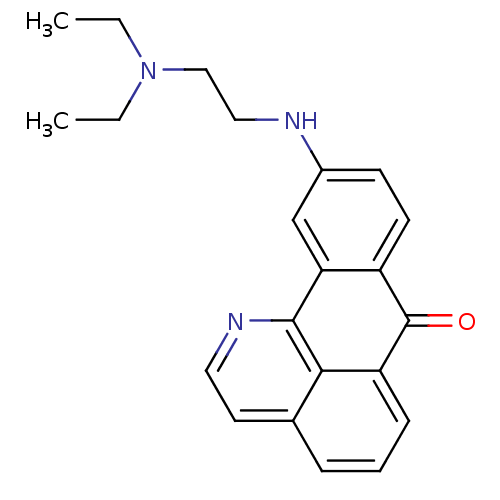
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50365781
(CHEMBL1956560)Show InChI InChI=1S/C24H25N3O/c28-24-19-9-8-18(25-11-5-15-27-13-2-1-3-14-27)16-21(19)23-22-17(10-12-26-23)6-4-7-20(22)24/h4,6-10,12,16,25H,1-3,5,11,13-15H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method |
Bioorg Med Chem Lett 22: 2257-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.090
BindingDB Entry DOI: 10.7270/Q2HH6KJX |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50606613

(CHEMBL5219865)Show SMILES O[C@@H]1CCCC[C@H]1CNc1cc(Br)cc2[nH]ncc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50606629

(CHEMBL5219595)Show SMILES N[C@@H]1CCCC[C@H]1CNc1cc(Br)cc2[nH]ncc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50365772
(CHEMBL1956551)Show InChI InChI=1S/C22H23N3O/c1-3-25(4-2)13-12-23-16-8-9-17-19(14-16)21-20-15(10-11-24-21)6-5-7-18(20)22(17)26/h5-11,14,23H,3-4,12-13H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method |
Bioorg Med Chem Lett 22: 2257-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.090
BindingDB Entry DOI: 10.7270/Q2HH6KJX |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50365779

(CHEMBL1956558)Show InChI InChI=1S/C23H23N3O/c27-23-18-8-7-17(24-10-4-14-26-12-1-2-13-26)15-20(18)22-21-16(9-11-25-22)5-3-6-19(21)23/h3,5-9,11,15,24H,1-2,4,10,12-14H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method |
Bioorg Med Chem Lett 22: 2257-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.090
BindingDB Entry DOI: 10.7270/Q2HH6KJX |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50606608
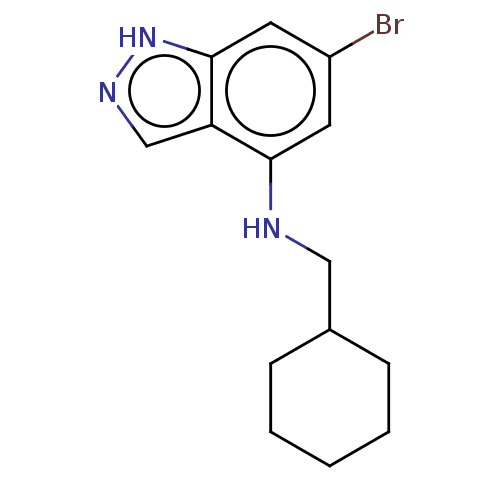
(CHEMBL5218714) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50606630
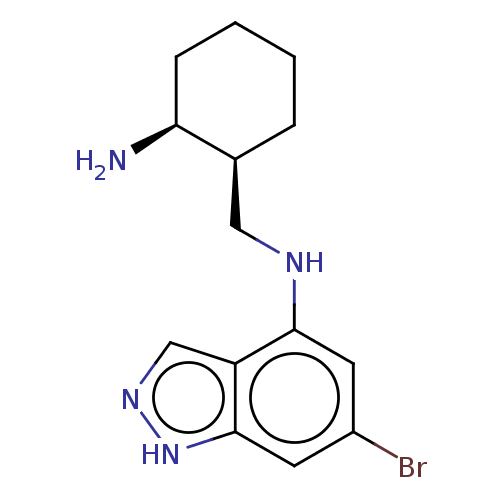
(CHEMBL5219723)Show SMILES N[C@H]1CCCC[C@H]1CNc1cc(Br)cc2[nH]ncc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50606608

(CHEMBL5218714) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50365780
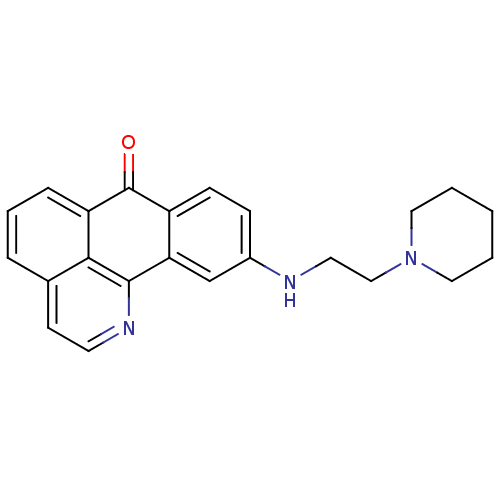
(CHEMBL1956559)Show InChI InChI=1S/C23H23N3O/c27-23-18-8-7-17(24-11-14-26-12-2-1-3-13-26)15-20(18)22-21-16(9-10-25-22)5-4-6-19(21)23/h4-10,15,24H,1-3,11-14H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method |
Bioorg Med Chem Lett 22: 2257-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.090
BindingDB Entry DOI: 10.7270/Q2HH6KJX |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50365772

(CHEMBL1956551)Show InChI InChI=1S/C22H23N3O/c1-3-25(4-2)13-12-23-16-8-9-17-19(14-16)21-20-15(10-11-24-21)6-5-7-18(20)22(17)26/h5-11,14,23H,3-4,12-13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BChE after 15 mins incubation by spectrophotometry based Ellman's method |
Bioorg Med Chem Lett 22: 2257-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.090
BindingDB Entry DOI: 10.7270/Q2HH6KJX |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50365771

(CHEMBL1956550)Show InChI InChI=1S/C21H21N3O/c1-24(2)12-4-10-22-15-7-8-16-18(13-15)20-19-14(9-11-23-20)5-3-6-17(19)21(16)25/h3,5-9,11,13,22H,4,10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BChE after 15 mins incubation by spectrophotometry based Ellman's method |
Bioorg Med Chem Lett 22: 2257-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.090
BindingDB Entry DOI: 10.7270/Q2HH6KJX |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50504430

(CHEMBL4474330)Show SMILES [H][C@@]12C[C@]3(OC(=O)C=C3C[C@@H]1N(CCC)C(=O)CCCCCCC(=O)N(CCC)[C@H]1CC3=CC(=O)O[C@]33C[C@@]1([H])N1CCCC[C@@]31[H])[C@]1([H])CCCCN21 |r,c:7,t:33| Show InChI InChI=1S/C40H58N4O6/c1-3-17-43(29-21-27-23-37(47)49-39(27)25-31(29)41-19-11-9-13-33(39)41)35(45)15-7-5-6-8-16-36(46)44(18-4-2)30-22-28-24-38(48)50-40(28)26-32(30)42-20-12-10-14-34(40)42/h23-24,29-34H,3-22,25-26H2,1-2H3/t29-,30-,31+,32+,33-,34-,39+,40+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jinan University
Curated by ChEMBL
| Assay Description
Reversal of P-gp mediated multidrug resistance in human MCF7/ADM cells assessed as doxorubicin IC50 at 10 uM measured within 48 hrs by MTT assay (Rvb... |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111726
BindingDB Entry DOI: 10.7270/Q20R9SNN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50606626
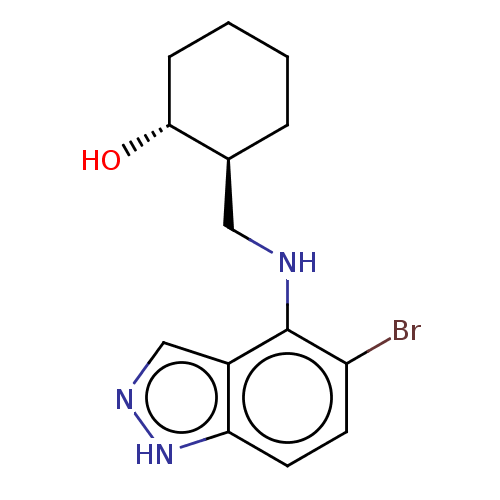
(CHEMBL5221020)Show SMILES O[C@@H]1CCCC[C@H]1CNc1c(Br)ccc2[nH]ncc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM81939
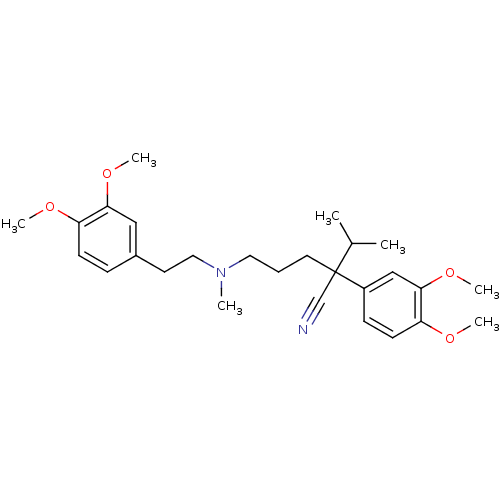
(CAS_52-53-9 | NSC_62969 | VERAPAMIL)Show SMILES COc1ccc(CCN(C)CCCC(C#N)(C(C)C)c2ccc(OC)c(OC)c2)cc1OC Show InChI InChI=1S/C27H38N2O4/c1-20(2)27(19-28,22-10-12-24(31-5)26(18-22)33-7)14-8-15-29(3)16-13-21-9-11-23(30-4)25(17-21)32-6/h9-12,17-18,20H,8,13-16H2,1-7H3 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jinan University
Curated by ChEMBL
| Assay Description
Reversal of P-gp mediated multidrug resistance in human HepG2/DOX cells assessed as doxorubicin IC50 at 10 uM measured within 48 hrs by MTT assay (Rv... |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111726
BindingDB Entry DOI: 10.7270/Q20R9SNN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data