

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533570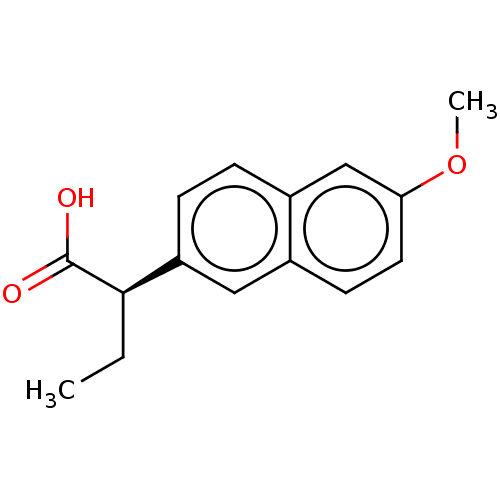 (CHEMBL4435662 | US11459295, Compound LM5750A 8b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preinc... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533570 (CHEMBL4435662 | US11459295, Compound LM5750A 8b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant AKR1C3 using assessed as reduction in NADPH-dependent reduction of delat4-androsten-3,17-dione preincubat... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50437324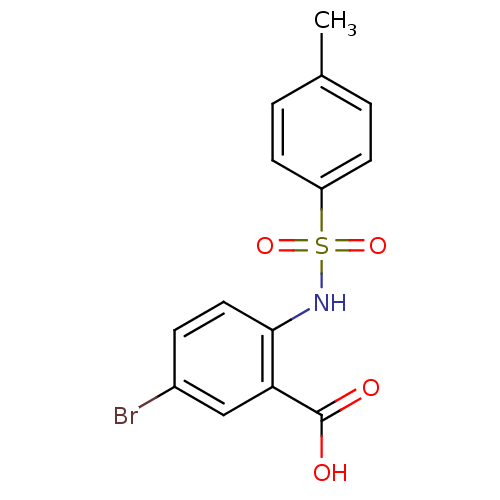 (CHEMBL2407597) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University Curated by ChEMBL | Assay Description Inhibition of human methionine aminopeptidase 2 | ACS Med Chem Lett 4: (2013) Article DOI: 10.1021/ml400034m BindingDB Entry DOI: 10.7270/Q2XW4M72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533573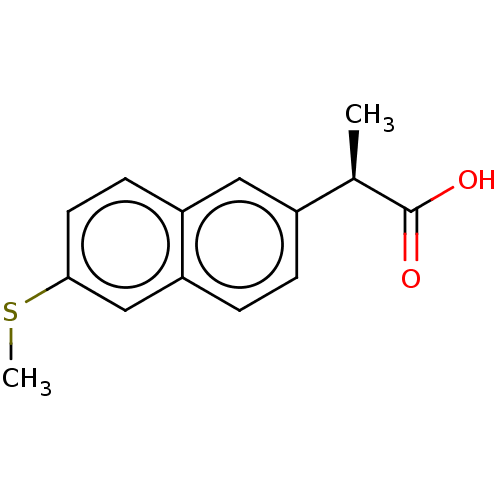 (CHEMBL4437291 | US11459295, Compound (R) 4a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533571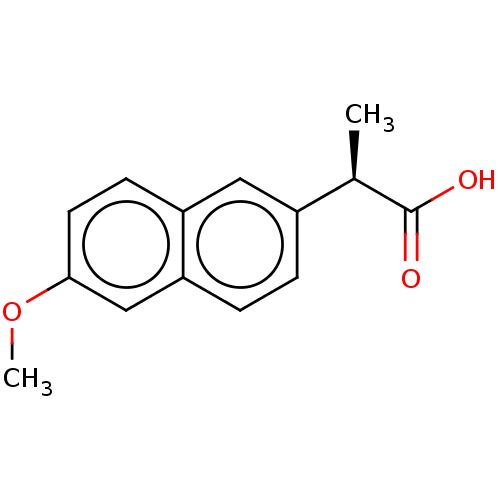 (CHEMBL1618254 | US11459295, Compound R-Naproxen (1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM64987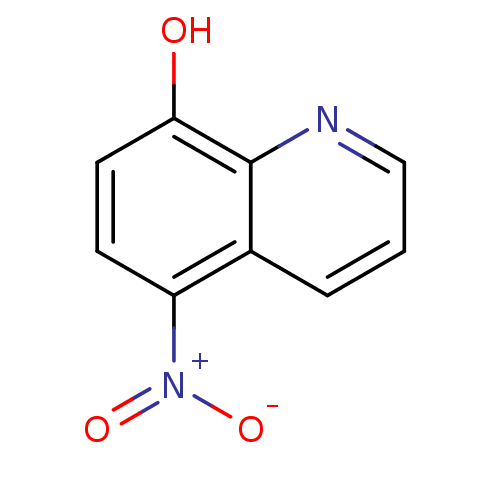 (5-nitro-8-quinolinol | 5-nitroquinolin-8-ol | 8-HY...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University Curated by ChEMBL | Assay Description Inhibition of human methionine aminopeptidase 2 | ACS Med Chem Lett 4: (2013) Article DOI: 10.1021/ml400034m BindingDB Entry DOI: 10.7270/Q2XW4M72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM76305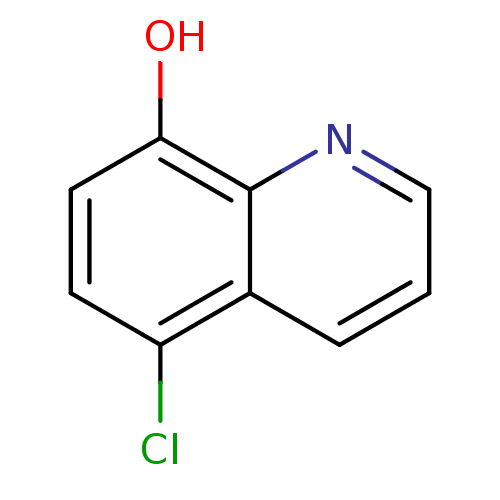 (5-chloranylquinolin-8-ol | 5-chloro-8-quinolinol |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal GST/His6-tagged methionine aminopeptidase 2 expressed in baculovirus infected sf9 cells using ... | Bioorg Med Chem 25: 813-824 (2017) Article DOI: 10.1016/j.bmc.2016.11.013 BindingDB Entry DOI: 10.7270/Q2125VV2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533576 (CHEMBL4551048 | US11459295, Compound LM5752(+-) 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50339185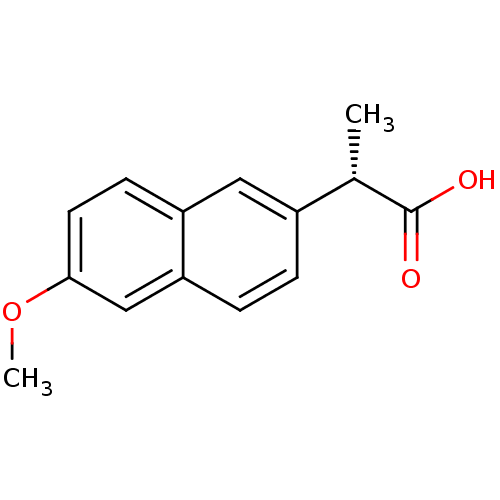 ((2S)-2-(6-methoxynaphthalen-2-yl)propanoic acid | ...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of COX1 in ram seminal vesicles using arachidonic acid as substrate assessed as reduction in PGH2 conversion to PGG2 by measuring TMPD oxi... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533577 (CHEMBL1236131 | US11459295, Compound (S) 4b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533572 (CHEMBL4457933 | US11459295, Compound LM5753(+-) 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533570 (CHEMBL4435662 | US11459295, Compound LM5750A 8b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533569 (CHEMBL4446810 | US11459295, Compound LM5751(+-) 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533574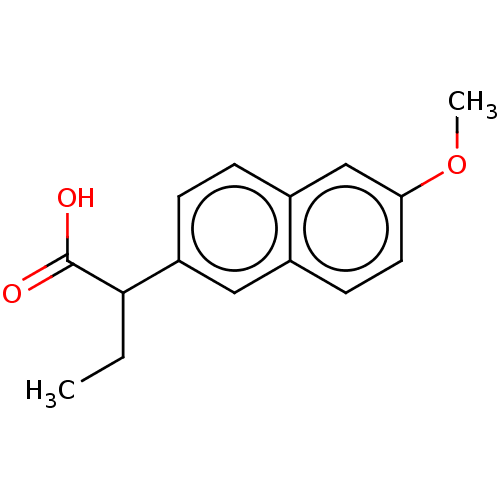 (CHEMBL4437071 | US11459295, Compound LM5750(+-) 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533568 (CHEMBL4466610 | US11459295, Compound LM5750B 8a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50339185 ((2S)-2-(6-methoxynaphthalen-2-yl)propanoic acid | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM54706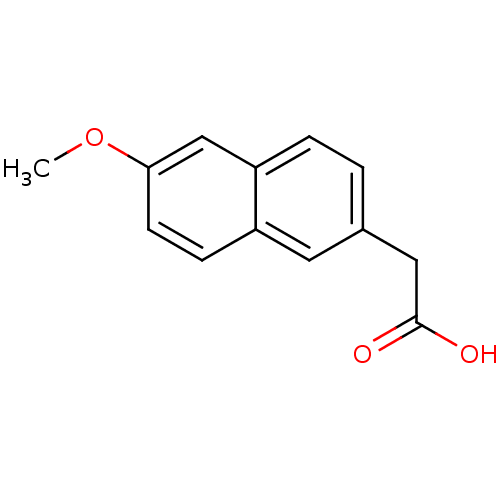 (2-(6-methoxy-2-naphthalenyl)acetic acid | 2-(6-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533578 (CHEMBL4459662) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50339185 ((2S)-2-(6-methoxynaphthalen-2-yl)propanoic acid | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) using arachidonic acid as substrate assessed as reduction in PGH2 conversion to PGG2 by measuring TMPD oxidation ... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533567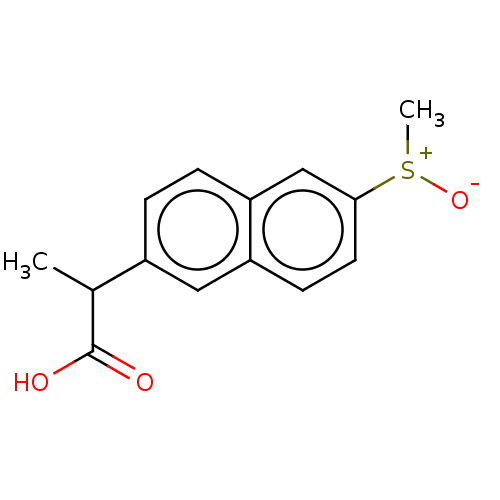 (CHEMBL4473378) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50339185 ((2S)-2-(6-methoxynaphthalen-2-yl)propanoic acid | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM32203 (8-quinolinol | CHEMBL310555 | US10005735, Table 1....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal GST/His6-tagged methionine aminopeptidase 2 expressed in baculovirus infected sf9 cells using ... | Bioorg Med Chem 25: 813-824 (2017) Article DOI: 10.1016/j.bmc.2016.11.013 BindingDB Entry DOI: 10.7270/Q2125VV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM76305 (5-chloranylquinolin-8-ol | 5-chloro-8-quinolinol |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University Curated by ChEMBL | Assay Description Inhibition of human methionine aminopeptidase 2 | ACS Med Chem Lett 4: (2013) Article DOI: 10.1021/ml400034m BindingDB Entry DOI: 10.7270/Q2XW4M72 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50533577 (CHEMBL1236131 | US11459295, Compound (S) 4b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50533576 (CHEMBL4551048 | US11459295, Compound LM5752(+-) 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50533568 (CHEMBL4466610 | US11459295, Compound LM5750B 8a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50533569 (CHEMBL4446810 | US11459295, Compound LM5751(+-) 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50533568 (CHEMBL4466610 | US11459295, Compound LM5750B 8a) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of COX1 in ram seminal vesicles using arachidonic acid as substrate assessed as reduction in PGH2 conversion to PGG2 by measuring TMPD oxi... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50065785 (2-Methyl-quinolin-8-ol | CHEMBL316892) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal GST/His6-tagged methionine aminopeptidase 2 expressed in baculovirus infected sf9 cells using ... | Bioorg Med Chem 25: 813-824 (2017) Article DOI: 10.1016/j.bmc.2016.11.013 BindingDB Entry DOI: 10.7270/Q2125VV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM32203 (8-quinolinol | CHEMBL310555 | US10005735, Table 1....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University Curated by ChEMBL | Assay Description Inhibition of human methionine aminopeptidase 2 | ACS Med Chem Lett 4: (2013) Article DOI: 10.1021/ml400034m BindingDB Entry DOI: 10.7270/Q2XW4M72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50533572 (CHEMBL4457933 | US11459295, Compound LM5753(+-) 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50437326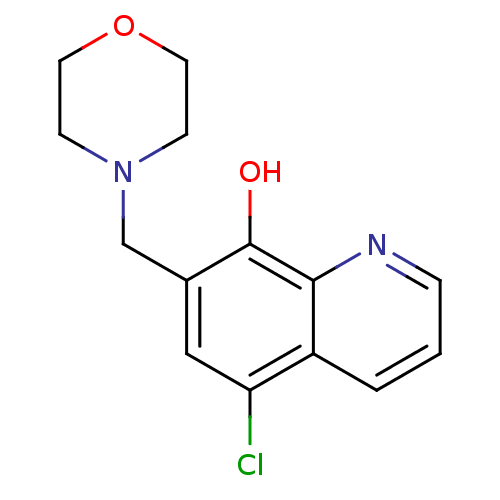 (CHEMBL2407599) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University Curated by ChEMBL | Assay Description Inhibition of human methionine aminopeptidase 2 | ACS Med Chem Lett 4: (2013) Article DOI: 10.1021/ml400034m BindingDB Entry DOI: 10.7270/Q2XW4M72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50533571 (CHEMBL1618254 | US11459295, Compound R-Naproxen (1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50533578 (CHEMBL4459662) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50437325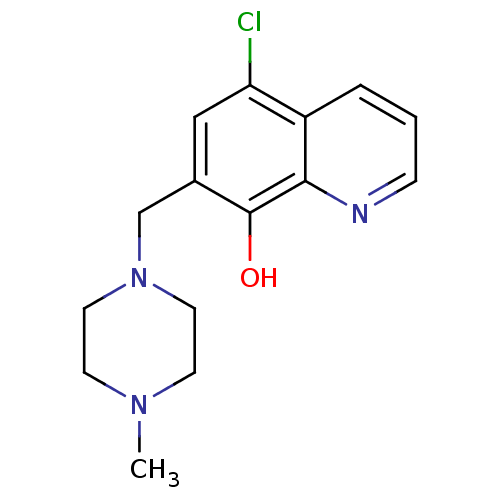 (CHEMBL1368981) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University Curated by ChEMBL | Assay Description Inhibition of human methionine aminopeptidase 2 | ACS Med Chem Lett 4: (2013) Article DOI: 10.1021/ml400034m BindingDB Entry DOI: 10.7270/Q2XW4M72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50533573 (CHEMBL4437291 | US11459295, Compound (R) 4a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50533575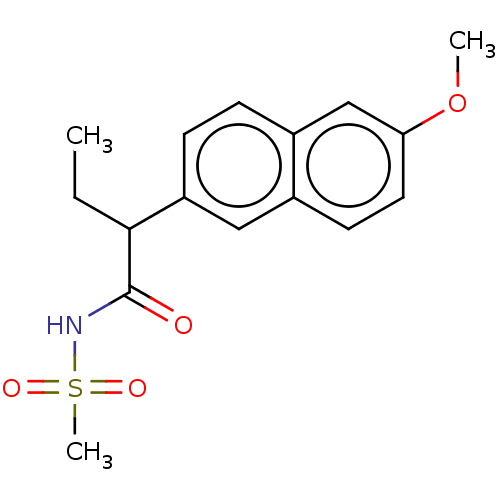 (CHEMBL4447175 | US11459295, Compound LM5885 (+-) 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50533567 (CHEMBL4473378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50533574 (CHEMBL4437071 | US11459295, Compound LM5750(+-) 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... | J Med Chem 59: 7431-44 (2016) Article DOI: 10.1021/acs.jmedchem.6b00160 BindingDB Entry DOI: 10.7270/Q21Z47WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50437327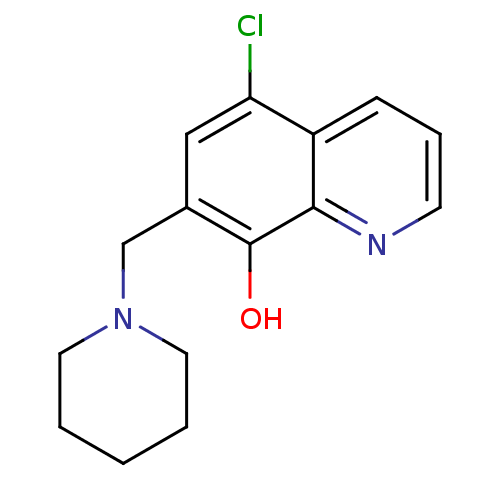 (CHEMBL2023325) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University Curated by ChEMBL | Assay Description Inhibition of human methionine aminopeptidase 2 | ACS Med Chem Lett 4: (2013) Article DOI: 10.1021/ml400034m BindingDB Entry DOI: 10.7270/Q2XW4M72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM32203 (8-quinolinol | CHEMBL310555 | US10005735, Table 1....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University Curated by ChEMBL | Assay Description Inhibition of recombinant full length human His-tagged methionine aminopeptidase 1 expressed in Escherichia coli BL21(DE3) using methionylprolyl-p-ni... | Bioorg Med Chem 25: 813-824 (2017) Article DOI: 10.1016/j.bmc.2016.11.013 BindingDB Entry DOI: 10.7270/Q2125VV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM76305 (5-chloranylquinolin-8-ol | 5-chloro-8-quinolinol |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University Curated by ChEMBL | Assay Description Inhibition of human methionine aminopeptidase 1 | ACS Med Chem Lett 4: (2013) Article DOI: 10.1021/ml400034m BindingDB Entry DOI: 10.7270/Q2XW4M72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM50065785 (2-Methyl-quinolin-8-ol | CHEMBL316892) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University Curated by ChEMBL | Assay Description Inhibition of human methionine aminopeptidase 1 | ACS Med Chem Lett 4: (2013) Article DOI: 10.1021/ml400034m BindingDB Entry DOI: 10.7270/Q2XW4M72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM50437328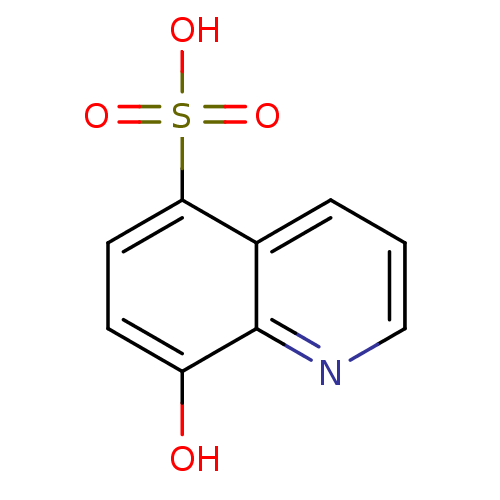 (CHEMBL2407598) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University Curated by ChEMBL | Assay Description Inhibition of human methionine aminopeptidase 1 | ACS Med Chem Lett 4: (2013) Article DOI: 10.1021/ml400034m BindingDB Entry DOI: 10.7270/Q2XW4M72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM76305 (5-chloranylquinolin-8-ol | 5-chloro-8-quinolinol |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University Curated by ChEMBL | Assay Description Inhibition of recombinant full length human His-tagged methionine aminopeptidase 1 expressed in Escherichia coli BL21(DE3) using methionylprolyl-p-ni... | Bioorg Med Chem 25: 813-824 (2017) Article DOI: 10.1016/j.bmc.2016.11.013 BindingDB Entry DOI: 10.7270/Q2125VV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM50065785 (2-Methyl-quinolin-8-ol | CHEMBL316892) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University Curated by ChEMBL | Assay Description Inhibition of recombinant full length human His-tagged methionine aminopeptidase 1 expressed in Escherichia coli BL21(DE3) using methionylprolyl-p-ni... | Bioorg Med Chem 25: 813-824 (2017) Article DOI: 10.1016/j.bmc.2016.11.013 BindingDB Entry DOI: 10.7270/Q2125VV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50211290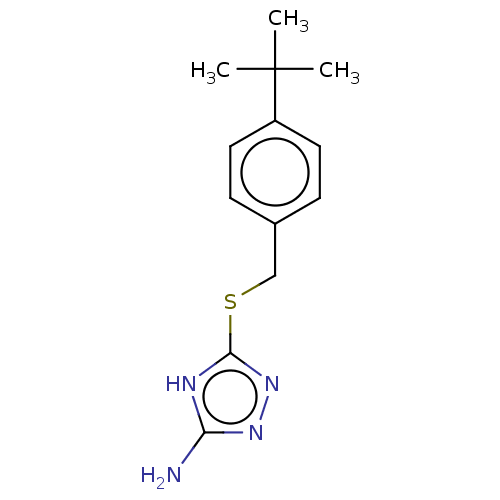 (CHEMBL2407096) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University Curated by ChEMBL | Assay Description Inhibition of recombinant full length human N-terminal GST/His6-tagged methionine aminopeptidase 2 expressed in baculovirus infected sf9 cells using ... | Bioorg Med Chem 25: 813-824 (2017) Article DOI: 10.1016/j.bmc.2016.11.013 BindingDB Entry DOI: 10.7270/Q2125VV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM50211290 (CHEMBL2407096) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University Curated by ChEMBL | Assay Description Inhibition of recombinant full length human His-tagged methionine aminopeptidase 1 expressed in Escherichia coli BL21(DE3) using methionylprolyl-p-ni... | Bioorg Med Chem 25: 813-824 (2017) Article DOI: 10.1016/j.bmc.2016.11.013 BindingDB Entry DOI: 10.7270/Q2125VV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM32203 (8-quinolinol | CHEMBL310555 | US10005735, Table 1....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University Curated by ChEMBL | Assay Description Inhibition of human methionine aminopeptidase 1 | ACS Med Chem Lett 4: (2013) Article DOI: 10.1021/ml400034m BindingDB Entry DOI: 10.7270/Q2XW4M72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50065785 (2-Methyl-quinolin-8-ol | CHEMBL316892) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northern Illinois University Curated by ChEMBL | Assay Description Inhibition of human methionine aminopeptidase 2 | ACS Med Chem Lett 4: (2013) Article DOI: 10.1021/ml400034m BindingDB Entry DOI: 10.7270/Q2XW4M72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 62 total ) | Next | Last >> |