 Found 14155 hits with Last Name = 'ward' and Initial = 'p'
Found 14155 hits with Last Name = 'ward' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340336
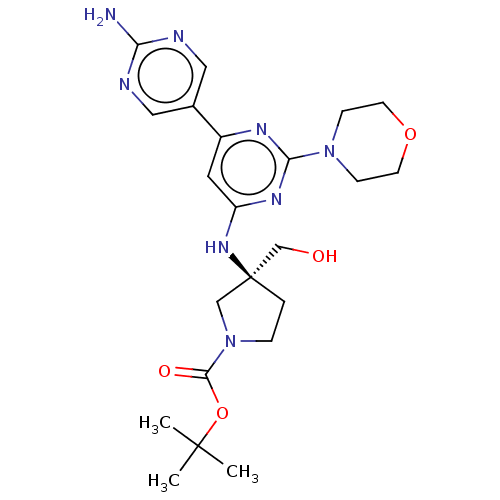
(US9758538, Example 24)Show SMILES CC(C)(C)OC(=O)N1CC[C@@](CO)(C1)Nc1cc(nc(n1)N1CCOCC1)-c1cnc(N)nc1 |r| Show InChI InChI=1S/C22H32N8O4/c1-21(2,3)34-20(32)30-5-4-22(13-30,14-31)28-17-10-16(15-11-24-18(23)25-12-15)26-19(27-17)29-6-8-33-9-7-29/h10-12,31H,4-9,13-14H2,1-3H3,(H2,23,24,25)(H,26,27,28)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340384
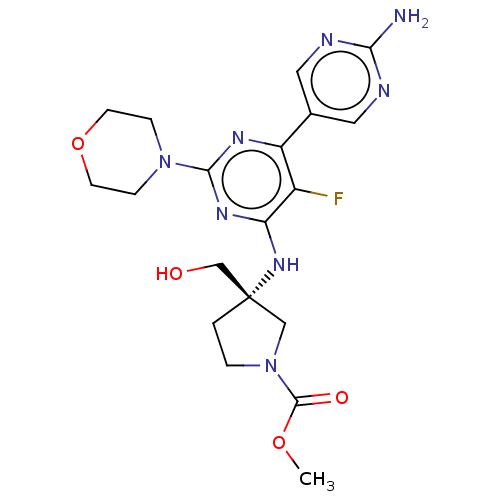
(US9758538, Example 72)Show SMILES COC(=O)N1CC[C@@](CO)(C1)Nc1nc(nc(c1F)-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C19H25FN8O4/c1-31-18(30)28-3-2-19(10-28,11-29)26-15-13(20)14(12-8-22-16(21)23-9-12)24-17(25-15)27-4-6-32-7-5-27/h8-9,29H,2-7,10-11H2,1H3,(H2,21,22,23)(H,24,25,26)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207217
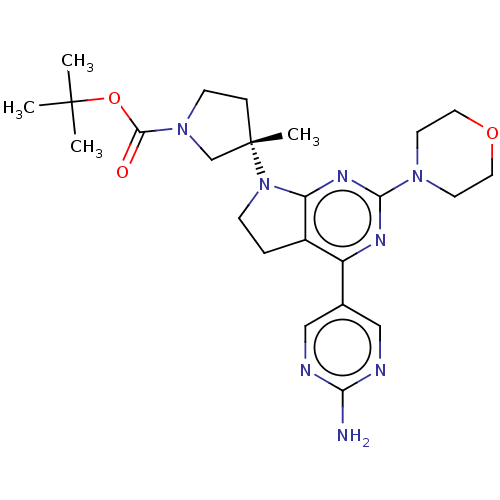
(US9260439, 194 | US9260439, 238 | US9260439, 239)Show SMILES CC(C)(C)OC(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C24H34N8O3/c1-23(2,3)35-22(33)31-8-6-24(4,15-31)32-7-5-17-18(16-13-26-20(25)27-14-16)28-21(29-19(17)32)30-9-11-34-12-10-30/h13-14H,5-12,15H2,1-4H3,(H2,25,26,27)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207378
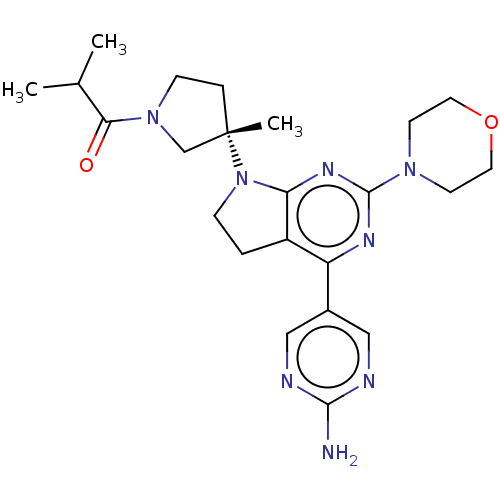
(US9260439, 262)Show SMILES CC(C)C(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C23H32N8O2/c1-15(2)20(32)30-7-5-23(3,14-30)31-6-4-17-18(16-12-25-21(24)26-13-16)27-22(28-19(17)31)29-8-10-33-11-9-29/h12-13,15H,4-11,14H2,1-3H3,(H2,24,25,26)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340314
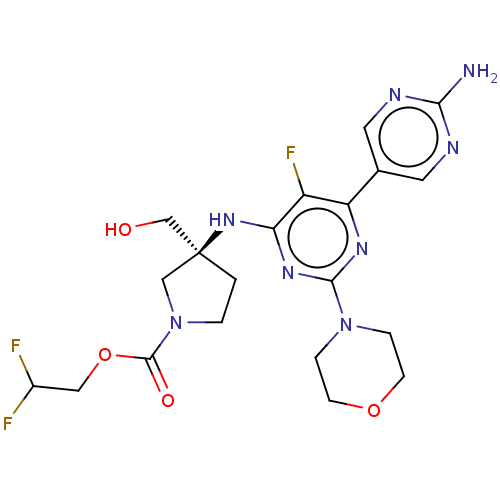
((Scheme A): Preparation of 2,2-difluoroethyl (3S)-...)Show SMILES Nc1ncc(cn1)-c1nc(nc(N[C@@]2(CO)CCN(C2)C(=O)OCC(F)F)c1F)N1CCOCC1 |r| Show InChI InChI=1S/C20H25F3N8O4/c21-13(22)9-35-19(33)31-2-1-20(10-31,11-32)29-16-14(23)15(12-7-25-17(24)26-8-12)27-18(28-16)30-3-5-34-6-4-30/h7-8,13,32H,1-6,9-11H2,(H2,24,25,26)(H,27,28,29)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207196
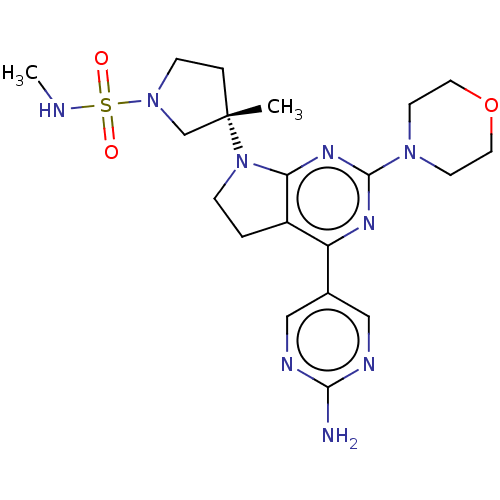
(US9260439, 173)Show SMILES CNS(=O)(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C20H29N9O3S/c1-20(4-6-28(13-20)33(30,31)22-2)29-5-3-15-16(14-11-23-18(21)24-12-14)25-19(26-17(15)29)27-7-9-32-10-8-27/h11-12,22H,3-10,13H2,1-2H3,(H2,21,23,24)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340346

(US9758538, Example 34)Show SMILES COC(=O)N1CC[C@@](CO)(C1)Nc1cc(nc(n1)N1CCOCC1)-c1cnc(N)nc1 |r| Show InChI InChI=1S/C19H26N8O4/c1-30-18(29)27-3-2-19(11-27,12-28)25-15-8-14(13-9-21-16(20)22-10-13)23-17(24-15)26-4-6-31-7-5-26/h8-10,28H,2-7,11-12H2,1H3,(H2,20,21,22)(H,23,24,25)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase ROS
(Homo sapiens (Human)) | BDBM50018830
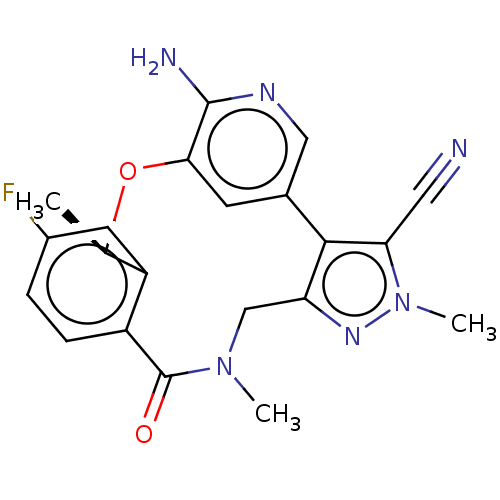
(CHEMBL3286830 | US10543199, Compound PF-06463922 |...)Show SMILES C[C@H]1Oc2cc(cnc2N)-c2c(CN(C)C(=O)c3ccc(F)cc13)nn(C)c2C#N |r| Show InChI InChI=1S/C21H19FN6O2/c1-11-15-7-13(22)4-5-14(15)21(29)27(2)10-16-19(17(8-23)28(3)26-16)12-6-18(30-11)20(24)25-9-12/h4-7,9,11H,10H2,1-3H3,(H2,24,25)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROS1 (unknown origin) by off-chip mobility shift assay |
J Med Chem 57: 4720-44 (2014)
Article DOI: 10.1021/jm500261q
BindingDB Entry DOI: 10.7270/Q2K35W68 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50018836
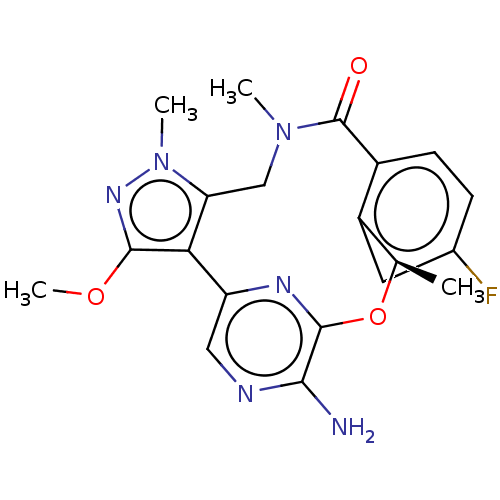
(CHEMBL3286826)Show SMILES COc1nn(C)c2CN(C)C(=O)c3ccc(F)cc3[C@@H](C)Oc3nc(cnc3N)-c12 |r| Show InChI InChI=1S/C20H21FN6O3/c1-10-13-7-11(21)5-6-12(13)20(28)26(2)9-15-16(18(29-4)25-27(15)3)14-8-23-17(22)19(24-14)30-10/h5-8,10H,9H2,1-4H3,(H2,22,23)/t10-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ALK L1196M mutant kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-... |
J Med Chem 57: 4720-44 (2014)
Article DOI: 10.1021/jm500261q
BindingDB Entry DOI: 10.7270/Q2K35W68 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase ROS
(Homo sapiens (Human)) | BDBM50448785

(CHEMBL3128069)Show SMILES C[C@@H](Oc1cc(cnc1N)-c1sc(nc1C)[C@](C)(O)CO)c1cc(F)ccc1-n1nccn1 |r| Show InChI InChI=1S/C22H23FN6O3S/c1-12-19(33-21(28-12)22(3,31)11-30)14-8-18(20(24)25-10-14)32-13(2)16-9-15(23)4-5-17(16)29-26-6-7-27-29/h4-10,13,30-31H,11H2,1-3H3,(H2,24,25)/t13-,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ROS1 (unknown origin) by Pfizer mobility shift assay |
J Med Chem 57: 1170-87 (2014)
Article DOI: 10.1021/jm401805h
BindingDB Entry DOI: 10.7270/Q29C6ZX5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340391
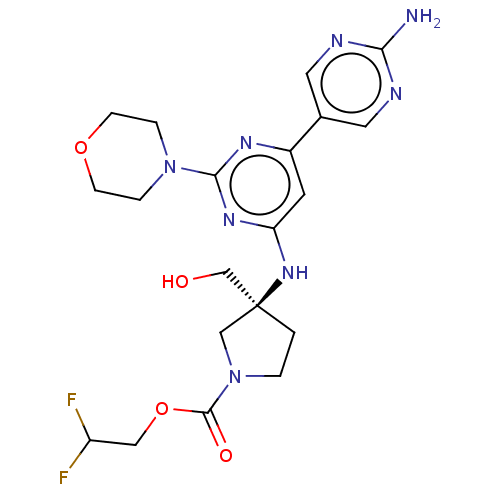
(US9758538, Example 79)Show SMILES Nc1ncc(cn1)-c1cc(N[C@@]2(CO)CCN(C2)C(=O)OCC(F)F)nc(n1)N1CCOCC1 |r| Show InChI InChI=1S/C20H26F2N8O4/c21-15(22)10-34-19(32)30-2-1-20(11-30,12-31)28-16-7-14(13-8-24-17(23)25-9-13)26-18(27-16)29-3-5-33-6-4-29/h7-9,15,31H,1-6,10-12H2,(H2,23,24,25)(H,26,27,28)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207028
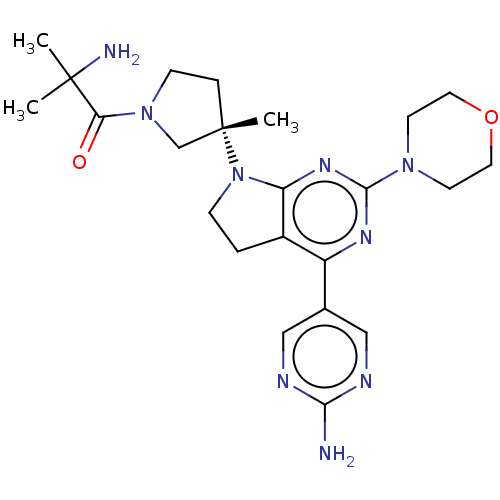
(US9260439, 10 | US9260439, 4)Show SMILES CC(C)(N)C(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C23H33N9O2/c1-22(2,25)19(33)31-7-5-23(3,14-31)32-6-4-16-17(15-12-26-20(24)27-13-15)28-21(29-18(16)32)30-8-10-34-11-9-30/h12-13H,4-11,14,25H2,1-3H3,(H2,24,26,27)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207236
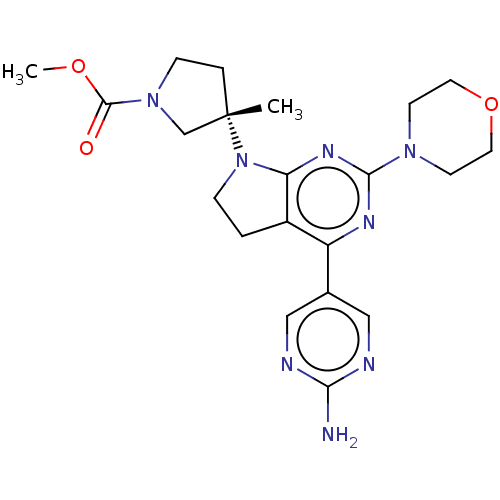
(US9260439, 213)Show SMILES COC(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C21H28N8O3/c1-21(4-6-28(13-21)20(30)31-2)29-5-3-15-16(14-11-23-18(22)24-12-14)25-19(26-17(15)29)27-7-9-32-10-8-27/h11-12H,3-10,13H2,1-2H3,(H2,22,23,24)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207172
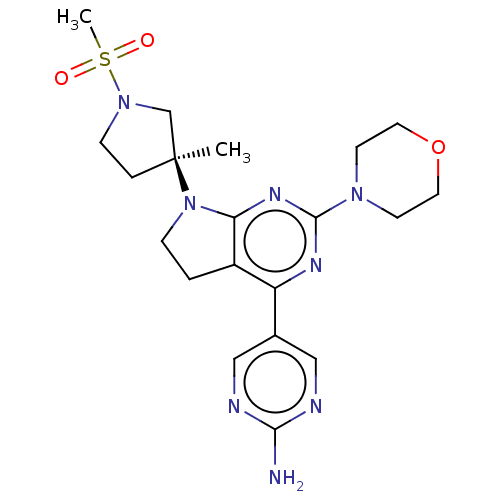
(US9260439, 149)Show SMILES C[C@@]1(CCN(C1)S(C)(=O)=O)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C20H28N8O3S/c1-20(4-6-27(13-20)32(2,29)30)28-5-3-15-16(14-11-22-18(21)23-12-14)24-19(25-17(15)28)26-7-9-31-10-8-26/h11-12H,3-10,13H2,1-2H3,(H2,21,22,23)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207391
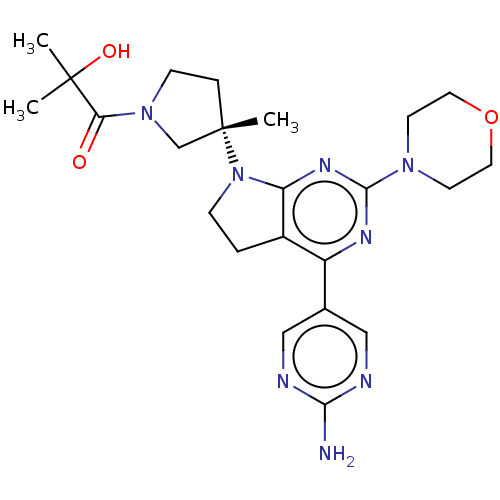
(US9260439, 275)Show SMILES CC(C)(O)C(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C23H32N8O3/c1-22(2,33)19(32)30-7-5-23(3,14-30)31-6-4-16-17(15-12-25-20(24)26-13-15)27-21(28-18(16)31)29-8-10-34-11-9-29/h12-13,33H,4-11,14H2,1-3H3,(H2,24,25,26)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50470670
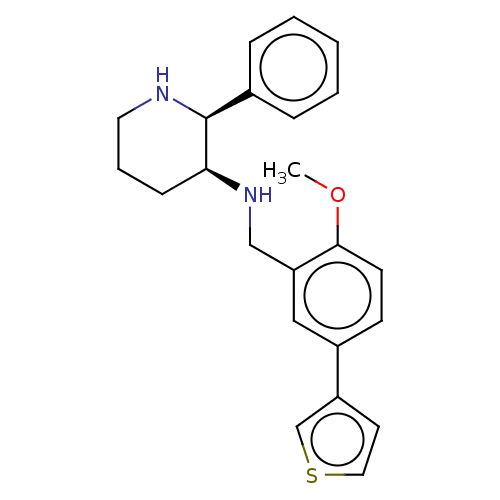
(CHEMBL149557)Show SMILES COc1ccc(cc1CN[C@H]1CCCN[C@H]1c1ccccc1)-c1ccsc1 Show InChI InChI=1S/C23H26N2OS/c1-26-22-10-9-18(19-11-13-27-16-19)14-20(22)15-25-21-8-5-12-24-23(21)17-6-3-2-4-7-17/h2-4,6-7,9-11,13-14,16,21,23-25H,5,8,12,15H2,1H3/t21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK UK
Curated by ChEMBL
| Assay Description
Binding affinity to Tachykinin receptor 1 stably expressed in chinese hamster ovary (CHO) cells |
J Med Chem 38: 4985-92 (1995)
Article DOI: 10.1021/jm00026a005
BindingDB Entry DOI: 10.7270/Q2S46VQ5 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50343025

((R)-4-(3-(methylamino)pyrrolidin-1-yl)-N2-neopenty...)Show SMILES CN[C@@H]1CCN(C1)c1cc(N)nc(NCC(C)(C)C)c1 |r| Show InChI InChI=1S/C15H27N5/c1-15(2,3)10-18-14-8-12(7-13(16)19-14)20-6-5-11(9-20)17-4/h7-8,11,17H,5-6,9-10H2,1-4H3,(H3,16,18,19)/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor |
Bioorg Med Chem Lett 21: 3113-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.017
BindingDB Entry DOI: 10.7270/Q24X5845 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50410193
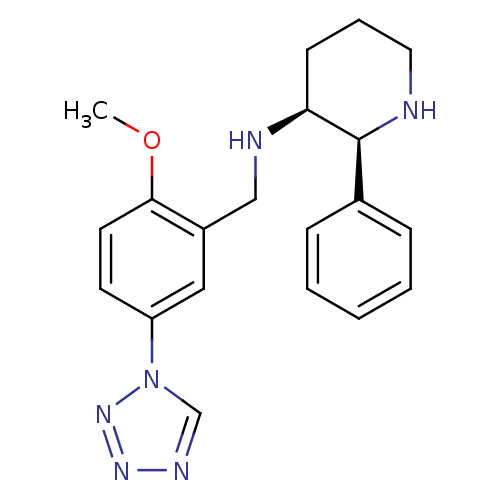
(CHEMBL356062 | GR-203040)Show SMILES COc1ccc(cc1CN[C@H]1CCCN[C@H]1c1ccccc1)-n1cnnn1 Show InChI InChI=1S/C20H24N6O/c1-27-19-10-9-17(26-14-23-24-25-26)12-16(19)13-22-18-8-5-11-21-20(18)15-6-3-2-4-7-15/h2-4,6-7,9-10,12,14,18,20-22H,5,8,11,13H2,1H3/t18-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK UK
Curated by ChEMBL
| Assay Description
Binding affinity to Tachykinin receptor 1 stably expressed in chinese hamster ovary (CHO) cells |
J Med Chem 38: 4985-92 (1995)
Article DOI: 10.1021/jm00026a005
BindingDB Entry DOI: 10.7270/Q2S46VQ5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50470678
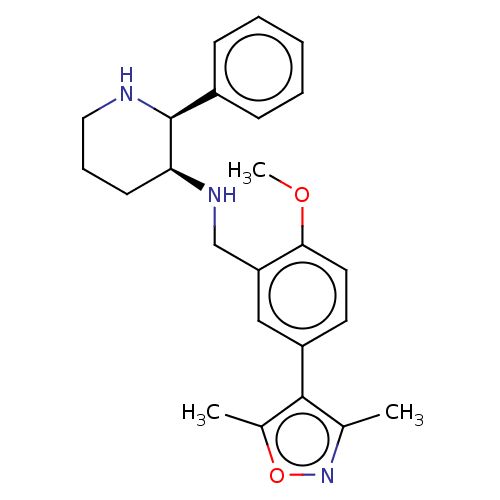
(CHEMBL146885)Show SMILES COc1ccc(cc1CN[C@H]1CCCN[C@H]1c1ccccc1)-c1c(C)noc1C Show InChI InChI=1S/C24H29N3O2/c1-16-23(17(2)29-27-16)19-11-12-22(28-3)20(14-19)15-26-21-10-7-13-25-24(21)18-8-5-4-6-9-18/h4-6,8-9,11-12,14,21,24-26H,7,10,13,15H2,1-3H3/t21-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK UK
Curated by ChEMBL
| Assay Description
Binding affinity to Tachykinin receptor 1 stably expressed in chinese hamster ovary (CHO) cells |
J Med Chem 38: 4985-92 (1995)
Article DOI: 10.1021/jm00026a005
BindingDB Entry DOI: 10.7270/Q2S46VQ5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50470675
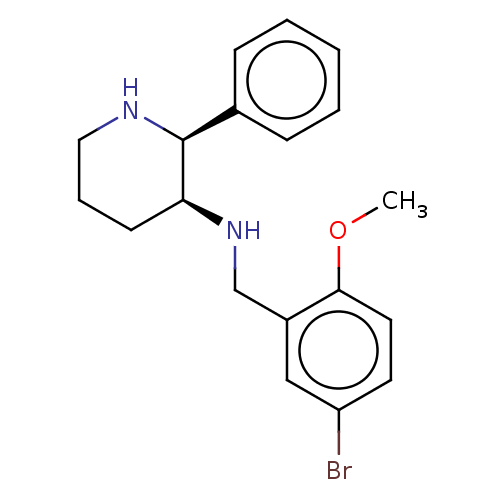
(CHEMBL344536)Show InChI InChI=1S/C19H23BrN2O/c1-23-18-10-9-16(20)12-15(18)13-22-17-8-5-11-21-19(17)14-6-3-2-4-7-14/h2-4,6-7,9-10,12,17,19,21-22H,5,8,11,13H2,1H3/t17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK UK
Curated by ChEMBL
| Assay Description
Binding affinity to Tachykinin receptor 1 stably expressed in chinese hamster ovary (CHO) cells |
J Med Chem 38: 4985-92 (1995)
Article DOI: 10.1021/jm00026a005
BindingDB Entry DOI: 10.7270/Q2S46VQ5 |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Homo sapiens (Human)) | BDBM50091753
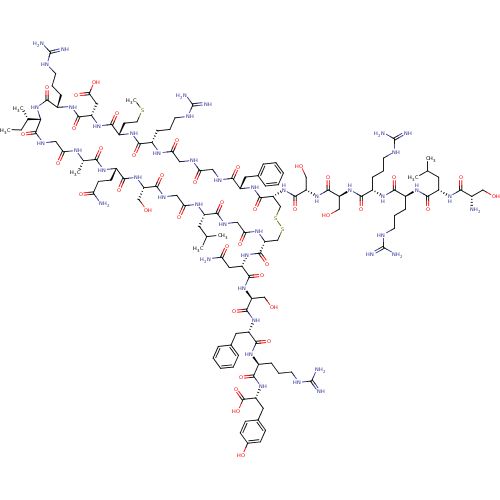
(CHEMBL405854 | H-Ser-Leu-Arg-Arg-Ser-Ser-cyclic(Cy...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)CNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CSSC[C@@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)CNC1=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](Cc1ccc(O)cc1)C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CO Show InChI InChI=1S/C127H203N45O39S3/c1-9-64(6)99-121(209)150-52-94(182)151-65(7)100(188)155-76(34-35-91(129)179)109(197)167-85(56-174)104(192)149-53-96(184)153-78(43-62(2)3)102(190)148-54-97(185)154-89(119(207)164-82(48-92(130)180)114(202)169-86(57-175)116(204)163-81(46-67-23-14-11-15-24-67)113(201)158-73(27-18-39-143-125(135)136)107(195)166-84(122(210)211)47-68-30-32-69(178)33-31-68)60-213-214-61-90(171-118(206)88(59-177)170-117(205)87(58-176)168-108(196)74(28-19-40-144-126(137)138)156-106(194)72(26-17-38-142-124(133)134)157-112(200)79(44-63(4)5)161-101(189)70(128)55-173)120(208)162-80(45-66-21-12-10-13-22-66)103(191)147-50-93(181)146-51-95(183)152-71(25-16-37-141-123(131)132)105(193)160-77(36-42-212-8)110(198)165-83(49-98(186)187)115(203)159-75(111(199)172-99)29-20-41-145-127(139)140/h10-15,21-24,30-33,62-65,70-90,99,173-178H,9,16-20,25-29,34-61,128H2,1-8H3,(H2,129,179)(H2,130,180)(H,146,181)(H,147,191)(H,148,190)(H,149,192)(H,150,209)(H,151,182)(H,152,183)(H,153,184)(H,154,185)(H,155,188)(H,156,194)(H,157,200)(H,158,201)(H,159,203)(H,160,193)(H,161,189)(H,162,208)(H,163,204)(H,164,207)(H,165,198)(H,166,195)(H,167,197)(H,168,196)(H,169,202)(H,170,205)(H,171,206)(H,172,199)(H,186,187)(H,210,211)(H4,131,132,141)(H4,133,134,142)(H4,135,136,143)(H4,137,138,144)(H4,139,140,145)/t64-,65-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84+,85-,86-,87-,88-,89+,90+,99-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ANP from the Atrial Natriuretic Peptide Clearance Receptor. |
Bioorg Med Chem Lett 10: 1949-52 (2001)
BindingDB Entry DOI: 10.7270/Q2BK1BKQ |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50031196
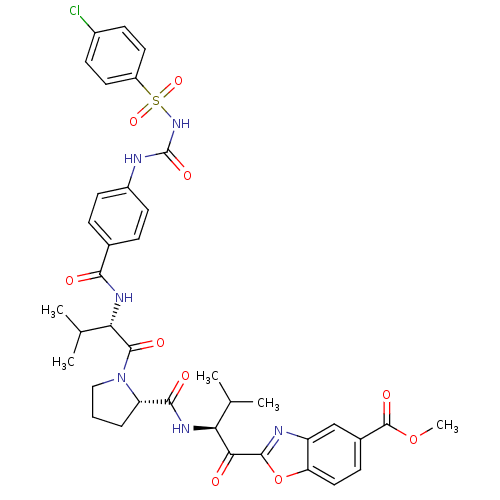
(2-[3-Methyl-2-({1-[3-methyl-2-(4-chlorophenyl-sulf...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)c1ccc(NC(=O)NS(=O)(=O)c2ccc(Cl)cc2)cc1)C(C)C)C(C)C Show InChI InChI=1S/C38H41ClN6O10S/c1-20(2)30(32(46)35-41-27-19-23(37(50)54-5)10-17-29(27)55-35)42-34(48)28-7-6-18-45(28)36(49)31(21(3)4)43-33(47)22-8-13-25(14-9-22)40-38(51)44-56(52,53)26-15-11-24(39)12-16-26/h8-17,19-21,28,30-31H,6-7,18H2,1-5H3,(H,42,48)(H,43,47)(H2,40,44,51)/t28-,30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa |
J Med Chem 38: 3972-82 (1995)
BindingDB Entry DOI: 10.7270/Q2T152NN |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061031
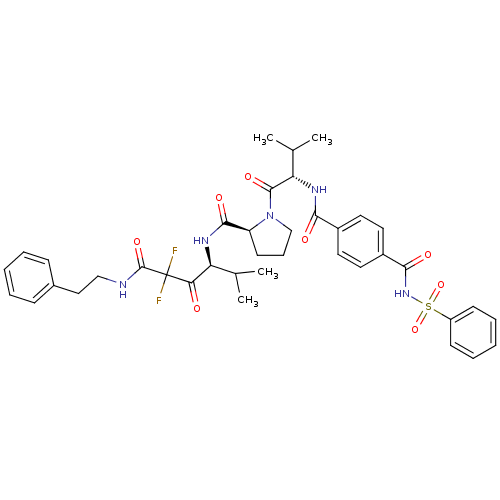
((S)-1-[(S)-2-(4-Benzenesulfonylaminocarbonyl-benzo...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C39H45F2N5O8S/c1-24(2)31(33(47)39(40,41)38(52)42-22-21-26-12-7-5-8-13-26)43-36(50)30-16-11-23-46(30)37(51)32(25(3)4)44-34(48)27-17-19-28(20-18-27)35(49)45-55(53,54)29-14-9-6-10-15-29/h5-10,12-15,17-20,24-25,30-32H,11,16,21-23H2,1-4H3,(H,42,52)(H,43,50)(H,44,48)(H,45,49)/t30-,31-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50470669

(CHEMBL359188)Show SMILES COc1ccc(cc1CN[C@H]1CCCN[C@H]1c1ccccc1)-c1ccncc1 Show InChI InChI=1S/C24H27N3O/c1-28-23-10-9-20(18-11-14-25-15-12-18)16-21(23)17-27-22-8-5-13-26-24(22)19-6-3-2-4-7-19/h2-4,6-7,9-12,14-16,22,24,26-27H,5,8,13,17H2,1H3/t22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK UK
Curated by ChEMBL
| Assay Description
Binding affinity to Tachykinin receptor 1 stably expressed in chinese hamster ovary (CHO) cells |
J Med Chem 38: 4985-92 (1995)
Article DOI: 10.1021/jm00026a005
BindingDB Entry DOI: 10.7270/Q2S46VQ5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50470683
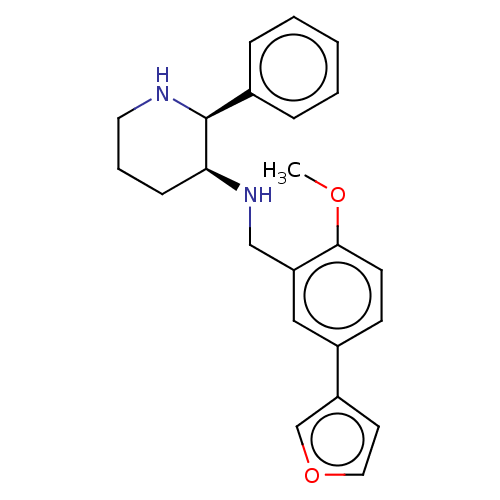
(CHEMBL356786)Show SMILES COc1ccc(cc1CN[C@H]1CCCN[C@H]1c1ccccc1)-c1ccoc1 Show InChI InChI=1S/C23H26N2O2/c1-26-22-10-9-18(19-11-13-27-16-19)14-20(22)15-25-21-8-5-12-24-23(21)17-6-3-2-4-7-17/h2-4,6-7,9-11,13-14,16,21,23-25H,5,8,12,15H2,1H3/t21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK UK
Curated by ChEMBL
| Assay Description
Binding affinity to Tachykinin receptor 1 stably expressed in chinese hamster ovary (CHO) cells |
J Med Chem 38: 4985-92 (1995)
Article DOI: 10.1021/jm00026a005
BindingDB Entry DOI: 10.7270/Q2S46VQ5 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50018830

(CHEMBL3286830 | US10543199, Compound PF-06463922 |...)Show SMILES C[C@H]1Oc2cc(cnc2N)-c2c(CN(C)C(=O)c3ccc(F)cc13)nn(C)c2C#N |r| Show InChI InChI=1S/C21H19FN6O2/c1-11-15-7-13(22)4-5-14(15)21(29)27(2)10-16-19(17(8-23)28(3)26-16)12-6-18(30-11)20(24)25-9-12/h4-7,9,11H,10H2,1-3H3,(H2,24,25)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant ALK kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-CONH... |
J Med Chem 57: 4720-44 (2014)
Article DOI: 10.1021/jm500261q
BindingDB Entry DOI: 10.7270/Q2K35W68 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50018837
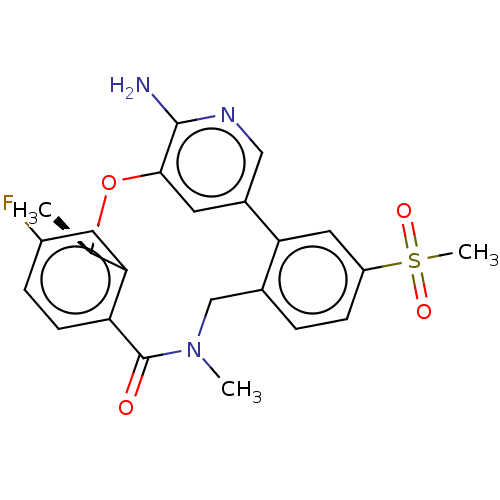
(CHEMBL3286827)Show SMILES C[C@H]1Oc2cc(cnc2N)-c2cc(ccc2CN(C)C(=O)c2ccc(F)cc12)S(C)(=O)=O |r| Show InChI InChI=1S/C23H22FN3O4S/c1-13-19-9-16(24)5-7-18(19)23(28)27(2)12-14-4-6-17(32(3,29)30)10-20(14)15-8-21(31-13)22(25)26-11-15/h4-11,13H,12H2,1-3H3,(H2,25,26)/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant ALK kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-CONH... |
J Med Chem 57: 4720-44 (2014)
Article DOI: 10.1021/jm500261q
BindingDB Entry DOI: 10.7270/Q2K35W68 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207234
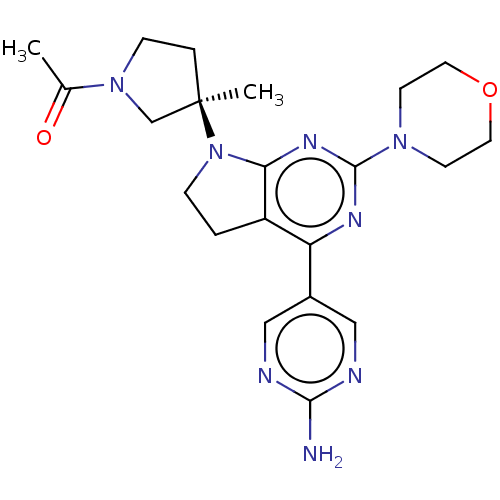
(US9260439, 211)Show SMILES CC(=O)N1CC[C@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C21H28N8O2/c1-14(30)28-6-4-21(2,13-28)29-5-3-16-17(15-11-23-19(22)24-12-15)25-20(26-18(16)29)27-7-9-31-10-8-27/h11-12H,3-10,13H2,1-2H3,(H2,22,23,24)/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50470672
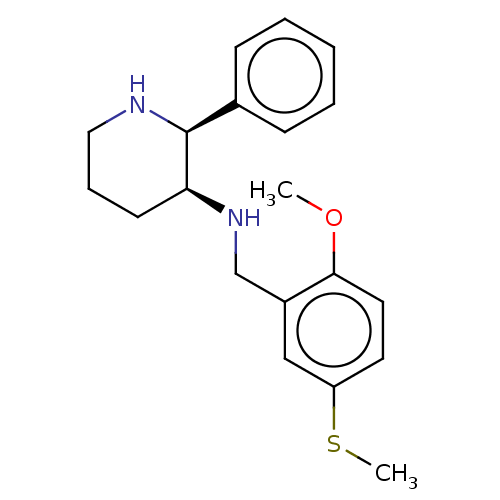
(CHEMBL434884)Show InChI InChI=1S/C20H26N2OS/c1-23-19-11-10-17(24-2)13-16(19)14-22-18-9-6-12-21-20(18)15-7-4-3-5-8-15/h3-5,7-8,10-11,13,18,20-22H,6,9,12,14H2,1-2H3/t18-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK UK
Curated by ChEMBL
| Assay Description
Binding affinity to Tachykinin receptor 1 stably expressed in chinese hamster ovary (CHO) cells |
J Med Chem 38: 4985-92 (1995)
Article DOI: 10.1021/jm00026a005
BindingDB Entry DOI: 10.7270/Q2S46VQ5 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50018836

(CHEMBL3286826)Show SMILES COc1nn(C)c2CN(C)C(=O)c3ccc(F)cc3[C@@H](C)Oc3nc(cnc3N)-c12 |r| Show InChI InChI=1S/C20H21FN6O3/c1-10-13-7-11(21)5-6-12(13)20(28)26(2)9-15-16(18(29-4)25-27(15)3)14-8-23-17(22)19(24-14)30-10/h5-8,10H,9H2,1-4H3,(H2,22,23)/t10-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant ALK kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-CONH... |
J Med Chem 57: 4720-44 (2014)
Article DOI: 10.1021/jm500261q
BindingDB Entry DOI: 10.7270/Q2K35W68 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207239
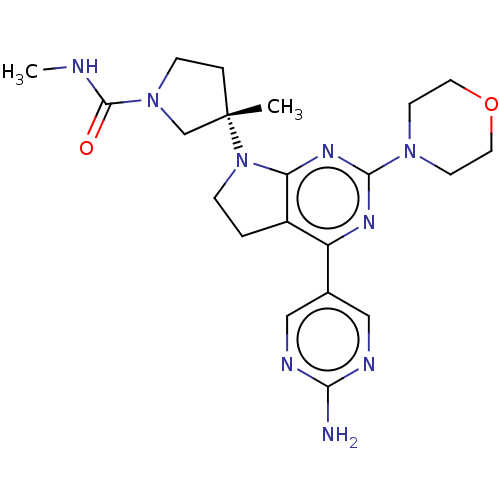
(US9260439, 216)Show SMILES CNC(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C21H29N9O2/c1-21(4-6-29(13-21)20(31)23-2)30-5-3-15-16(14-11-24-18(22)25-12-14)26-19(27-17(15)30)28-7-9-32-10-8-28/h11-12H,3-10,13H2,1-2H3,(H,23,31)(H2,22,24,25)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Rattus norvegicus) | BDBM50165931
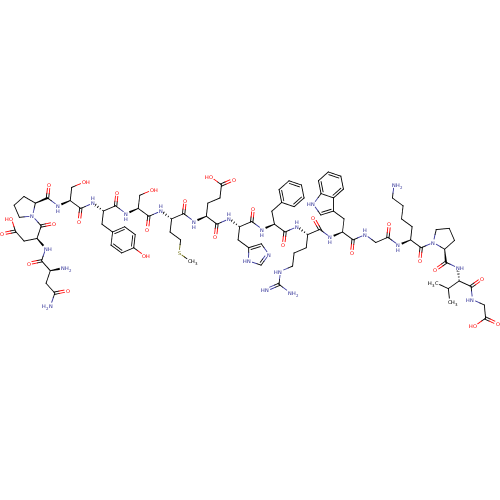
(CHEMBL415165 | NDP-SYSMEHFRWGKPVG)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O Show InChI InChI=1S/C90H127N25O26S/c1-47(2)74(87(139)100-43-73(125)126)113-86(138)68-21-13-31-114(68)88(140)59(18-9-10-29-91)102-70(120)42-99-76(128)62(36-50-40-98-55-17-8-7-16-53(50)55)108-77(129)56(19-11-30-97-90(94)95)103-80(132)60(34-48-14-5-4-6-15-48)106-82(134)63(37-51-41-96-46-101-51)109-78(130)57(26-27-71(121)122)104-79(131)58(28-33-142-3)105-83(135)65(44-116)111-81(133)61(35-49-22-24-52(118)25-23-49)107-84(136)66(45-117)112-85(137)67-20-12-32-115(67)89(141)64(39-72(123)124)110-75(127)54(92)38-69(93)119/h4-8,14-17,22-25,40-41,46-47,54,56-68,74,98,116-118H,9-13,18-21,26-39,42-45,91-92H2,1-3H3,(H2,93,119)(H,96,101)(H,99,128)(H,100,139)(H,102,120)(H,103,132)(H,104,131)(H,105,135)(H,106,134)(H,107,136)(H,108,129)(H,109,130)(H,110,127)(H,111,133)(H,112,137)(H,113,138)(H,121,122)(H,123,124)(H,125,126)(H4,94,95,97)/t54-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,74-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for rat melanocortin-4 receptor |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50165935
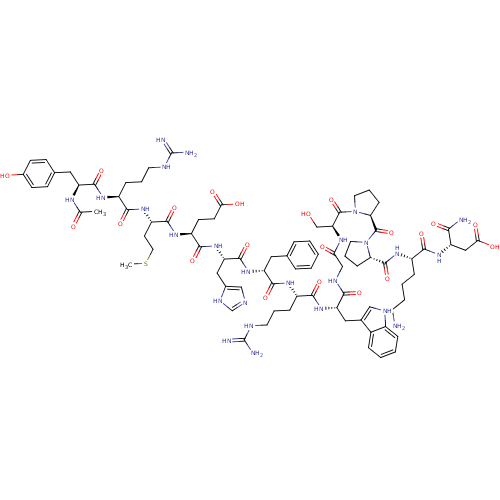
(Ac-YRMEHdFRWGSPPKD-NH2 | CHEMBL414718)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(N)=O Show InChI InChI=1S/C84H119N25O21S/c1-46(111)97-60(37-48-23-25-51(112)26-24-48)77(125)99-55(19-10-31-92-83(87)88)72(120)102-58(29-35-131-2)76(124)101-57(27-28-68(114)115)75(123)107-63(39-50-42-91-45-96-50)79(127)105-61(36-47-14-4-3-5-15-47)78(126)100-56(20-11-32-93-84(89)90)74(122)106-62(38-49-41-94-53-17-7-6-16-52(49)53)71(119)95-43-67(113)98-64(44-110)81(129)109-34-13-22-66(109)82(130)108-33-12-21-65(108)80(128)103-54(18-8-9-30-85)73(121)104-59(70(86)118)40-69(116)117/h3-7,14-17,23-26,41-42,45,54-66,94,110,112H,8-13,18-22,27-40,43-44,85H2,1-2H3,(H2,86,118)(H,91,96)(H,95,119)(H,97,111)(H,98,113)(H,99,125)(H,100,126)(H,101,124)(H,102,120)(H,103,128)(H,104,121)(H,105,127)(H,106,122)(H,107,123)(H,114,115)(H,116,117)(H4,87,88,92)(H4,89,90,93)/t54-,55-,56-,57-,58-,59-,60-,61+,62-,63-,64-,65-,66-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-1 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340352
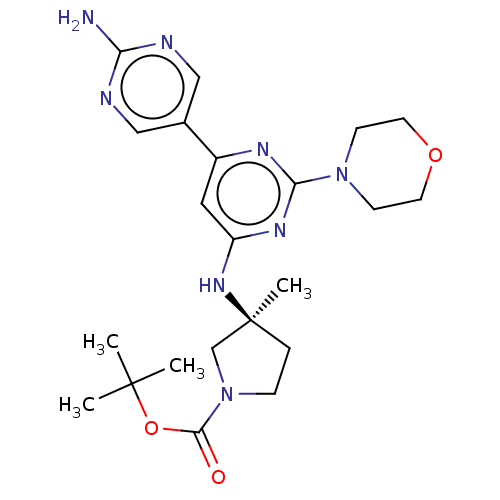
(US9758538, Example 40)Show SMILES CC(C)(C)OC(=O)N1CC[C@@](C)(C1)Nc1cc(nc(n1)N1CCOCC1)-c1cnc(N)nc1 |r| Show InChI InChI=1S/C22H32N8O3/c1-21(2,3)33-20(31)30-6-5-22(4,14-30)28-17-11-16(15-12-24-18(23)25-13-15)26-19(27-17)29-7-9-32-10-8-29/h11-13H,5-10,14H2,1-4H3,(H2,23,24,25)(H,26,27,28)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50031185

(2-{(S)-2-[((S)-1-{(S)-2-[4-(4-Chloro-benzenesulfon...)Show SMILES COC(=O)c1ccc2oc(nc2c1)C(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(C)C)C(C)C Show InChI InChI=1S/C38H40ClN5O10S/c1-20(2)30(32(45)36-40-27-19-24(38(50)53-5)12-17-29(27)54-36)41-35(48)28-7-6-18-44(28)37(49)31(21(3)4)42-33(46)22-8-10-23(11-9-22)34(47)43-55(51,52)26-15-13-25(39)14-16-26/h8-17,19-21,28,30-31H,6-7,18H2,1-5H3,(H,41,48)(H,42,46)(H,43,47)/t28-,30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-pNa |
J Med Chem 38: 3972-82 (1995)
BindingDB Entry DOI: 10.7270/Q2T152NN |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 3
(Homo sapiens (Human)) | BDBM50091751
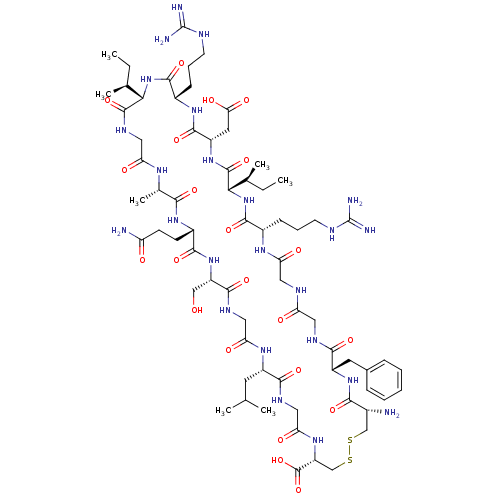
(CHEMBL411542 | Cyclic-(Cys-Phe-Gly-Gly-Arg-Ile-Asp...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)CNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](N)CSSC[C@@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)CNC1=O)C(O)=O)[C@@H](C)CC Show InChI InChI=1S/C70H114N24O22S2/c1-8-35(5)55-66(113)83-28-50(98)84-37(7)57(104)88-42(19-20-48(72)96)62(109)92-46(31-95)61(108)82-29-52(100)86-43(23-34(3)4)59(106)81-30-53(101)87-47(68(115)116)33-118-117-32-39(71)58(105)90-44(24-38-15-11-10-12-16-38)60(107)80-26-49(97)79-27-51(99)85-40(17-13-21-77-69(73)74)63(110)94-56(36(6)9-2)67(114)91-45(25-54(102)103)65(112)89-41(64(111)93-55)18-14-22-78-70(75)76/h10-12,15-16,34-37,39-47,55-56,95H,8-9,13-14,17-33,71H2,1-7H3,(H2,72,96)(H,79,97)(H,80,107)(H,81,106)(H,82,108)(H,83,113)(H,84,98)(H,85,99)(H,86,100)(H,87,101)(H,88,104)(H,89,112)(H,90,105)(H,91,114)(H,92,109)(H,93,111)(H,94,110)(H,102,103)(H,115,116)(H4,73,74,77)(H4,75,76,78)/t35-,36-,37-,39+,40-,41-,42-,43-,44-,45-,46-,47+,55-,56-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ANP from the Atrial Natriuretic Peptide Clearance Receptor. |
Bioorg Med Chem Lett 10: 1949-52 (2001)
BindingDB Entry DOI: 10.7270/Q2BK1BKQ |
More data for this
Ligand-Target Pair | |
Atrial natriuretic peptide receptor 1
(Homo sapiens (Human)) | BDBM50091751

(CHEMBL411542 | Cyclic-(Cys-Phe-Gly-Gly-Arg-Ile-Asp...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)CNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](N)CSSC[C@@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)CNC1=O)C(O)=O)[C@@H](C)CC Show InChI InChI=1S/C70H114N24O22S2/c1-8-35(5)55-66(113)83-28-50(98)84-37(7)57(104)88-42(19-20-48(72)96)62(109)92-46(31-95)61(108)82-29-52(100)86-43(23-34(3)4)59(106)81-30-53(101)87-47(68(115)116)33-118-117-32-39(71)58(105)90-44(24-38-15-11-10-12-16-38)60(107)80-26-49(97)79-27-51(99)85-40(17-13-21-77-69(73)74)63(110)94-56(36(6)9-2)67(114)91-45(25-54(102)103)65(112)89-41(64(111)93-55)18-14-22-78-70(75)76/h10-12,15-16,34-37,39-47,55-56,95H,8-9,13-14,17-33,71H2,1-7H3,(H2,72,96)(H,79,97)(H,80,107)(H,81,106)(H,82,108)(H,83,113)(H,84,98)(H,85,99)(H,86,100)(H,87,101)(H,88,104)(H,89,112)(H,90,105)(H,91,114)(H,92,109)(H,93,111)(H,94,110)(H,102,103)(H,115,116)(H4,73,74,77)(H4,75,76,78)/t35-,36-,37-,39+,40-,41-,42-,43-,44-,45-,46-,47+,55-,56-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]- ANP from the Atrial natriuretic peptide receptor A. |
Bioorg Med Chem Lett 10: 1949-52 (2001)
BindingDB Entry DOI: 10.7270/Q2BK1BKQ |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50018825
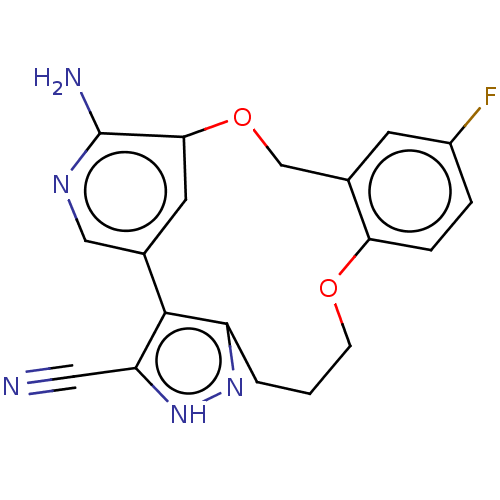
(CHEMBL3286815)Show SMILES Nc1ncc-2cc1OCc1cc(F)ccc1OCCCc1n[nH]c(C#N)c-21 Show InChI InChI=1S/C19H16FN5O2/c20-13-3-4-16-12(6-13)10-27-17-7-11(9-23-19(17)22)18-14(2-1-5-26-16)24-25-15(18)8-21/h3-4,6-7,9H,1-2,5,10H2,(H2,22,23)(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ALK L1196M mutant kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-... |
J Med Chem 57: 4720-44 (2014)
Article DOI: 10.1021/jm500261q
BindingDB Entry DOI: 10.7270/Q2K35W68 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50018833
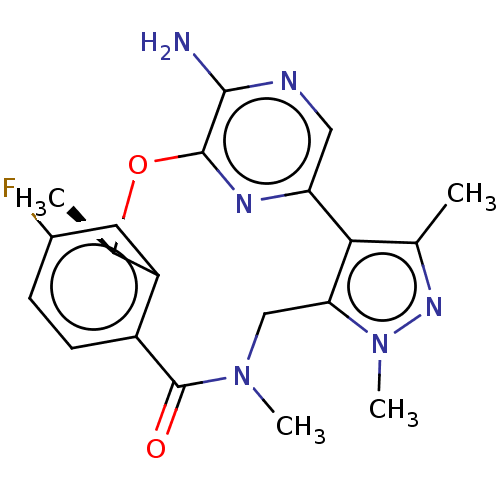
(CHEMBL3286823)Show SMILES C[C@H]1Oc2nc(cnc2N)-c2c(C)nn(C)c2CN(C)C(=O)c2ccc(F)cc12 |r| Show InChI InChI=1S/C20H21FN6O2/c1-10-17-15-8-23-18(22)19(24-15)29-11(2)14-7-12(21)5-6-13(14)20(28)26(3)9-16(17)27(4)25-10/h5-8,11H,9H2,1-4H3,(H2,22,23)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ALK L1196M mutant kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-... |
J Med Chem 57: 4720-44 (2014)
Article DOI: 10.1021/jm500261q
BindingDB Entry DOI: 10.7270/Q2K35W68 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50018835
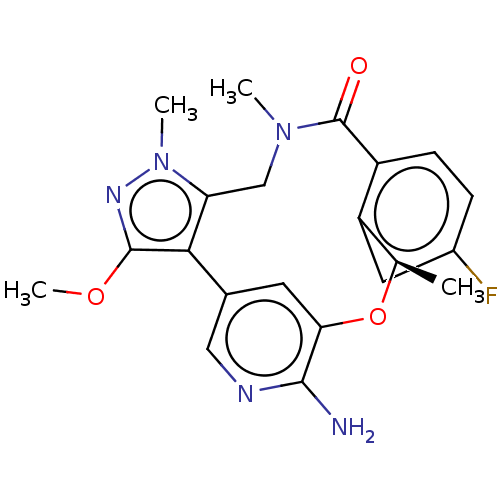
(CHEMBL3286825)Show SMILES COc1nn(C)c2CN(C)C(=O)c3ccc(F)cc3[C@@H](C)Oc3cc(cnc3N)-c12 |r| Show InChI InChI=1S/C21H22FN5O3/c1-11-15-8-13(22)5-6-14(15)21(28)26(2)10-16-18(20(29-4)25-27(16)3)12-7-17(30-11)19(23)24-9-12/h5-9,11H,10H2,1-4H3,(H2,23,24)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ALK L1196M mutant kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-... |
J Med Chem 57: 4720-44 (2014)
Article DOI: 10.1021/jm500261q
BindingDB Entry DOI: 10.7270/Q2K35W68 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50165931

(CHEMBL415165 | NDP-SYSMEHFRWGKPVG)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O Show InChI InChI=1S/C90H127N25O26S/c1-47(2)74(87(139)100-43-73(125)126)113-86(138)68-21-13-31-114(68)88(140)59(18-9-10-29-91)102-70(120)42-99-76(128)62(36-50-40-98-55-17-8-7-16-53(50)55)108-77(129)56(19-11-30-97-90(94)95)103-80(132)60(34-48-14-5-4-6-15-48)106-82(134)63(37-51-41-96-46-101-51)109-78(130)57(26-27-71(121)122)104-79(131)58(28-33-142-3)105-83(135)65(44-116)111-81(133)61(35-49-22-24-52(118)25-23-49)107-84(136)66(45-117)112-85(137)67-20-12-32-115(67)89(141)64(39-72(123)124)110-75(127)54(92)38-69(93)119/h4-8,14-17,22-25,40-41,46-47,54,56-68,74,98,116-118H,9-13,18-21,26-39,42-45,91-92H2,1-3H3,(H2,93,119)(H,96,101)(H,99,128)(H,100,139)(H,102,120)(H,103,132)(H,104,131)(H,105,135)(H,106,134)(H,107,136)(H,108,129)(H,109,130)(H,110,127)(H,111,133)(H,112,137)(H,113,138)(H,121,122)(H,123,124)(H,125,126)(H4,94,95,97)/t54-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,74-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-1 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50448785

(CHEMBL3128069)Show SMILES C[C@@H](Oc1cc(cnc1N)-c1sc(nc1C)[C@](C)(O)CO)c1cc(F)ccc1-n1nccn1 |r| Show InChI InChI=1S/C22H23FN6O3S/c1-12-19(33-21(28-12)22(3,31)11-30)14-8-18(20(24)25-10-14)32-13(2)16-9-15(23)4-5-17(16)29-26-6-7-27-29/h4-10,13,30-31H,11H2,1-3H3,(H2,24,25)/t13-,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant ALK kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-CONH... |
J Med Chem 57: 4720-44 (2014)
Article DOI: 10.1021/jm500261q
BindingDB Entry DOI: 10.7270/Q2K35W68 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50018828
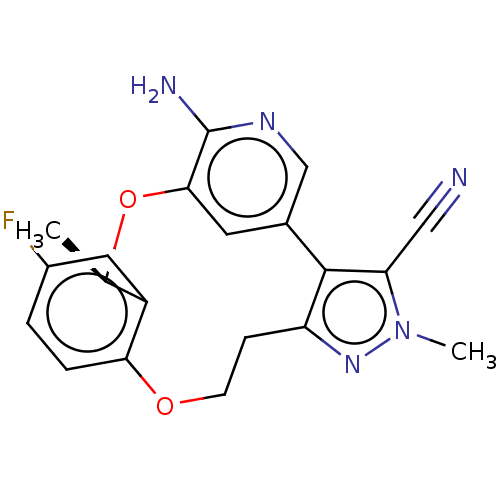
(CHEMBL3286818)Show SMILES C[C@H]1Oc2cc(cnc2N)-c2c(CCOc3ccc(F)cc13)nn(C)c2C#N |r| Show InChI InChI=1S/C20H18FN5O2/c1-11-14-8-13(21)3-4-17(14)27-6-5-15-19(16(9-22)26(2)25-15)12-7-18(28-11)20(23)24-10-12/h3-4,7-8,10-11H,5-6H2,1-2H3,(H2,23,24)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant ALK kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-CONH... |
J Med Chem 57: 4720-44 (2014)
Article DOI: 10.1021/jm500261q
BindingDB Entry DOI: 10.7270/Q2K35W68 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50018829
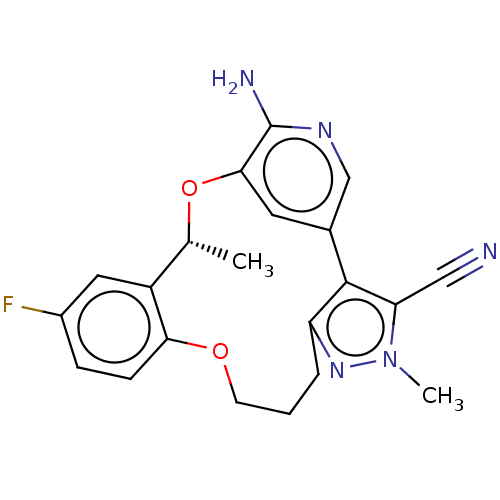
(CHEMBL3286819)Show SMILES C[C@H]1Oc2cc(cnc2N)-c2c(CCCOc3ccc(F)cc13)nn(C)c2C#N |r| Show InChI InChI=1S/C21H20FN5O2/c1-12-15-9-14(22)5-6-18(15)28-7-3-4-16-20(17(10-23)27(2)26-16)13-8-19(29-12)21(24)25-11-13/h5-6,8-9,11-12H,3-4,7H2,1-2H3,(H2,24,25)/t12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant ALK kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-CONH... |
J Med Chem 57: 4720-44 (2014)
Article DOI: 10.1021/jm500261q
BindingDB Entry DOI: 10.7270/Q2K35W68 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50018838
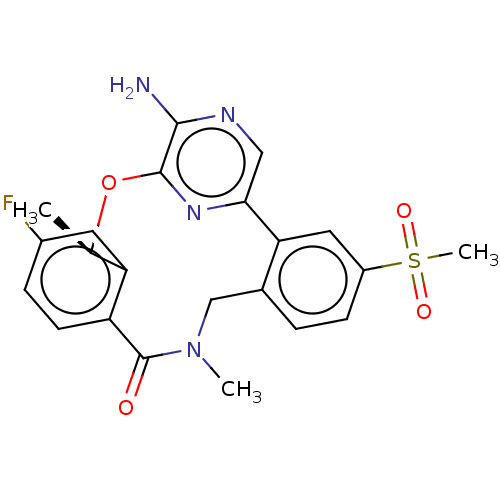
(CHEMBL3286828)Show SMILES C[C@H]1Oc2nc(cnc2N)-c2cc(ccc2CN(C)C(=O)c2ccc(F)cc12)S(C)(=O)=O |r| Show InChI InChI=1S/C22H21FN4O4S/c1-12-17-8-14(23)5-7-16(17)22(28)27(2)11-13-4-6-15(32(3,29)30)9-18(13)19-10-25-20(24)21(26-19)31-12/h4-10,12H,11H2,1-3H3,(H2,24,25)/t12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant ALK kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-CONH... |
J Med Chem 57: 4720-44 (2014)
Article DOI: 10.1021/jm500261q
BindingDB Entry DOI: 10.7270/Q2K35W68 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356873
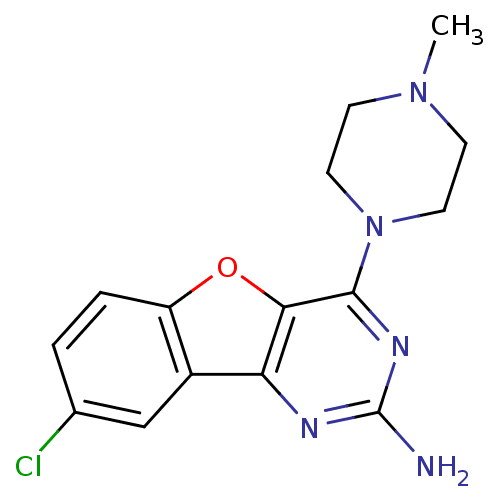
(CHEMBL1914541)Show InChI InChI=1S/C15H16ClN5O/c1-20-4-6-21(7-5-20)14-13-12(18-15(17)19-14)10-8-9(16)2-3-11(10)22-13/h2-3,8H,4-7H2,1H3,(H2,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from recombinant human histamine H4 receptor |
Bioorg Med Chem Lett 21: 6577-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.014
BindingDB Entry DOI: 10.7270/Q2CN749X |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50018839
(CHEMBL3286829)Show SMILES C[C@H]1Oc2cc(cnc2N)-c2c(nn(C)c2CN(C)C(=O)c2ccc(F)cc12)C1CC1 |r| Show InChI InChI=1S/C23H24FN5O2/c1-12-17-9-15(24)6-7-16(17)23(30)28(2)11-18-20(21(13-4-5-13)27-29(18)3)14-8-19(31-12)22(25)26-10-14/h6-10,12-13H,4-5,11H2,1-3H3,(H2,25,26)/t12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ALK L1196M mutant kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-... |
J Med Chem 57: 4720-44 (2014)
Article DOI: 10.1021/jm500261q
BindingDB Entry DOI: 10.7270/Q2K35W68 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50165935

(Ac-YRMEHdFRWGSPPKD-NH2 | CHEMBL414718)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(N)=O Show InChI InChI=1S/C84H119N25O21S/c1-46(111)97-60(37-48-23-25-51(112)26-24-48)77(125)99-55(19-10-31-92-83(87)88)72(120)102-58(29-35-131-2)76(124)101-57(27-28-68(114)115)75(123)107-63(39-50-42-91-45-96-50)79(127)105-61(36-47-14-4-3-5-15-47)78(126)100-56(20-11-32-93-84(89)90)74(122)106-62(38-49-41-94-53-17-7-6-16-52(49)53)71(119)95-43-67(113)98-64(44-110)81(129)109-34-13-22-66(109)82(130)108-33-12-21-65(108)80(128)103-54(18-8-9-30-85)73(121)104-59(70(86)118)40-69(116)117/h3-7,14-17,23-26,41-42,45,54-66,94,110,112H,8-13,18-22,27-40,43-44,85H2,1-2H3,(H2,86,118)(H,91,96)(H,95,119)(H,97,111)(H,98,113)(H,99,125)(H,100,126)(H,101,124)(H,102,120)(H,103,128)(H,104,121)(H,105,127)(H,106,122)(H,107,123)(H,114,115)(H,116,117)(H4,87,88,92)(H4,89,90,93)/t54-,55-,56-,57-,58-,59-,60-,61+,62-,63-,64-,65-,66-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-3 receptor expressed in HEK293 cells |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50018838

(CHEMBL3286828)Show SMILES C[C@H]1Oc2nc(cnc2N)-c2cc(ccc2CN(C)C(=O)c2ccc(F)cc12)S(C)(=O)=O |r| Show InChI InChI=1S/C22H21FN4O4S/c1-12-17-8-14(23)5-7-16(17)22(28)27(2)11-13-4-6-15(32(3,29)30)9-18(13)19-10-25-20(24)21(26-19)31-12/h4-10,12H,11H2,1-3H3,(H2,24,25)/t12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ALK L1196M mutant kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-... |
J Med Chem 57: 4720-44 (2014)
Article DOI: 10.1021/jm500261q
BindingDB Entry DOI: 10.7270/Q2K35W68 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data