 Found 28 hits with Last Name = 'weldon' and Initial = 'sm'
Found 28 hits with Last Name = 'weldon' and Initial = 'sm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50004182
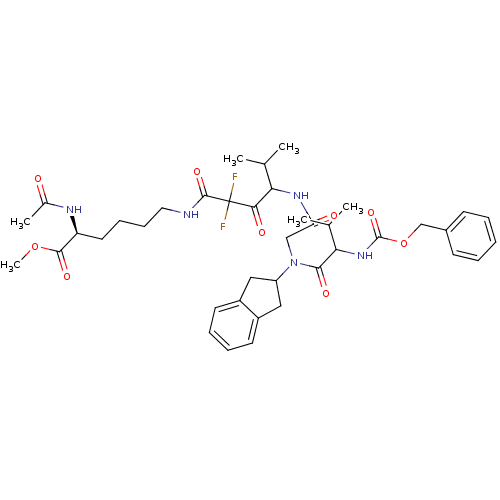
(2-Acetylamino-6-(4-{2-[(2-benzyloxycarbonylamino-3...)Show SMILES COC(=O)[C@H](CCCCNC(=O)C(F)(F)C(=O)C(NC(=O)CN(C1Cc2ccccc2C1)C(=O)C(NC(=O)OCc1ccccc1)C(C)C)C(C)C)NC(C)=O Show InChI InChI=1S/C40H53F2N5O9/c1-24(2)33(35(50)40(41,42)38(53)43-19-13-12-18-31(37(52)55-6)44-26(5)48)45-32(49)22-47(30-20-28-16-10-11-17-29(28)21-30)36(51)34(25(3)4)46-39(54)56-23-27-14-8-7-9-15-27/h7-11,14-17,24-25,30-31,33-34H,12-13,18-23H2,1-6H3,(H,43,53)(H,44,48)(H,45,49)(H,46,54)/t31-,33?,34?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 |
J Med Chem 35: 4795-808 (1993)
BindingDB Entry DOI: 10.7270/Q2HX1BM5 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50004192
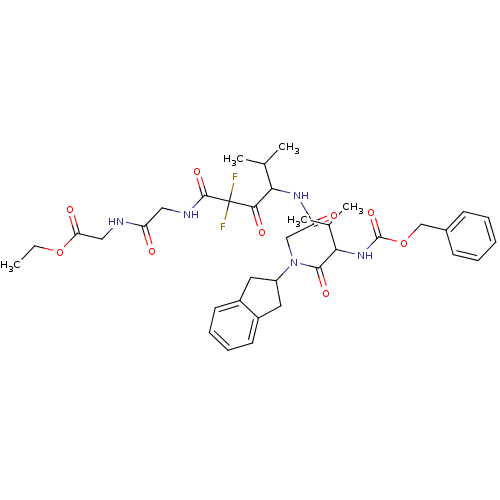
(CHEMBL344981 | [2-(4-{2-[(2-Benzyloxycarbonylamino...)Show SMILES CCOC(=O)CNC(=O)CNC(=O)C(F)(F)C(=O)C(NC(=O)CN(C1Cc2ccccc2C1)C(=O)C(NC(=O)OCc1ccccc1)C(C)C)C(C)C Show InChI InChI=1S/C37H47F2N5O9/c1-6-52-30(47)19-40-28(45)18-41-35(50)37(38,39)33(48)31(22(2)3)42-29(46)20-44(27-16-25-14-10-11-15-26(25)17-27)34(49)32(23(4)5)43-36(51)53-21-24-12-8-7-9-13-24/h7-15,22-23,27,31-32H,6,16-21H2,1-5H3,(H,40,45)(H,41,50)(H,42,46)(H,43,51) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 |
J Med Chem 35: 4795-808 (1993)
BindingDB Entry DOI: 10.7270/Q2HX1BM5 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50004184
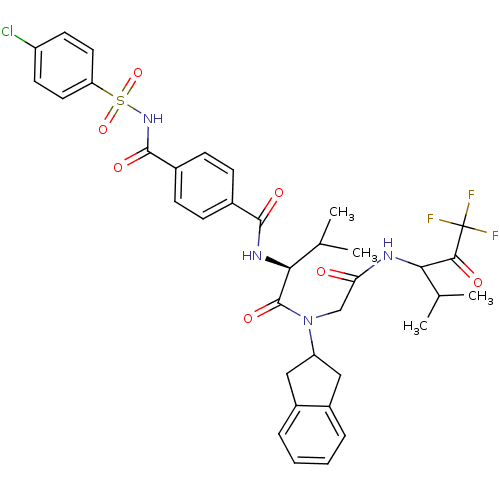
(4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-((S)-1...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(=O)N(CC(=O)NC(C(C)C)C(=O)C(F)(F)F)C1Cc2ccccc2C1 Show InChI InChI=1S/C36H38ClF3N4O7S/c1-20(2)30(32(46)36(38,39)40)41-29(45)19-44(27-17-24-7-5-6-8-25(24)18-27)35(49)31(21(3)4)42-33(47)22-9-11-23(12-10-22)34(48)43-52(50,51)28-15-13-26(37)14-16-28/h5-16,20-21,27,30-31H,17-19H2,1-4H3,(H,41,45)(H,42,47)(H,43,48)/t30?,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 |
J Med Chem 35: 4795-808 (1993)
BindingDB Entry DOI: 10.7270/Q2HX1BM5 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50273776
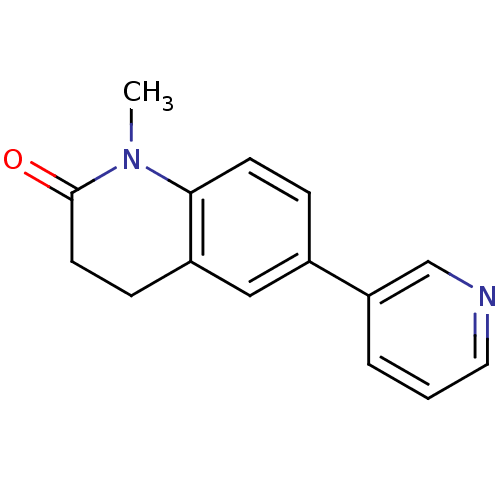
(1-Methyl-6-pyridin-3-yl-3,4-dihydroquinolin-2(1H)-...)Show InChI InChI=1S/C15H14N2O/c1-17-14-6-4-11(13-3-2-8-16-10-13)9-12(14)5-7-15(17)18/h2-4,6,8-10H,5,7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP19A1 (unknown origin) |
Bioorg Med Chem Lett 28: 979-984 (2018)
Article DOI: 10.1016/j.bmcl.2017.12.015
BindingDB Entry DOI: 10.7270/Q2XG9TQK |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50004190
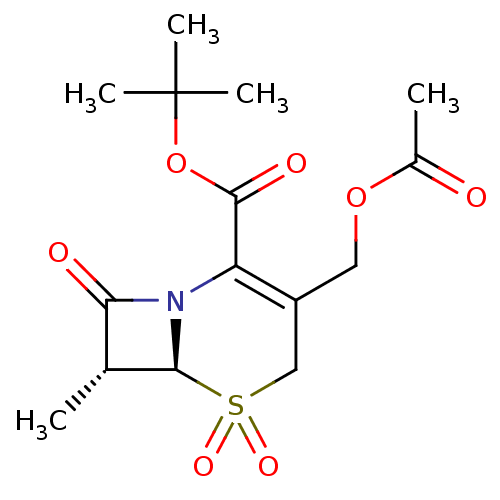
(3-Acetoxymethyl-7-methyl-5,5,8-trioxo-5lambda*6*-t...)Show SMILES C[C@H]1[C@H]2N(C1=O)C(C(=O)OC(C)(C)C)=C(COC(C)=O)CS2(=O)=O |t:14| Show InChI InChI=1S/C15H21NO7S/c1-8-12(18)16-11(14(19)23-15(3,4)5)10(6-22-9(2)17)7-24(20,21)13(8)16/h8,13H,6-7H2,1-5H3/t8-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 |
J Med Chem 35: 4795-808 (1993)
BindingDB Entry DOI: 10.7270/Q2HX1BM5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50273776

(1-Methyl-6-pyridin-3-yl-3,4-dihydroquinolin-2(1H)-...)Show InChI InChI=1S/C15H14N2O/c1-17-14-6-4-11(13-3-2-8-16-10-13)9-12(14)5-7-15(17)18/h2-4,6,8-10H,5,7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP17A1 (unknown origin) |
Bioorg Med Chem Lett 28: 979-984 (2018)
Article DOI: 10.1016/j.bmcl.2017.12.015
BindingDB Entry DOI: 10.7270/Q2XG9TQK |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50004187
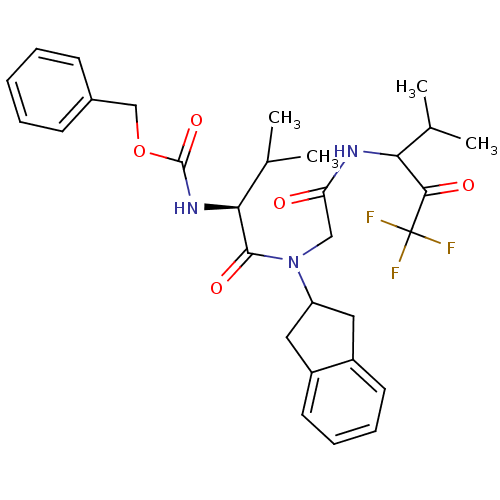
(((S)-1-{Indan-2-yl-[(3,3,3-trifluoro-1-isopropyl-2...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N(CC(=O)NC(C(C)C)C(=O)C(F)(F)F)C1Cc2ccccc2C1 Show InChI InChI=1S/C30H36F3N3O5/c1-18(2)25(27(38)30(31,32)33)34-24(37)16-36(23-14-21-12-8-9-13-22(21)15-23)28(39)26(19(3)4)35-29(40)41-17-20-10-6-5-7-11-20/h5-13,18-19,23,25-26H,14-17H2,1-4H3,(H,34,37)(H,35,40)/t25?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 365 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 |
J Med Chem 35: 4795-808 (1993)
BindingDB Entry DOI: 10.7270/Q2HX1BM5 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50004185
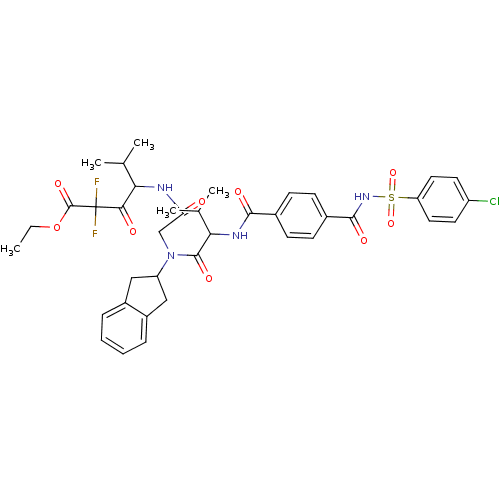
(4-[2-({2-[4-(4-Chloro-benzenesulfonylaminocarbonyl...)Show SMILES CCOC(=O)C(F)(F)C(=O)C(NC(=O)CN(C1Cc2ccccc2C1)C(=O)C(NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(C)C)C(C)C Show InChI InChI=1S/C39H43ClF2N4O9S/c1-6-55-38(52)39(41,42)34(48)32(22(2)3)43-31(47)21-46(29-19-26-9-7-8-10-27(26)20-29)37(51)33(23(4)5)44-35(49)24-11-13-25(14-12-24)36(50)45-56(53,54)30-17-15-28(40)16-18-30/h7-18,22-23,29,32-33H,6,19-21H2,1-5H3,(H,43,47)(H,44,49)(H,45,50) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 404 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 |
J Med Chem 35: 4795-808 (1993)
BindingDB Entry DOI: 10.7270/Q2HX1BM5 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50004183

(4-{2-[(2-Benzyloxycarbonylamino-3-methyl-butyryl)-...)Show SMILES CCOC(=O)C(F)(F)C(=O)C(NC(=O)CN(C1Cc2ccccc2C1)C(=O)C(NC(=O)OCc1ccccc1)C(C)C)C(C)C Show InChI InChI=1S/C33H41F2N3O7/c1-6-44-31(42)33(34,35)29(40)27(20(2)3)36-26(39)18-38(25-16-23-14-10-11-15-24(23)17-25)30(41)28(21(4)5)37-32(43)45-19-22-12-8-7-9-13-22/h7-15,20-21,25,27-28H,6,16-19H2,1-5H3,(H,36,39)(H,37,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 635 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 |
J Med Chem 35: 4795-808 (1993)
BindingDB Entry DOI: 10.7270/Q2HX1BM5 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50047262
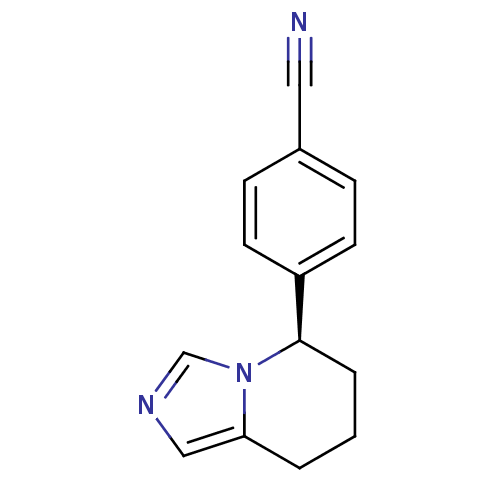
((R)-4-(5,6,7,8-tetrahydroimidazo[1,5-a]pyridin-5-y...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 794 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP19A1 (unknown origin) |
Bioorg Med Chem Lett 28: 979-984 (2018)
Article DOI: 10.1016/j.bmcl.2017.12.015
BindingDB Entry DOI: 10.7270/Q2XG9TQK |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50444549
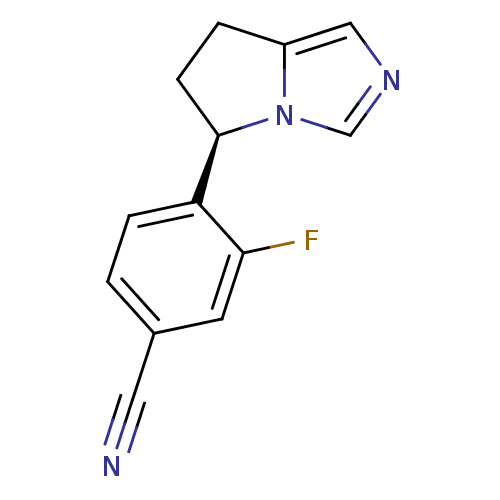
(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP19A1 (unknown origin) |
Bioorg Med Chem Lett 28: 979-984 (2018)
Article DOI: 10.1016/j.bmcl.2017.12.015
BindingDB Entry DOI: 10.7270/Q2XG9TQK |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP17A1 (unknown origin) |
Bioorg Med Chem Lett 28: 979-984 (2018)
Article DOI: 10.1016/j.bmcl.2017.12.015
BindingDB Entry DOI: 10.7270/Q2XG9TQK |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50004189

(4-[2-({2-[4-(4-Chloro-benzenesulfonylaminocarbonyl...)Show SMILES CCOC(=O)C(F)(F)C(=O)C(CC(C)C)NC(=O)CN(C1Cc2ccccc2C1)C(=O)C(NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(C)C Show InChI InChI=1S/C40H45ClF2N4O9S/c1-6-56-39(53)40(42,43)35(49)32(19-23(2)3)44-33(48)22-47(30-20-27-9-7-8-10-28(27)21-30)38(52)34(24(4)5)45-36(50)25-11-13-26(14-12-25)37(51)46-57(54,55)31-17-15-29(41)16-18-31/h7-18,23-24,30,32,34H,6,19-22H2,1-5H3,(H,44,48)(H,45,50)(H,46,51) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 |
J Med Chem 35: 4795-808 (1993)
BindingDB Entry DOI: 10.7270/Q2HX1BM5 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50004188
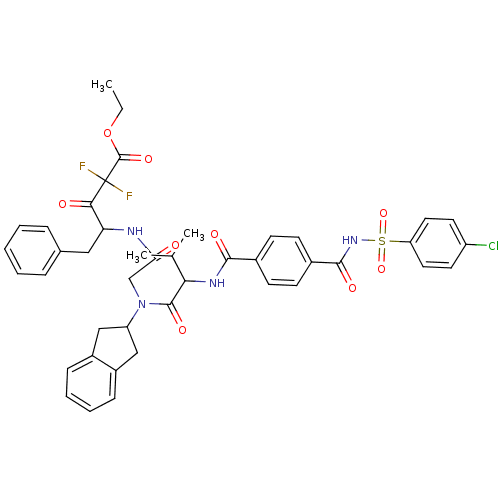
(4-[2-({2-[4-(4-Chloro-benzenesulfonylaminocarbonyl...)Show SMILES CCOC(=O)C(F)(F)C(=O)C(Cc1ccccc1)NC(=O)CN(C1Cc2ccccc2C1)C(=O)C(NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(C)C Show InChI InChI=1S/C43H43ClF2N4O9S/c1-4-59-42(56)43(45,46)38(52)35(22-27-10-6-5-7-11-27)47-36(51)25-50(33-23-30-12-8-9-13-31(30)24-33)41(55)37(26(2)3)48-39(53)28-14-16-29(17-15-28)40(54)49-60(57,58)34-20-18-32(44)19-21-34/h5-21,26,33,35,37H,4,22-25H2,1-3H3,(H,47,51)(H,48,53)(H,49,54) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 |
J Med Chem 35: 4795-808 (1993)
BindingDB Entry DOI: 10.7270/Q2HX1BM5 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50004186
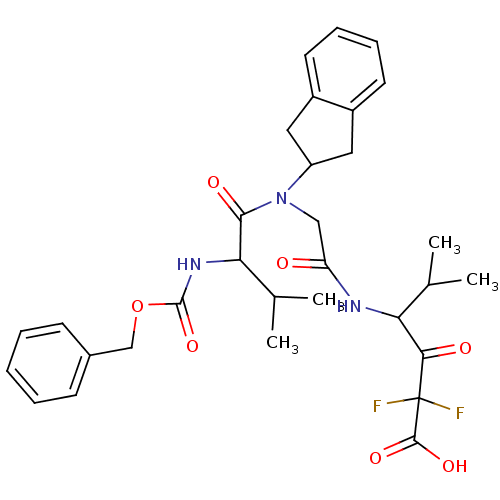
(4-{2-[(2-Benzyloxycarbonylamino-3-methyl-butyryl)-...)Show SMILES CC(C)C(NC(=O)OCc1ccccc1)C(=O)N(CC(=O)NC(C(C)C)C(=O)C(F)(F)C(O)=O)C1Cc2ccccc2C1 Show InChI InChI=1S/C31H37F2N3O7/c1-18(2)25(27(38)31(32,33)29(40)41)34-24(37)16-36(23-14-21-12-8-9-13-22(21)15-23)28(39)26(19(3)4)35-30(42)43-17-20-10-6-5-7-11-20/h5-13,18-19,23,25-26H,14-17H2,1-4H3,(H,34,37)(H,35,42)(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 |
J Med Chem 35: 4795-808 (1993)
BindingDB Entry DOI: 10.7270/Q2HX1BM5 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50004191
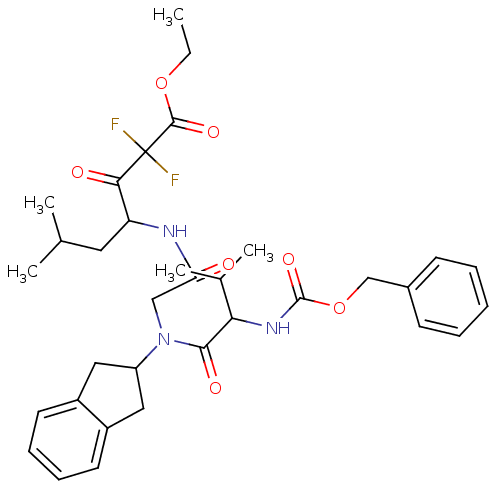
(4-{2-[(2-Benzyloxycarbonylamino-3-methyl-butyryl)-...)Show SMILES CCOC(=O)C(F)(F)C(=O)C(CC(C)C)NC(=O)CN(C1Cc2ccccc2C1)C(=O)C(NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C34H43F2N3O7/c1-6-45-32(43)34(35,36)30(41)27(16-21(2)3)37-28(40)19-39(26-17-24-14-10-11-15-25(24)18-26)31(42)29(22(4)5)38-33(44)46-20-23-12-8-7-9-13-23/h7-15,21-22,26-27,29H,6,16-20H2,1-5H3,(H,37,40)(H,38,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 |
J Med Chem 35: 4795-808 (1993)
BindingDB Entry DOI: 10.7270/Q2HX1BM5 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50454115
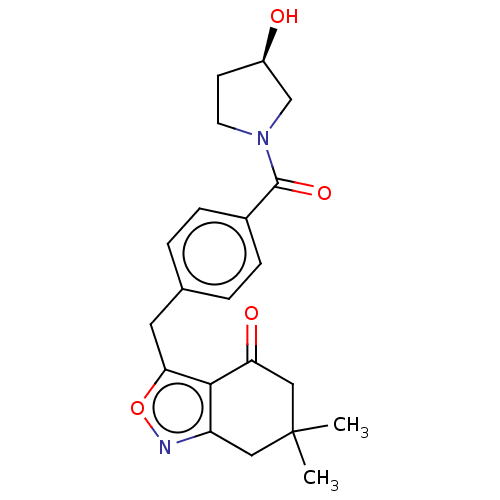
(CHEMBL4207348)Show SMILES CC1(C)Cc2noc(Cc3ccc(cc3)C(=O)N3CC[C@@H](O)C3)c2C(=O)C1 |r| Show InChI InChI=1S/C21H24N2O4/c1-21(2)10-16-19(17(25)11-21)18(27-22-16)9-13-3-5-14(6-4-13)20(26)23-8-7-15(24)12-23/h3-6,15,24H,7-12H2,1-2H3/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP19A1 (unknown origin) |
Bioorg Med Chem Lett 28: 979-984 (2018)
Article DOI: 10.1016/j.bmcl.2017.12.015
BindingDB Entry DOI: 10.7270/Q2XG9TQK |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50047262

((R)-4-(5,6,7,8-tetrahydroimidazo[1,5-a]pyridin-5-y...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP17A1 (unknown origin) |
Bioorg Med Chem Lett 28: 979-984 (2018)
Article DOI: 10.1016/j.bmcl.2017.12.015
BindingDB Entry DOI: 10.7270/Q2XG9TQK |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50454114
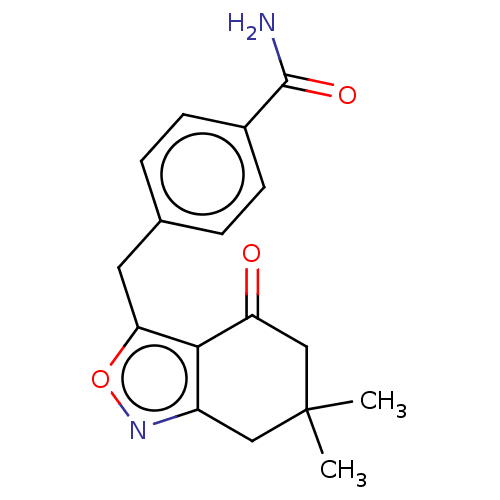
(CHEMBL4211193)Show InChI InChI=1S/C17H18N2O3/c1-17(2)8-12-15(13(20)9-17)14(22-19-12)7-10-3-5-11(6-4-10)16(18)21/h3-6H,7-9H2,1-2H3,(H2,18,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP19A1 (unknown origin) |
Bioorg Med Chem Lett 28: 979-984 (2018)
Article DOI: 10.1016/j.bmcl.2017.12.015
BindingDB Entry DOI: 10.7270/Q2XG9TQK |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50454120
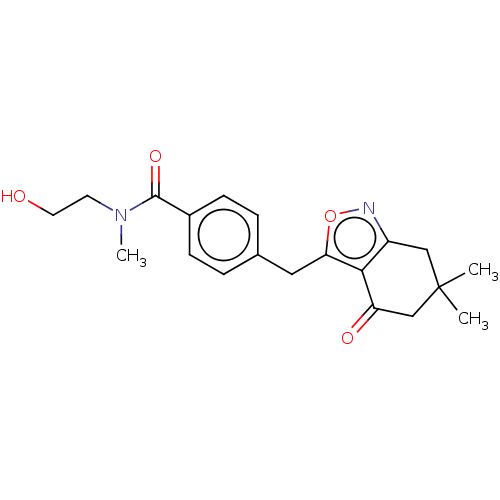
(CHEMBL4214618)Show SMILES CN(CCO)C(=O)c1ccc(Cc2onc3CC(C)(C)CC(=O)c23)cc1 Show InChI InChI=1S/C20H24N2O4/c1-20(2)11-15-18(16(24)12-20)17(26-21-15)10-13-4-6-14(7-5-13)19(25)22(3)8-9-23/h4-7,23H,8-12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP17A1 (unknown origin) |
Bioorg Med Chem Lett 28: 979-984 (2018)
Article DOI: 10.1016/j.bmcl.2017.12.015
BindingDB Entry DOI: 10.7270/Q2XG9TQK |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50454116

(CHEMBL4207995)Show InChI InChI=1S/C16H17NO2/c1-16(2)9-12-15(13(18)10-16)14(19-17-12)8-11-6-4-3-5-7-11/h3-7H,8-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP19A1 (unknown origin) |
Bioorg Med Chem Lett 28: 979-984 (2018)
Article DOI: 10.1016/j.bmcl.2017.12.015
BindingDB Entry DOI: 10.7270/Q2XG9TQK |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50454120

(CHEMBL4214618)Show SMILES CN(CCO)C(=O)c1ccc(Cc2onc3CC(C)(C)CC(=O)c23)cc1 Show InChI InChI=1S/C20H24N2O4/c1-20(2)11-15-18(16(24)12-20)17(26-21-15)10-13-4-6-14(7-5-13)19(25)22(3)8-9-23/h4-7,23H,8-12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP19A1 (unknown origin) |
Bioorg Med Chem Lett 28: 979-984 (2018)
Article DOI: 10.1016/j.bmcl.2017.12.015
BindingDB Entry DOI: 10.7270/Q2XG9TQK |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50454119
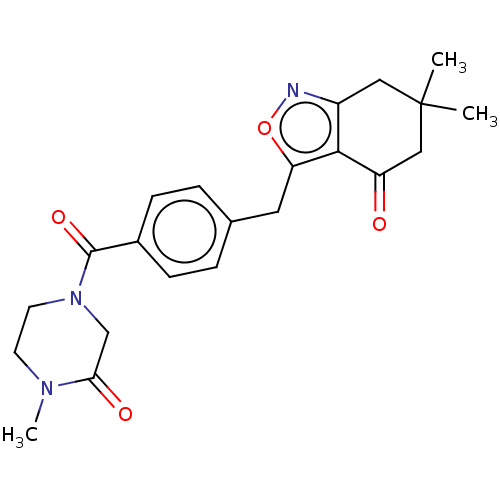
(CHEMBL4212918)Show SMILES CN1CCN(CC1=O)C(=O)c1ccc(Cc2onc3CC(C)(C)CC(=O)c23)cc1 Show InChI InChI=1S/C22H25N3O4/c1-22(2)11-16-20(17(26)12-22)18(29-23-16)10-14-4-6-15(7-5-14)21(28)25-9-8-24(3)19(27)13-25/h4-7H,8-13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP19A1 (unknown origin) |
Bioorg Med Chem Lett 28: 979-984 (2018)
Article DOI: 10.1016/j.bmcl.2017.12.015
BindingDB Entry DOI: 10.7270/Q2XG9TQK |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50454113
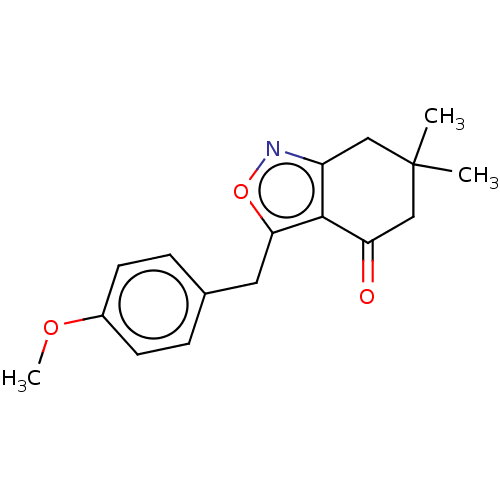
(CHEMBL4212612)Show InChI InChI=1S/C17H19NO3/c1-17(2)9-13-16(14(19)10-17)15(21-18-13)8-11-4-6-12(20-3)7-5-11/h4-7H,8-10H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP19A1 (unknown origin) |
Bioorg Med Chem Lett 28: 979-984 (2018)
Article DOI: 10.1016/j.bmcl.2017.12.015
BindingDB Entry DOI: 10.7270/Q2XG9TQK |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50454118
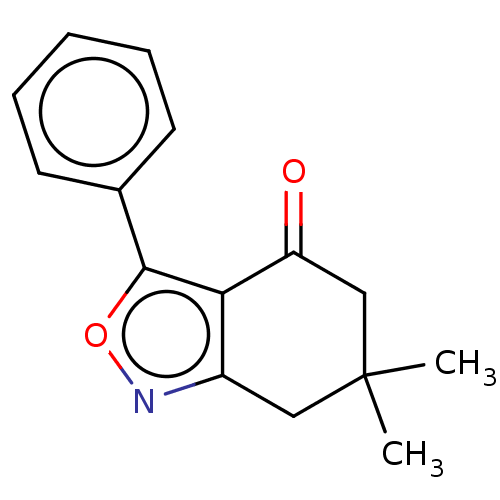
(CHEMBL1490952)Show InChI InChI=1S/C15H15NO2/c1-15(2)8-11-13(12(17)9-15)14(18-16-11)10-6-4-3-5-7-10/h3-7H,8-9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP17A1 (unknown origin) |
Bioorg Med Chem Lett 28: 979-984 (2018)
Article DOI: 10.1016/j.bmcl.2017.12.015
BindingDB Entry DOI: 10.7270/Q2XG9TQK |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50454117
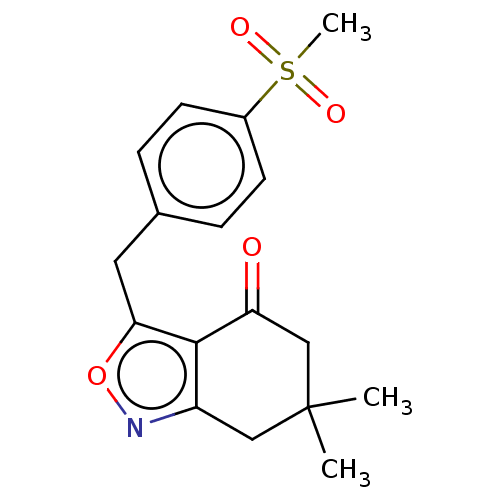
(CHEMBL4211126)Show SMILES CC1(C)Cc2noc(Cc3ccc(cc3)S(C)(=O)=O)c2C(=O)C1 Show InChI InChI=1S/C17H19NO4S/c1-17(2)9-13-16(14(19)10-17)15(22-18-13)8-11-4-6-12(7-5-11)23(3,20)21/h4-7H,8-10H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP17A1 (unknown origin) |
Bioorg Med Chem Lett 28: 979-984 (2018)
Article DOI: 10.1016/j.bmcl.2017.12.015
BindingDB Entry DOI: 10.7270/Q2XG9TQK |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50454113

(CHEMBL4212612)Show InChI InChI=1S/C17H19NO3/c1-17(2)9-13-16(14(19)10-17)15(21-18-13)8-11-4-6-12(20-3)7-5-11/h4-7H,8-10H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP17A1 (unknown origin) |
Bioorg Med Chem Lett 28: 979-984 (2018)
Article DOI: 10.1016/j.bmcl.2017.12.015
BindingDB Entry DOI: 10.7270/Q2XG9TQK |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50454116

(CHEMBL4207995)Show InChI InChI=1S/C16H17NO2/c1-16(2)9-12-15(13(18)10-16)14(19-17-12)8-11-6-4-3-5-7-11/h3-7H,8-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP17A1 (unknown origin) |
Bioorg Med Chem Lett 28: 979-984 (2018)
Article DOI: 10.1016/j.bmcl.2017.12.015
BindingDB Entry DOI: 10.7270/Q2XG9TQK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data