 Found 134 hits with Last Name = 'wenandy' and Initial = 't'
Found 134 hits with Last Name = 'wenandy' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50409610

(CHEMBL2021596)Show SMILES CC[C@@H]1NC(=O)[C@H](C(C)C(C)C\C=C\C)N(C)C(=O)[C@@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O11/c1-26-28-29-41(15)42(16)53-57(79)66-45(27-2)59(81)68(19)34-50(75)69(20)46(30-35(3)4)56(78)67-51(39(11)12)62(84)70(21)47(31-36(5)6)55(77)64-43(17)54(76)65-44(18)58(80)71(22)48(32-37(7)8)60(82)72(23)49(33-38(9)10)61(83)73(24)52(40(13)14)63(85)74(53)25/h26,28,35-49,51-53H,27,29-34H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b28-26+/t41?,42?,43-,44+,45-,46-,47-,48-,49-,51-,52+,53-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against human formylpeptide receptor (FPR) of human leukemia HL-60 cells |
J Med Chem 45: 4613-28 (2002)
BindingDB Entry DOI: 10.7270/Q2571CQX |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50369565
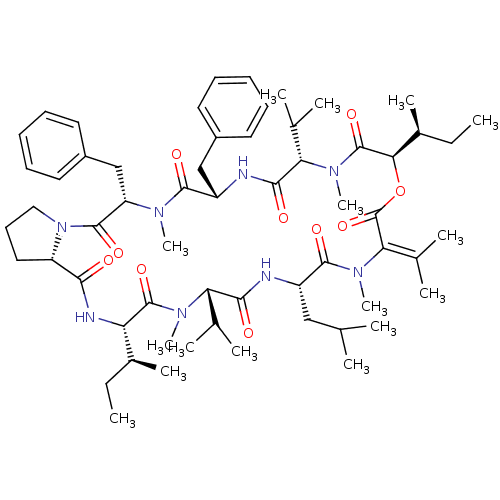
(CHEMBL1790683)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7](-[#6])-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6](-[#6])-[#6])-[#7](-[#6])-[#6](=O)-[#6@H](-[#8]-[#6](=O)\[#6](-[#7](-[#6])-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6](-[#6])-[#6])-[#7](-[#6])-[#6]-1=O)=[#6](\[#6])-[#6])-[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C60H90N8O10/c1-17-39(11)47-58(75)65(14)48(36(5)6)53(70)61-43(32-35(3)4)56(73)67(16)50(38(9)10)60(77)78-51(40(12)18-2)59(76)66(15)49(37(7)8)54(71)62-44(33-41-26-21-19-22-27-41)55(72)64(13)46(34-42-28-23-20-24-29-42)57(74)68-31-25-30-45(68)52(69)63-47/h19-24,26-29,35-37,39-40,43-49,51H,17-18,25,30-34H2,1-16H3,(H,61,70)(H,62,71)(H,63,69)/t39-,40-,43-,44-,45-,46-,47-,48-,49-,51+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Human MDR1 Pgp inhibitory activity by using standard calcein-AM efflux method with the human leukemia CEM cells. |
J Med Chem 43: 2547-56 (2000)
BindingDB Entry DOI: 10.7270/Q2PG1SFC |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50026897

(CHEMBL2372488)Show SMILES [H][C@]1(NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@](O)(NC(=O)[C@H](C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)CNC1=O)C(C)C)[C@@H](C)O |r| Show InChI InChI=1S/C63H113N11O14/c1-24-25-26-40(16)52(77)51-58(83)68-49(42(18)75)57(82)64-32-48(76)70(19)45(29-35(6)7)55(80)66-43(27-33(2)3)59(84)71(20)46(30-36(8)9)54(79)65-41(17)53(78)69-63(88,39(14)15)62(87)72(21)47(31-37(10)11)56(81)67-44(28-34(4)5)60(85)73(22)50(38(12)13)61(86)74(51)23/h24-25,33-47,49-52,75,77,88H,26-32H2,1-23H3,(H,64,82)(H,65,79)(H,66,80)(H,67,81)(H,68,83)(H,69,78)/b25-24+/t40-,41+,42-,43+,44+,45-,46-,47+,49+,50+,51+,52-,63+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against human formylpeptide receptor (FPR) of human leukemia HL-60 cells |
J Med Chem 45: 4613-28 (2002)
BindingDB Entry DOI: 10.7270/Q2571CQX |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50369557
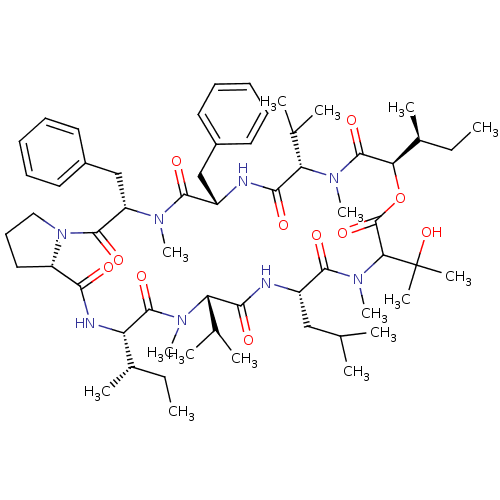
(CHEMBL1790680)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](OC(=O)C(N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C1=O)C(C)(C)O)[C@@H](C)CC Show InChI InChI=1S/C60H92N8O11/c1-17-38(9)46-57(75)65(14)47(36(5)6)52(70)61-42(32-35(3)4)55(73)67(16)50(60(11,12)78)59(77)79-49(39(10)18-2)58(76)66(15)48(37(7)8)53(71)62-43(33-40-26-21-19-22-27-40)54(72)64(13)45(34-41-28-23-20-24-29-41)56(74)68-31-25-30-44(68)51(69)63-46/h19-24,26-29,35-39,42-50,78H,17-18,25,30-34H2,1-16H3,(H,61,70)(H,62,71)(H,63,69)/t38-,39-,42-,43-,44-,45-,46-,47-,48-,49+,50?/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Human MDR1 Pgp inhibitory activity by using standard calcein-AM efflux method with the human leukemia CEM cells. |
J Med Chem 43: 2547-56 (2000)
BindingDB Entry DOI: 10.7270/Q2PG1SFC |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50369564
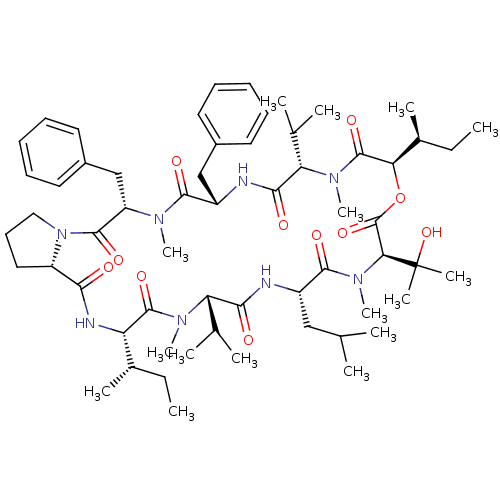
(CHEMBL1790681)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](OC(=O)[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C1=O)C(C)(C)O)[C@@H](C)CC Show InChI InChI=1S/C60H92N8O11/c1-17-38(9)46-57(75)65(14)47(36(5)6)52(70)61-42(32-35(3)4)55(73)67(16)50(60(11,12)78)59(77)79-49(39(10)18-2)58(76)66(15)48(37(7)8)53(71)62-43(33-40-26-21-19-22-27-40)54(72)64(13)45(34-41-28-23-20-24-29-41)56(74)68-31-25-30-44(68)51(69)63-46/h19-24,26-29,35-39,42-50,78H,17-18,25,30-34H2,1-16H3,(H,61,70)(H,62,71)(H,63,69)/t38-,39-,42-,43-,44-,45-,46-,47-,48-,49+,50-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Human MDR1 Pgp inhibitory activity by using standard calcein-AM efflux method with the human leukemia CEM cells. |
J Med Chem 43: 2547-56 (2000)
BindingDB Entry DOI: 10.7270/Q2PG1SFC |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50026915
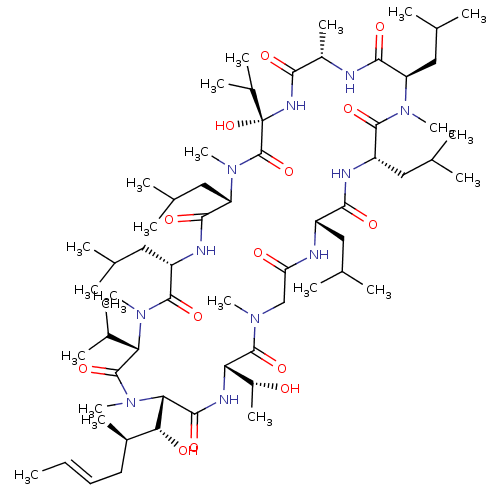
(CHEMBL2372486)Show SMILES [H][C@]1(NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@](O)(NC(=O)[C@H](C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)CN(C)C1=O)C(C)C)[C@@H](C)O |r| Show InChI InChI=1S/C63H113N11O14/c1-24-25-26-40(16)52(77)51-57(82)68-49(42(18)75)60(85)70(19)32-48(76)65-43(27-33(2)3)54(79)66-44(28-34(4)5)58(83)71(20)46(30-36(8)9)55(80)64-41(17)53(78)69-63(88,39(14)15)62(87)72(21)47(31-37(10)11)56(81)67-45(29-35(6)7)59(84)73(22)50(38(12)13)61(86)74(51)23/h24-25,33-47,49-52,75,77,88H,26-32H2,1-23H3,(H,64,80)(H,65,76)(H,66,79)(H,67,81)(H,68,82)(H,69,78)/b25-24+/t40-,41+,42-,43+,44+,45+,46-,47+,49+,50+,51+,52-,63+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against human formylpeptide receptor (FPR) of human leukemia HL-60 cells |
J Med Chem 45: 4613-28 (2002)
BindingDB Entry DOI: 10.7270/Q2571CQX |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50026911

(CHEMBL2372507)Show SMILES [H][C@]1(NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@](O)(NC(=O)[C@H](C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C)[C@@H](C)O |r| Show InChI InChI=1S/C64H115N11O14/c1-25-26-27-41(16)53(78)52-58(83)68-50(43(18)76)61(86)70(19)33-49(77)71(20)46(30-36(6)7)56(81)66-44(28-34(2)3)59(84)72(21)47(31-37(8)9)55(80)65-42(17)54(79)69-64(89,40(14)15)63(88)73(22)48(32-38(10)11)57(82)67-45(29-35(4)5)60(85)74(23)51(39(12)13)62(87)75(52)24/h25-26,34-48,50-53,76,78,89H,27-33H2,1-24H3,(H,65,80)(H,66,81)(H,67,82)(H,68,83)(H,69,79)/b26-25+/t41-,42+,43-,44+,45+,46-,47-,48+,50+,51+,52+,53-,64+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against human formylpeptide receptor (FPR) of human leukemia HL-60 cells |
J Med Chem 45: 4613-28 (2002)
BindingDB Entry DOI: 10.7270/Q2571CQX |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50026906

(CHEMBL2372505)Show SMILES [H][C@]1(NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C1=O)[C@@H](C)O |r| Show InChI InChI=1S/C62H111N11O13/c1-24-25-26-39(14)52(76)51-57(81)67-49(42(17)74)61(85)68(18)32-48(75)69(19)45(29-35(6)7)55(79)65-43(27-33(2)3)59(83)71(21)46(30-36(8)9)54(78)63-40(15)53(77)64-41(16)58(82)70(20)47(31-37(10)11)56(80)66-44(28-34(4)5)60(84)72(22)50(38(12)13)62(86)73(51)23/h24-25,33-47,49-52,74,76H,26-32H2,1-23H3,(H,63,78)(H,64,77)(H,65,79)(H,66,80)(H,67,81)/b25-24+/t39-,40+,41-,42-,43+,44+,45-,46-,47+,49+,50+,51+,52-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against human formylpeptide receptor (FPR) of human leukemia HL-60 cells |
J Med Chem 45: 4613-28 (2002)
BindingDB Entry DOI: 10.7270/Q2571CQX |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50026895
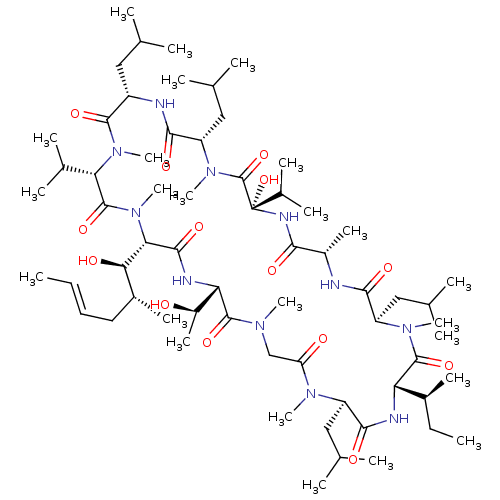
(CHEMBL2372487)Show SMILES [H][C@]1(NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@](O)(NC(=O)[C@H](C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@@]([H])(NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C1=O)[C@@H](C)CC)C(C)C)[C@@H](C)O |r| Show InChI InChI=1S/C64H115N11O14/c1-25-27-28-41(16)53(78)52-58(83)68-50(43(18)76)60(85)70(19)33-48(77)71(20)45(30-35(5)6)57(82)67-49(40(15)26-2)61(86)72(21)46(31-36(7)8)55(80)65-42(17)54(79)69-64(89,39(13)14)63(88)73(22)47(32-37(9)10)56(81)66-44(29-34(3)4)59(84)74(23)51(38(11)12)62(87)75(52)24/h25,27,34-47,49-53,76,78,89H,26,28-33H2,1-24H3,(H,65,80)(H,66,81)(H,67,82)(H,68,83)(H,69,79)/b27-25+/t40-,41+,42-,43+,44-,45+,46+,47-,49-,50-,51-,52-,53+,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against human formylpeptide receptor (FPR) of human leukemia HL-60 cells |
J Med Chem 45: 4613-28 (2002)
BindingDB Entry DOI: 10.7270/Q2571CQX |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50369570
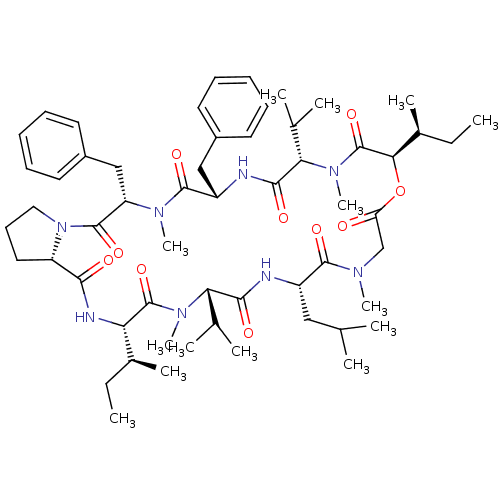
(CHEMBL1790684)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](OC(=O)CN(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C1=O)[C@@H](C)CC Show InChI InChI=1S/C57H86N8O10/c1-15-37(9)46-56(73)63(13)47(35(5)6)51(68)58-41(30-34(3)4)53(70)61(11)33-45(66)75-49(38(10)16-2)57(74)64(14)48(36(7)8)52(69)59-42(31-39-24-19-17-20-25-39)54(71)62(12)44(32-40-26-21-18-22-27-40)55(72)65-29-23-28-43(65)50(67)60-46/h17-22,24-27,34-38,41-44,46-49H,15-16,23,28-33H2,1-14H3,(H,58,68)(H,59,69)(H,60,67)/t37-,38-,41-,42-,43-,44-,46-,47-,48-,49+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Human MDR1 Pgp inhibitory activity by using standard calcein-AM efflux method with the human leukemia CEM cells. |
J Med Chem 43: 2547-56 (2000)
BindingDB Entry DOI: 10.7270/Q2PG1SFC |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50369561
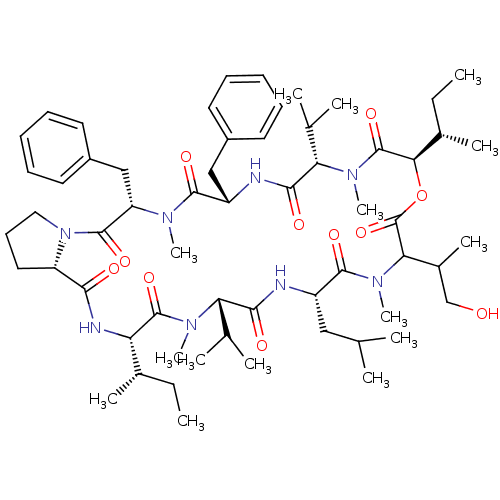
(CHEMBL1790685)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](OC(=O)C(C(C)CO)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C1=O)[C@@H](C)CC Show InChI InChI=1S/C60H92N8O11/c1-16-38(9)47-58(76)65(13)48(36(5)6)53(71)61-43(31-35(3)4)56(74)67(15)50(40(11)34-69)60(78)79-51(39(10)17-2)59(77)66(14)49(37(7)8)54(72)62-44(32-41-25-20-18-21-26-41)55(73)64(12)46(33-42-27-22-19-23-28-42)57(75)68-30-24-29-45(68)52(70)63-47/h18-23,25-28,35-40,43-51,69H,16-17,24,29-34H2,1-15H3,(H,61,71)(H,62,72)(H,63,70)/t38-,39-,40?,43-,44-,45-,46-,47-,48-,49-,50?,51+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Human MDR1 Pgp inhibitory activity by using standard calcein-AM efflux method with the human leukemia CEM cells. |
J Med Chem 43: 2547-56 (2000)
BindingDB Entry DOI: 10.7270/Q2PG1SFC |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50369551
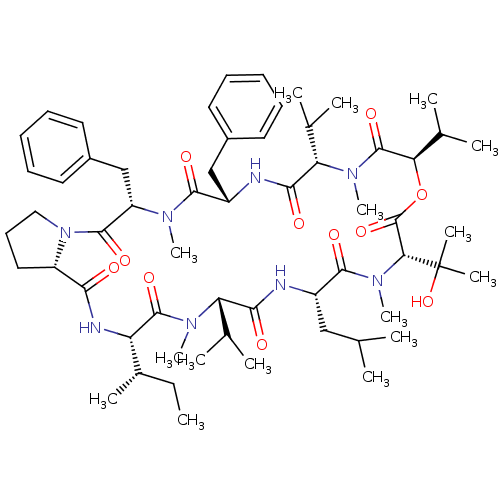
(CHEMBL1790690)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](OC(=O)[C@@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C1=O)C(C)(C)O)C(C)C Show InChI InChI=1S/C59H90N8O11/c1-17-38(10)45-56(74)64(14)46(35(4)5)51(69)60-41(31-34(2)3)54(72)66(16)49(59(11,12)77)58(76)78-48(37(8)9)57(75)65(15)47(36(6)7)52(70)61-42(32-39-25-20-18-21-26-39)53(71)63(13)44(33-40-27-22-19-23-28-40)55(73)67-30-24-29-43(67)50(68)62-45/h18-23,25-28,34-38,41-49,77H,17,24,29-33H2,1-16H3,(H,60,69)(H,61,70)(H,62,68)/t38-,41-,42-,43-,44-,45-,46-,47-,48+,49+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Human MDR1 Pgp inhibitory activity by using standard calcein-AM efflux method with the human leukemia CEM cells. |
J Med Chem 43: 2547-56 (2000)
BindingDB Entry DOI: 10.7270/Q2PG1SFC |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50369558

(CHEMBL1790700)Show SMILES CC[C@H](C)[C@H]1OC(=O)[C@@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C(=O)C(NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C(C)C)N(C)C1=O)C(C)C)C(C)(C)O Show InChI InChI=1S/C59H90N8O11/c1-17-38(10)48-57(75)65(15)47(37(8)9)52(70)61-42(32-39-25-20-18-21-26-39)53(71)63(13)44(33-40-27-22-19-23-28-40)55(73)67-30-24-29-43(67)50(68)62-45(35(4)5)56(74)64(14)46(36(6)7)51(69)60-41(31-34(2)3)54(72)66(16)49(58(76)78-48)59(11,12)77/h18-23,25-28,34-38,41-49,77H,17,24,29-33H2,1-16H3,(H,60,69)(H,61,70)(H,62,68)/t38-,41-,42-,43-,44-,45?,46-,47-,48+,49+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Human MDR1 Pgp inhibitory activity by using standard calcein-AM efflux method with the human leukemia CEM cells. |
J Med Chem 43: 2547-56 (2000)
BindingDB Entry DOI: 10.7270/Q2PG1SFC |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50119425
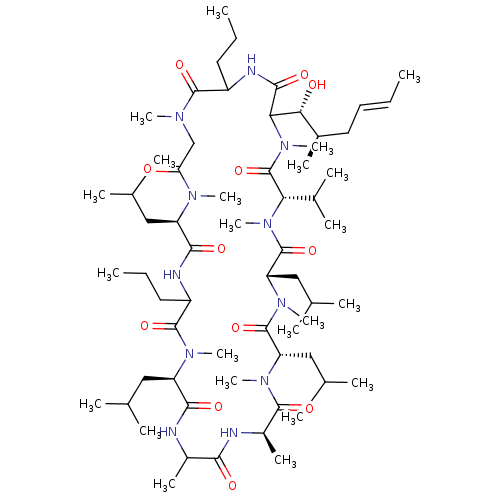
(33-(1-Hydroxy-2-methyl-hex-4-enyl)-6,9,18,24-tetra...)Show SMILES CCCC1NC(=O)C([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)C(C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)C(CCC)NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C1=O Show InChI InChI=1S/C63H113N11O12/c1-24-27-30-41(14)53(76)52-57(80)67-44(28-25-2)59(82)68(17)35-50(75)69(18)46(31-36(4)5)56(79)66-45(29-26-3)60(83)70(19)47(32-37(6)7)55(78)64-42(15)54(77)65-43(16)58(81)71(20)48(33-38(8)9)61(84)72(21)49(34-39(10)11)62(85)73(22)51(40(12)13)63(86)74(52)23/h24,27,36-49,51-53,76H,25-26,28-35H2,1-23H3,(H,64,78)(H,65,77)(H,66,79)(H,67,80)/b27-24+/t41-,42?,43-,44?,45?,46-,47-,48+,49+,51+,52?,53-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human MDR1 P-Glycoprotein ABC Transporter using leukemia CEM cells |
J Med Chem 45: 4598-612 (2002)
BindingDB Entry DOI: 10.7270/Q29024HJ |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50369547
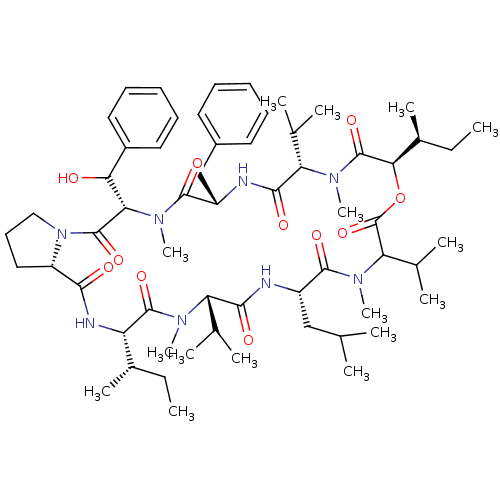
(CHEMBL1790679)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](C(O)c2ccccc2)N(C)C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](OC(=O)C(C(C)C)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C1=O)[C@@H](C)CC Show InChI InChI=1S/C60H92N8O11/c1-17-38(11)45-57(75)64(13)46(35(5)6)53(71)61-42(32-34(3)4)55(73)66(15)48(37(9)10)60(78)79-51(39(12)18-2)59(77)65(14)47(36(7)8)54(72)62-43(33-40-26-21-19-22-27-40)56(74)67(16)49(50(69)41-28-23-20-24-29-41)58(76)68-31-25-30-44(68)52(70)63-45/h19-24,26-29,34-39,42-51,69H,17-18,25,30-33H2,1-16H3,(H,61,71)(H,62,72)(H,63,70)/t38-,39-,42-,43-,44-,45-,46-,47-,48?,49-,50?,51+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Human MDR1 Pgp inhibitory activity by using standard calcein-AM efflux method with the human leukemia CEM cells. |
J Med Chem 43: 2547-56 (2000)
BindingDB Entry DOI: 10.7270/Q2PG1SFC |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50026921
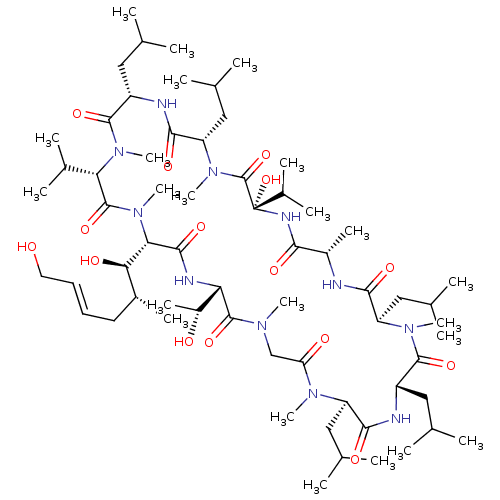
(CHEMBL2372498)Show SMILES [H][C@]1(NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\CO)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@](O)(NC(=O)[C@H](C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C)[C@@H](C)O |r| Show InChI InChI=1S/C64H115N11O15/c1-34(2)28-44-59(85)72(20)47(31-37(7)8)55(81)65-42(16)54(80)69-64(90,40(13)14)63(89)73(21)48(32-38(9)10)57(83)67-45(29-35(3)4)60(86)74(22)51(39(11)12)62(88)75(23)52(53(79)41(15)26-24-25-27-76)58(84)68-50(43(17)77)61(87)70(18)33-49(78)71(19)46(30-36(5)6)56(82)66-44/h24-25,34-48,50-53,76-77,79,90H,26-33H2,1-23H3,(H,65,81)(H,66,82)(H,67,83)(H,68,84)(H,69,80)/b25-24+/t41-,42+,43-,44+,45+,46-,47-,48+,50+,51+,52+,53-,64+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against human formylpeptide receptor (FPR) of human leukemia HL-60 cells |
J Med Chem 45: 4613-28 (2002)
BindingDB Entry DOI: 10.7270/Q2571CQX |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50119438

(3-sec-Butyl-30-ethyl-33-(1-hydroxy-2-methyl-hex-4-...)Show SMILES CCC(C)C1N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)C(C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C(=O)C(CC)NC(=O)C([C@H](O)[C@H](C)C\C=C\C)N(C)C1=O)C(C)C Show InChI InChI=1S/C63H113N11O12/c1-25-28-29-41(15)53(76)52-57(80)66-44(27-3)59(82)68(18)34-49(75)69(19)45(30-35(4)5)56(79)67-50(39(12)13)62(85)70(20)46(31-36(6)7)55(78)64-42(16)54(77)65-43(17)58(81)71(21)47(32-37(8)9)60(83)72(22)48(33-38(10)11)61(84)73(23)51(40(14)26-2)63(86)74(52)24/h25,28,35-48,50-53,76H,26-27,29-34H2,1-24H3,(H,64,78)(H,65,77)(H,66,80)(H,67,79)/b28-25+/t40?,41-,42?,43-,44?,45-,46-,47+,48+,50-,51?,52?,53-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human MDR1 P-Glycoprotein ABC Transporter using leukemia CEM cells |
J Med Chem 45: 4598-612 (2002)
BindingDB Entry DOI: 10.7270/Q29024HJ |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50119402
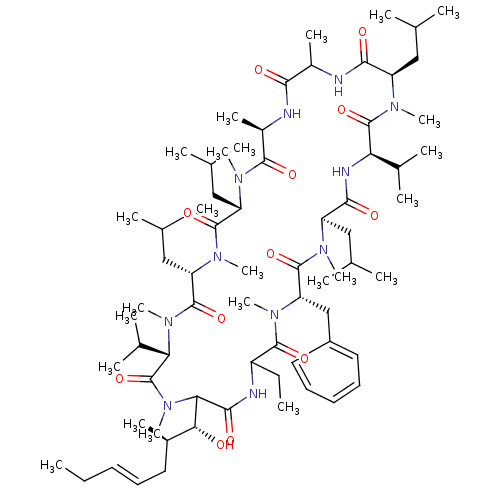
(27-Benzyl-30-ethyl-33-(1-hydroxy-2-methyl-hept-4-e...)Show SMILES CC\C=C\C[C@@H](C)[C@@H](O)C1N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)C(C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](Cc2ccccc2)N(C)C(=O)C(CC)NC1=O)C(C)C Show InChI InChI=1S/C70H119N11O12/c1-25-27-29-32-46(15)59(82)58-63(86)73-50(26-2)65(88)78(21)55(39-49-33-30-28-31-34-49)67(90)75(18)52(36-41(5)6)62(85)74-56(44(11)12)69(92)76(19)51(35-40(3)4)61(84)71-47(16)60(83)72-48(17)64(87)77(20)53(37-42(7)8)66(89)79(22)54(38-43(9)10)68(91)80(23)57(45(13)14)70(93)81(58)24/h27-31,33-34,40-48,50-59,82H,25-26,32,35-39H2,1-24H3,(H,71,84)(H,72,83)(H,73,86)(H,74,85)/b29-27+/t46-,47?,48-,50?,51-,52-,53+,54+,55+,56-,57+,58?,59-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human MDR1 P-Glycoprotein ABC Transporter using leukemia CEM cells |
J Med Chem 45: 4598-612 (2002)
BindingDB Entry DOI: 10.7270/Q29024HJ |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50369548
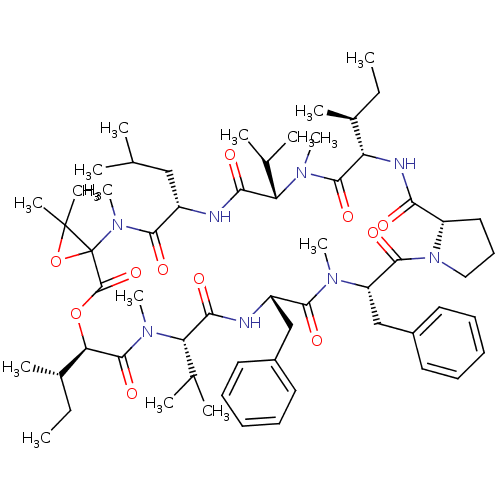
(CHEMBL1790682)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](OC(=O)C2(OC2(C)C)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C1=O)[C@@H](C)CC Show InChI InChI=1S/C60H90N8O11/c1-17-38(9)46-56(75)65(14)47(36(5)6)51(70)61-42(32-35(3)4)54(73)67(16)60(59(11,12)79-60)58(77)78-49(39(10)18-2)57(76)66(15)48(37(7)8)52(71)62-43(33-40-26-21-19-22-27-40)53(72)64(13)45(34-41-28-23-20-24-29-41)55(74)68-31-25-30-44(68)50(69)63-46/h19-24,26-29,35-39,42-49H,17-18,25,30-34H2,1-16H3,(H,61,70)(H,62,71)(H,63,69)/t38-,39-,42-,43-,44-,45-,46-,47-,48-,49+,60?/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Human MDR1 Pgp inhibitory activity by using standard calcein-AM efflux method with the human leukemia CEM cells. |
J Med Chem 43: 2547-56 (2000)
BindingDB Entry DOI: 10.7270/Q2PG1SFC |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50026920
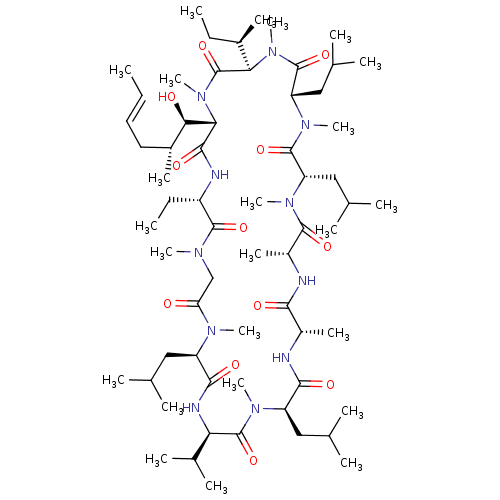
(CHEMBL2369724)Show SMILES [H][C@@]1([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@@]([H])([C@H](C)CC)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C(=O)[C@H](CC)NC1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O12/c1-25-28-29-41(15)53(76)52-57(80)66-44(27-3)59(82)68(18)34-49(75)69(19)45(30-35(4)5)56(79)67-50(39(12)13)62(85)70(20)46(31-36(6)7)55(78)64-42(16)54(77)65-43(17)58(81)71(21)47(32-37(8)9)60(83)72(22)48(33-38(10)11)61(84)73(23)51(40(14)26-2)63(86)74(52)24/h25,28,35-48,50-53,76H,26-27,29-34H2,1-24H3,(H,64,78)(H,65,77)(H,66,80)(H,67,79)/b28-25+/t40-,41-,42+,43-,44+,45-,46-,47+,48+,50-,51-,52+,53-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against human formylpeptide receptor (FPR) of human leukemia HL-60 cells |
J Med Chem 45: 4613-28 (2002)
BindingDB Entry DOI: 10.7270/Q2571CQX |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50119424
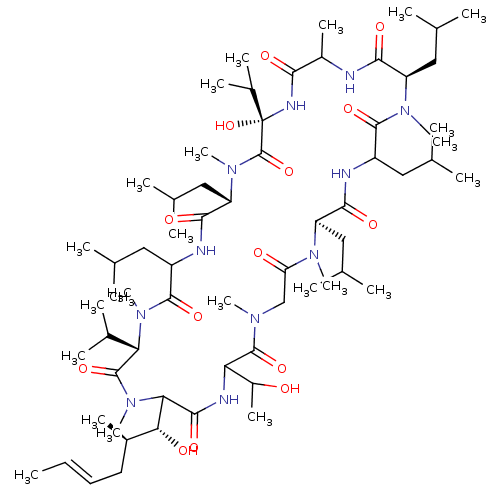
(3-Hydroxy-21-(1-hydroxy-ethyl)-24-(1-hydroxy-2-met...)Show SMILES C\C=C\C[C@@H](C)[C@@H](O)C1N(C)C(=O)[C@H](C(C)C)N(C)C(=O)C(CC(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@](O)(NC(=O)C(C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)C(CC(C)C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C(=O)C(NC1=O)C(C)O)C(C)C Show InChI InChI=1S/C64H115N11O14/c1-25-26-27-41(16)53(78)52-58(83)68-50(43(18)76)61(86)70(19)33-49(77)71(20)46(30-36(6)7)56(81)66-44(28-34(2)3)59(84)72(21)47(31-37(8)9)55(80)65-42(17)54(79)69-64(89,40(14)15)63(88)73(22)48(32-38(10)11)57(82)67-45(29-35(4)5)60(85)74(23)51(39(12)13)62(87)75(52)24/h25-26,34-48,50-53,76,78,89H,27-33H2,1-24H3,(H,65,80)(H,66,81)(H,67,82)(H,68,83)(H,69,79)/b26-25+/t41-,42?,43?,44?,45?,46-,47-,48+,50?,51+,52?,53-,64+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human MDR1 P-Glycoprotein ABC Transporter using leukemia CEM cells |
J Med Chem 45: 4598-612 (2002)
BindingDB Entry DOI: 10.7270/Q29024HJ |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50119450
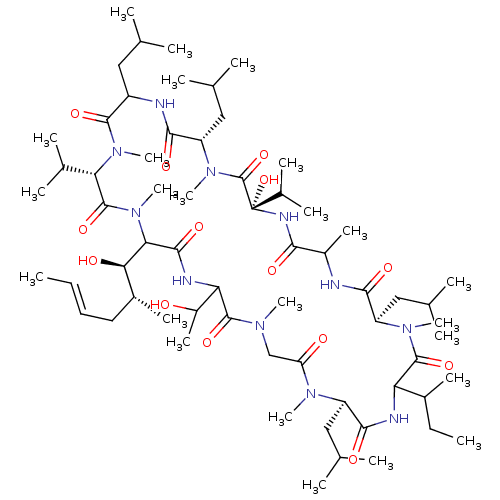
(12-sec-Butyl-3-hydroxy-21-(1-hydroxy-ethyl)-24-(1-...)Show SMILES CCC(C)C1NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C(=O)C(NC(=O)C([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)C(CC(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@](O)(NC(=O)C(C)NC(=O)[C@@H](CC(C)C)N(C)C1=O)C(C)C)C(C)O Show InChI InChI=1S/C64H115N11O14/c1-25-27-28-41(16)53(78)52-58(83)68-50(43(18)76)60(85)70(19)33-48(77)71(20)45(30-35(5)6)57(82)67-49(40(15)26-2)61(86)72(21)46(31-36(7)8)55(80)65-42(17)54(79)69-64(89,39(13)14)63(88)73(22)47(32-37(9)10)56(81)66-44(29-34(3)4)59(84)74(23)51(38(11)12)62(87)75(52)24/h25,27,34-47,49-53,76,78,89H,26,28-33H2,1-24H3,(H,65,80)(H,66,81)(H,67,82)(H,68,83)(H,69,79)/b27-25+/t40?,41-,42?,43?,44?,45-,46-,47+,49?,50?,51+,52?,53-,64+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human MDR1 P-Glycoprotein ABC Transporter using leukemia CEM cells |
J Med Chem 45: 4598-612 (2002)
BindingDB Entry DOI: 10.7270/Q29024HJ |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50369562
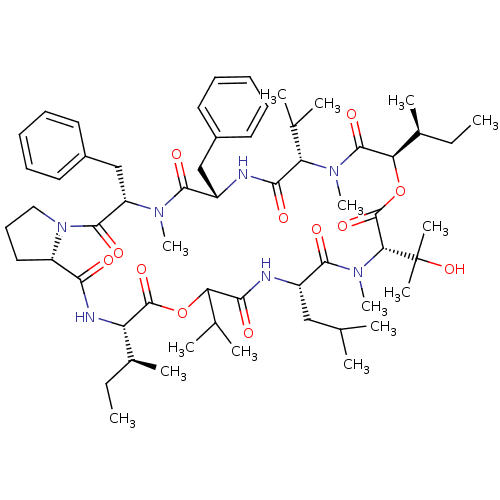
(CHEMBL1790698)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](OC(=O)[C@@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)C(OC1=O)C(C)C)C(C)(C)O)[C@@H](C)CC Show InChI InChI=1S/C59H89N7O12/c1-16-37(9)45-57(74)77-47(36(7)8)52(69)61-41(31-34(3)4)54(71)65(15)49(59(11,12)76)58(75)78-48(38(10)17-2)56(73)64(14)46(35(5)6)51(68)60-42(32-39-25-20-18-21-26-39)53(70)63(13)44(33-40-27-22-19-23-28-40)55(72)66-30-24-29-43(66)50(67)62-45/h18-23,25-28,34-38,41-49,76H,16-17,24,29-33H2,1-15H3,(H,60,68)(H,61,69)(H,62,67)/t37-,38-,41-,42-,43-,44-,45-,46-,47?,48+,49+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Human MDR1 Pgp inhibitory activity by using standard calcein-AM efflux method with the human leukemia CEM cells. |
J Med Chem 43: 2547-56 (2000)
BindingDB Entry DOI: 10.7270/Q2PG1SFC |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50026927

(CHEMBL2372478)Show SMILES [H][C@@]1([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C(=O)[C@H](CC)NC1=O)C(C)C |r| Show InChI InChI=1S/C60H107N11O12/c1-23-25-26-38(15)50(73)49-56(79)63-41(24-2)58(81)67(18)31-46(72)68(19)44(29-34(7)8)54(77)66-47(36(11)12)55(78)64-42(27-32(3)4)52(75)61-39(16)51(74)62-40(17)57(80)69(20)45(30-35(9)10)53(76)65-43(28-33(5)6)59(82)70(21)48(37(13)14)60(83)71(49)22/h23,25,32-45,47-50,73H,24,26-31H2,1-22H3,(H,61,75)(H,62,74)(H,63,79)(H,64,78)(H,65,76)(H,66,77)/b25-23+/t38-,39+,40-,41+,42+,43+,44-,45+,47-,48+,49+,50-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against human formylpeptide receptor (FPR) of human leukemia HL-60 cells |
J Med Chem 45: 4613-28 (2002)
BindingDB Entry DOI: 10.7270/Q2571CQX |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50119413

(33-(1-Hydroxy-2-methyl-hept-4-enyl)-6,9,18,24-tetr...)Show SMILES CCCC1NC(=O)C([C@H](O)[C@H](C)C\C=C\CC)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)C(C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C Show InChI InChI=1S/C64H115N11O12/c1-25-27-28-30-42(15)54(77)53-58(81)67-45(29-26-2)60(83)69(18)35-50(76)70(19)46(31-36(3)4)57(80)68-51(40(11)12)63(86)71(20)47(32-37(5)6)56(79)65-43(16)55(78)66-44(17)59(82)72(21)48(33-38(7)8)61(84)73(22)49(34-39(9)10)62(85)74(23)52(41(13)14)64(87)75(53)24/h27-28,36-49,51-54,77H,25-26,29-35H2,1-24H3,(H,65,79)(H,66,78)(H,67,81)(H,68,80)/b28-27+/t42-,43?,44-,45?,46-,47-,48+,49+,51-,52+,53?,54-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human MDR1 P-Glycoprotein ABC Transporter using leukemia CEM cells |
J Med Chem 45: 4598-612 (2002)
BindingDB Entry DOI: 10.7270/Q29024HJ |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50119443

(33-(1-Hydroxy-2-methyl-hex-4-enyl)-6,9,18,21,24-pe...)Show SMILES CCCC1NC(=O)C([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)C(C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)C(CC(C)C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C1=O Show InChI InChI=1S/C64H115N11O12/c1-25-27-29-42(15)54(77)53-58(81)67-45(28-26-2)60(83)69(18)35-51(76)70(19)47(31-37(5)6)57(80)68-46(30-36(3)4)61(84)71(20)48(32-38(7)8)56(79)65-43(16)55(78)66-44(17)59(82)72(21)49(33-39(9)10)62(85)73(22)50(34-40(11)12)63(86)74(23)52(41(13)14)64(87)75(53)24/h25,27,36-50,52-54,77H,26,28-35H2,1-24H3,(H,65,79)(H,66,78)(H,67,81)(H,68,80)/b27-25+/t42-,43?,44-,45?,46?,47-,48-,49+,50+,52+,53?,54-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human MDR1 P-Glycoprotein ABC Transporter using leukemia CEM cells |
J Med Chem 45: 4598-612 (2002)
BindingDB Entry DOI: 10.7270/Q29024HJ |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50119416
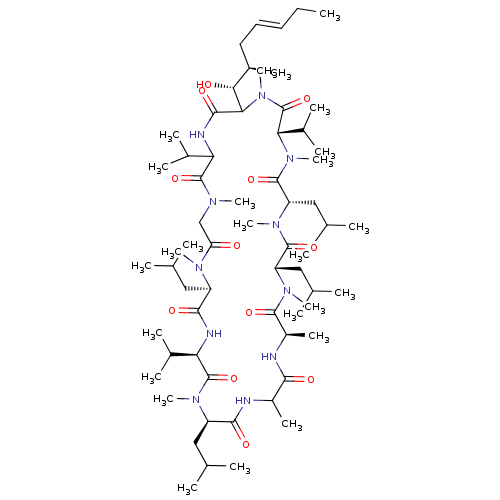
(33-(1-Hydroxy-2-methyl-hept-4-enyl)-6,9,18,24-tetr...)Show SMILES CC\C=C\C[C@@H](C)[C@@H](O)C1N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)C(C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C(=O)C(NC1=O)C(C)C)C(C)C Show InChI InChI=1S/C64H115N11O12/c1-26-27-28-29-42(16)54(77)53-58(81)68-50(39(10)11)62(85)69(19)34-49(76)70(20)45(30-35(2)3)57(80)67-51(40(12)13)63(86)71(21)46(31-36(4)5)56(79)65-43(17)55(78)66-44(18)59(82)72(22)47(32-37(6)7)60(83)73(23)48(33-38(8)9)61(84)74(24)52(41(14)15)64(87)75(53)25/h27-28,35-48,50-54,77H,26,29-34H2,1-25H3,(H,65,79)(H,66,78)(H,67,80)(H,68,81)/b28-27+/t42-,43?,44-,45-,46-,47+,48+,50?,51-,52+,53?,54-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human MDR1 P-Glycoprotein ABC Transporter using leukemia CEM cells |
J Med Chem 45: 4598-612 (2002)
BindingDB Entry DOI: 10.7270/Q29024HJ |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50119439
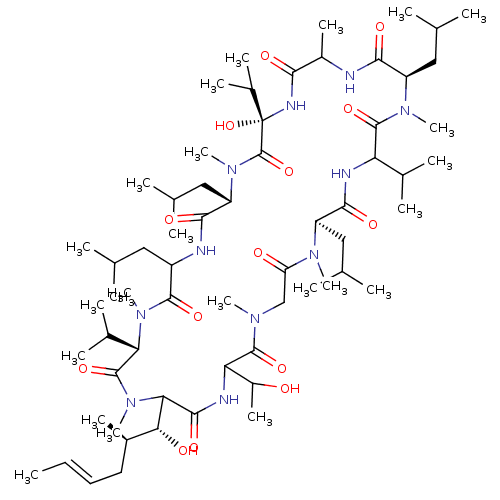
((cyclo-[MeBmt-Thr-MeGly-MeLeu-Val-MeLeu-Ala-D-Hiv-...)Show SMILES C\C=C\C[C@@H](C)[C@@H](O)C1N(C)C(=O)[C@H](C(C)C)N(C)C(=O)C(CC(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@](O)(NC(=O)C(C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)C(NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C(=O)C(NC1=O)C(C)O)C(C)C)C(C)C Show InChI InChI=1S/C63H113N11O14/c1-25-26-27-40(16)52(77)51-57(82)67-49(42(18)75)59(84)69(19)32-47(76)70(20)44(29-34(4)5)56(81)66-48(37(10)11)60(85)71(21)45(30-35(6)7)54(79)64-41(17)53(78)68-63(88,39(14)15)62(87)72(22)46(31-36(8)9)55(80)65-43(28-33(2)3)58(83)73(23)50(38(12)13)61(86)74(51)24/h25-26,33-46,48-52,75,77,88H,27-32H2,1-24H3,(H,64,79)(H,65,80)(H,66,81)(H,67,82)(H,68,78)/b26-25+/t40-,41?,42?,43?,44-,45-,46+,48?,49?,50+,51?,52-,63+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human MDR1 P-Glycoprotein ABC Transporter using leukemia CEM cells |
J Med Chem 45: 4598-612 (2002)
BindingDB Entry DOI: 10.7270/Q29024HJ |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50119415
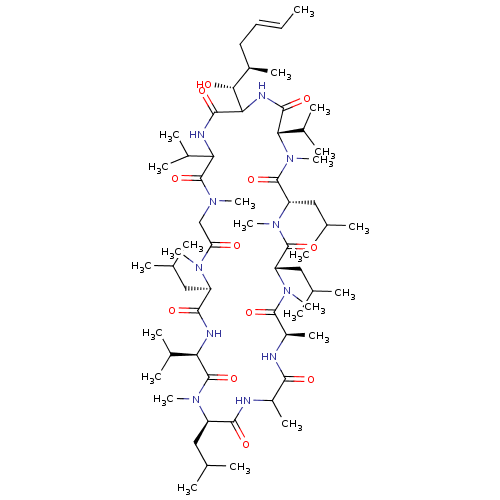
(30-(1-Hydroxy-2-methyl-hex-4-enyl)-3,6,15,21-tetra...)Show SMILES C\C=C\C[C@@H](C)[C@@H](O)C1NC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)C(C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C(=O)C(NC1=O)C(C)C)C(C)C Show InChI InChI=1S/C62H111N11O12/c1-25-26-27-40(16)52(75)50-56(79)66-48(37(10)11)61(84)68(19)32-47(74)69(20)43(28-33(2)3)55(78)65-49(38(12)13)62(85)70(21)44(29-34(4)5)54(77)63-41(17)53(76)64-42(18)58(81)71(22)45(30-35(6)7)59(82)72(23)46(31-36(8)9)60(83)73(24)51(39(14)15)57(80)67-50/h25-26,33-46,48-52,75H,27-32H2,1-24H3,(H,63,77)(H,64,76)(H,65,78)(H,66,79)(H,67,80)/b26-25+/t40-,41?,42-,43-,44-,45+,46+,48?,49-,50?,51+,52-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human MDR1 P-Glycoprotein ABC Transporter using leukemia CEM cells |
J Med Chem 45: 4598-612 (2002)
BindingDB Entry DOI: 10.7270/Q29024HJ |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50369550
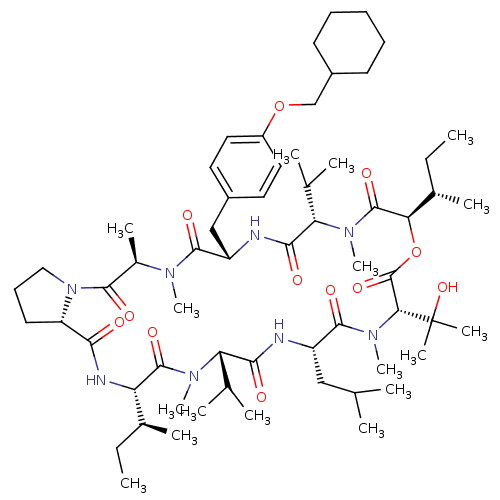
(CHEMBL1790696)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H](C)N(C)C(=O)[C@H](Cc2ccc(OCC3CCCCC3)cc2)NC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](OC(=O)[C@@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C1=O)C(C)(C)O)[C@@H](C)CC Show InChI InChI=1S/C61H100N8O12/c1-18-38(9)47-58(76)66(15)48(36(5)6)53(71)62-44(32-35(3)4)57(75)68(17)51(61(12,13)79)60(78)81-50(39(10)19-2)59(77)67(16)49(37(7)8)54(72)63-45(33-41-27-29-43(30-28-41)80-34-42-24-21-20-22-25-42)56(74)65(14)40(11)55(73)69-31-23-26-46(69)52(70)64-47/h27-30,35-40,42,44-51,79H,18-26,31-34H2,1-17H3,(H,62,71)(H,63,72)(H,64,70)/t38-,39-,40+,44-,45-,46-,47-,48-,49-,50+,51+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Human MDR1 Pgp inhibitory activity by using standard calcein-AM efflux method with the human leukemia CEM cells. |
J Med Chem 43: 2547-56 (2000)
BindingDB Entry DOI: 10.7270/Q2PG1SFC |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50369554
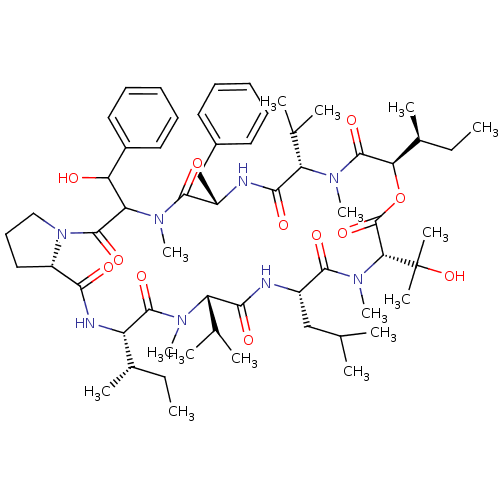
(CHEMBL1790691)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)C(C(O)c2ccccc2)N(C)C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](OC(=O)[C@@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C1=O)C(C)(C)O)[C@@H](C)CC Show InChI InChI=1S/C60H92N8O12/c1-17-37(9)44-56(75)64(13)45(35(5)6)52(71)61-41(32-34(3)4)55(74)67(16)50(60(11,12)79)59(78)80-49(38(10)18-2)58(77)65(14)46(36(7)8)53(72)62-42(33-39-26-21-19-22-27-39)54(73)66(15)47(48(69)40-28-23-20-24-29-40)57(76)68-31-25-30-43(68)51(70)63-44/h19-24,26-29,34-38,41-50,69,79H,17-18,25,30-33H2,1-16H3,(H,61,71)(H,62,72)(H,63,70)/t37-,38-,41-,42-,43-,44-,45-,46-,47?,48?,49+,50+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Human MDR1 Pgp inhibitory activity by using standard calcein-AM efflux method with the human leukemia CEM cells. |
J Med Chem 43: 2547-56 (2000)
BindingDB Entry DOI: 10.7270/Q2PG1SFC |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50026943

(CHEMBL2372474)Show SMILES [H][C@@]1([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C(=O)[C@H](C)NC1=O)C(C)C |r| Show InChI InChI=1S/C60H107N11O12/c1-24-25-26-38(14)50(73)49-55(78)63-40(16)56(79)66(18)31-46(72)67(19)43(28-33(4)5)54(77)65-47(36(10)11)59(82)69(21)44(29-34(6)7)52(75)61-39(15)51(74)62-41(17)57(80)68(20)45(30-35(8)9)53(76)64-42(27-32(2)3)58(81)70(22)48(37(12)13)60(83)71(49)23/h24-25,32-45,47-50,73H,26-31H2,1-23H3,(H,61,75)(H,62,74)(H,63,78)(H,64,76)(H,65,77)/b25-24+/t38-,39+,40+,41-,42+,43-,44-,45+,47-,48+,49+,50-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against human formylpeptide receptor (FPR) of human leukemia HL-60 cells |
J Med Chem 45: 4613-28 (2002)
BindingDB Entry DOI: 10.7270/Q2571CQX |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50119423
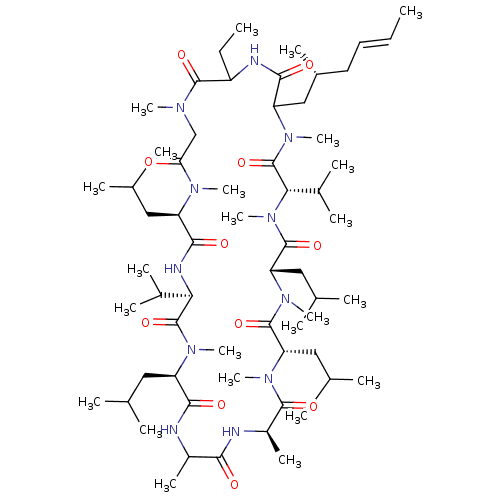
(30-Ethyl-6,9,18,24-tetraisobutyl-3,21-diisopropyl-...)Show SMILES CCC1NC(=O)C(C[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)C(C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C Show InChI InChI=1S/C62H111N11O11/c1-25-27-28-41(15)33-47-55(77)65-44(26-2)58(80)67(18)34-50(74)68(19)45(29-35(3)4)56(78)66-51(39(11)12)61(83)69(20)46(30-36(5)6)54(76)63-42(16)53(75)64-43(17)57(79)71(22)48(31-37(7)8)59(81)72(23)49(32-38(9)10)60(82)73(24)52(40(13)14)62(84)70(47)21/h25,27,35-49,51-52H,26,28-34H2,1-24H3,(H,63,76)(H,64,75)(H,65,77)(H,66,78)/b27-25+/t41-,42?,43-,44?,45-,46-,47?,48+,49+,51-,52+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human MDR1 P-Glycoprotein ABC Transporter using leukemia CEM cells |
J Med Chem 45: 4598-612 (2002)
BindingDB Entry DOI: 10.7270/Q29024HJ |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50369569
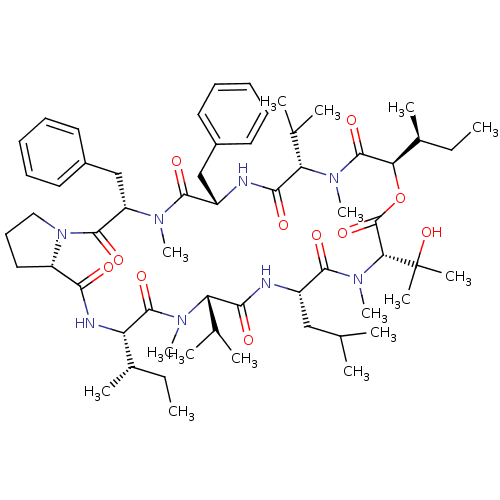
(CHEMBL1790689)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](OC(=O)[C@@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C1=O)C(C)(C)O)[C@@H](C)CC Show InChI InChI=1S/C60H92N8O11/c1-17-38(9)46-57(75)65(14)47(36(5)6)52(70)61-42(32-35(3)4)55(73)67(16)50(60(11,12)78)59(77)79-49(39(10)18-2)58(76)66(15)48(37(7)8)53(71)62-43(33-40-26-21-19-22-27-40)54(72)64(13)45(34-41-28-23-20-24-29-41)56(74)68-31-25-30-44(68)51(69)63-46/h19-24,26-29,35-39,42-50,78H,17-18,25,30-34H2,1-16H3,(H,61,70)(H,62,71)(H,63,69)/t38-,39-,42-,43-,44-,45-,46-,47-,48-,49+,50+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Human MDR1 Pgp inhibitory activity by using standard calcein-AM efflux method with the human leukemia CEM cells. |
J Med Chem 43: 2547-56 (2000)
BindingDB Entry DOI: 10.7270/Q2PG1SFC |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50408926
(Aureobasidin A | CHEMBL1793802)Show SMILES CC[C@@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](OC(=O)[C@@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C1=O)C(C)(C)O)[C@H](C)CC |r| Show InChI InChI=1S/C60H92N8O11/c1-17-38(9)46-57(75)65(14)47(36(5)6)52(70)61-42(32-35(3)4)55(73)67(16)50(60(11,12)78)59(77)79-49(39(10)18-2)58(76)66(15)48(37(7)8)53(71)62-43(33-40-26-21-19-22-27-40)54(72)64(13)45(34-41-28-23-20-24-29-41)56(74)68-31-25-30-44(68)51(69)63-46/h19-24,26-29,35-39,42-50,78H,17-18,25,30-34H2,1-16H3,(H,61,70)(H,62,71)(H,63,69)/t38-,39-,42+,43+,44+,45+,46+,47+,48+,49-,50-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of Calcein-AM efflux (Calcein-AM: 0.25 uM) in CEM/VLB100 cells |
J Med Chem 43: 2547-56 (2000)
BindingDB Entry DOI: 10.7270/Q2PG1SFC |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50119396
(27-Ethyl-30-(1-hydroxy-2-methyl-hex-4-enyl)-3,6,15...)Show SMILES CCC1NC(=O)C(NC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)C(C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C)[C@H](O)[C@H](C)C\C=C\C Show InChI InChI=1S/C61H109N11O12/c1-24-26-27-39(15)51(74)49-55(78)64-42(25-2)58(81)67(18)32-47(73)68(19)43(28-33(3)4)54(77)65-48(37(11)12)61(84)69(20)44(29-34(5)6)53(76)62-40(16)52(75)63-41(17)57(80)70(21)45(30-35(7)8)59(82)71(22)46(31-36(9)10)60(83)72(23)50(38(13)14)56(79)66-49/h24,26,33-46,48-51,74H,25,27-32H2,1-23H3,(H,62,76)(H,63,75)(H,64,78)(H,65,77)(H,66,79)/b26-24+/t39-,40?,41-,42?,43-,44-,45+,46+,48-,49?,50+,51-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human MDR1 P-Glycoprotein ABC Transporter using leukemia CEM cells |
J Med Chem 45: 4598-612 (2002)
BindingDB Entry DOI: 10.7270/Q29024HJ |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50026907

(CHEMBL2372461)Show SMILES [H][C@]1(NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@@]([H])(NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C1=O)[C@@H](C)CC)[C@@H](C)O |r| Show InChI InChI=1S/C63H113N11O13/c1-25-27-28-40(14)53(77)52-57(81)67-50(43(17)75)61(85)68(18)33-48(76)69(19)44(29-34(3)4)56(80)66-49(39(13)26-2)62(86)70(20)45(30-35(5)6)55(79)64-41(15)54(78)65-42(16)58(82)71(21)46(31-36(7)8)59(83)72(22)47(32-37(9)10)60(84)73(23)51(38(11)12)63(87)74(52)24/h25,27,34-47,49-53,75,77H,26,28-33H2,1-24H3,(H,64,79)(H,65,78)(H,66,80)(H,67,81)/b27-25+/t39-,40+,41-,42+,43+,44+,45+,46-,47-,49-,50-,51-,52-,53+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against human formylpeptide receptor (FPR) of human leukemia HL-60 cells |
J Med Chem 45: 4613-28 (2002)
BindingDB Entry DOI: 10.7270/Q2571CQX |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50119408
(24-Benzyl-30-ethyl-33-(1-hydroxy-2-methyl-hept-4-e...)Show SMILES CC\C=C\C[C@@H](C)[C@@H](O)C1N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)C(C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](NC(=O)C(Cc2ccccc2)N(C)C(=O)CN(C)C(=O)C(CC)NC1=O)C(C)C Show InChI InChI=1S/C66H111N11O12/c1-23-25-27-30-43(13)56(79)55-60(83)69-47(24-2)62(85)71(16)37-52(78)72(17)49(36-46-31-28-26-29-32-46)59(82)70-53(41(9)10)65(88)73(18)48(33-38(3)4)58(81)67-44(14)57(80)68-45(15)61(84)74(19)50(34-39(5)6)63(86)75(20)51(35-40(7)8)64(87)76(21)54(42(11)12)66(89)77(55)22/h25-29,31-32,38-45,47-51,53-56,79H,23-24,30,33-37H2,1-22H3,(H,67,81)(H,68,80)(H,69,83)(H,70,82)/b27-25+/t43-,44?,45-,47?,48-,49?,50+,51+,53-,54+,55?,56-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human MDR1 P-Glycoprotein ABC Transporter using leukemia CEM cells |
J Med Chem 45: 4598-612 (2002)
BindingDB Entry DOI: 10.7270/Q29024HJ |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50026904
(CHEMBL2372504)Show SMILES [H][C@]1(NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@](O)(NC(=O)[C@H](C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C)C(C)C)[C@@H](C)O |r| Show InChI InChI=1S/C63H113N11O14/c1-25-26-27-40(16)52(77)51-57(82)67-49(42(18)75)59(84)69(19)32-47(76)70(20)44(29-34(4)5)56(81)66-48(37(10)11)60(85)71(21)45(30-35(6)7)54(79)64-41(17)53(78)68-63(88,39(14)15)62(87)72(22)46(31-36(8)9)55(80)65-43(28-33(2)3)58(83)73(23)50(38(12)13)61(86)74(51)24/h25-26,33-46,48-52,75,77,88H,27-32H2,1-24H3,(H,64,79)(H,65,80)(H,66,81)(H,67,82)(H,68,78)/b26-25+/t40-,41+,42-,43+,44-,45-,46+,48+,49+,50+,51+,52-,63+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against human formylpeptide receptor (FPR) of human leukemia HL-60 cells |
J Med Chem 45: 4613-28 (2002)
BindingDB Entry DOI: 10.7270/Q2571CQX |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50026923
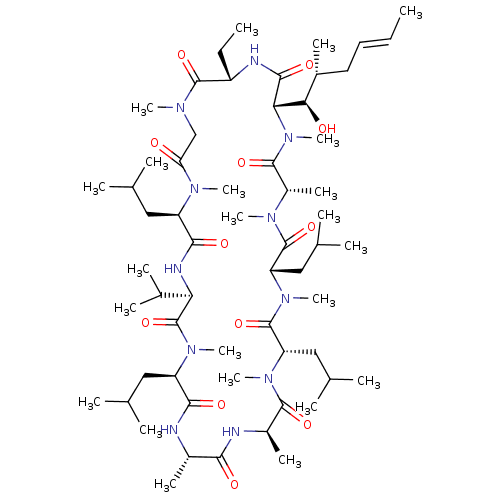
(CHEMBL2372477)Show SMILES [H][C@@]1([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C(=O)[C@H](CC)NC1=O)C(C)C |r| Show InChI InChI=1S/C60H107N11O12/c1-24-26-27-38(13)50(73)49-54(77)63-42(25-2)57(80)65(17)32-47(72)67(19)43(28-33(3)4)53(76)64-48(37(11)12)60(83)68(20)44(29-34(5)6)52(75)61-39(14)51(74)62-40(15)55(78)69(21)46(31-36(9)10)59(82)70(22)45(30-35(7)8)58(81)66(18)41(16)56(79)71(49)23/h24,26,33-46,48-50,73H,25,27-32H2,1-23H3,(H,61,75)(H,62,74)(H,63,77)(H,64,76)/b26-24+/t38-,39+,40-,41+,42+,43-,44-,45+,46+,48-,49+,50-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against human formylpeptide receptor (FPR) of human leukemia HL-60 cells |
J Med Chem 45: 4613-28 (2002)
BindingDB Entry DOI: 10.7270/Q2571CQX |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50369545
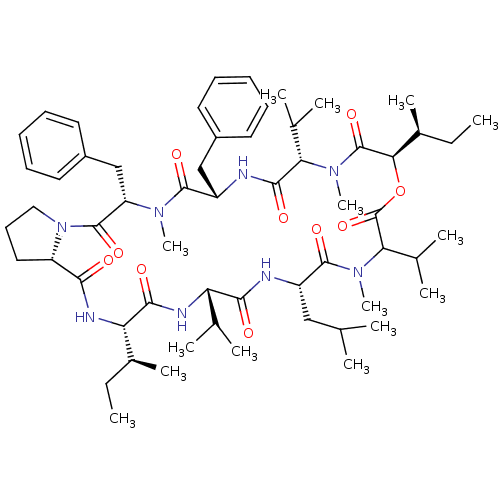
(CHEMBL1790686)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](OC(=O)C(C(C)C)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC1=O)C(C)C)[C@@H](C)CC Show InChI InChI=1S/C59H90N8O10/c1-16-38(11)47-53(70)62-46(35(5)6)52(69)60-42(31-34(3)4)56(73)66(15)49(37(9)10)59(76)77-50(39(12)17-2)58(75)65(14)48(36(7)8)54(71)61-43(32-40-25-20-18-21-26-40)55(72)64(13)45(33-41-27-22-19-23-28-41)57(74)67-30-24-29-44(67)51(68)63-47/h18-23,25-28,34-39,42-50H,16-17,24,29-33H2,1-15H3,(H,60,69)(H,61,71)(H,62,70)(H,63,68)/t38-,39-,42-,43-,44-,45-,46-,47-,48-,49?,50+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Human MDR1 Pgp inhibitory activity by using standard calcein-AM efflux method with the human leukemia CEM cells. |
J Med Chem 43: 2547-56 (2000)
BindingDB Entry DOI: 10.7270/Q2PG1SFC |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50369567
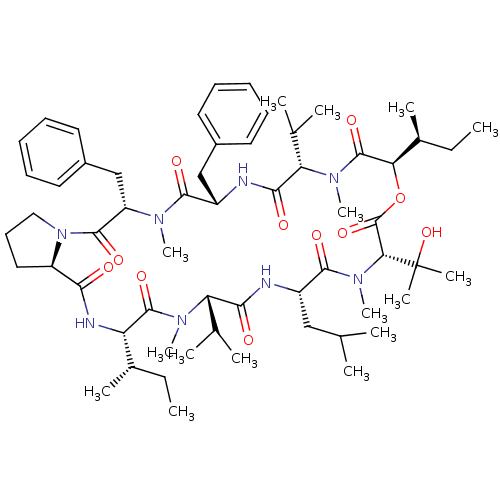
(CHEMBL1790695)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](OC(=O)[C@@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C1=O)C(C)(C)O)[C@@H](C)CC Show InChI InChI=1S/C60H92N8O11/c1-17-38(9)46-57(75)65(14)47(36(5)6)52(70)61-42(32-35(3)4)55(73)67(16)50(60(11,12)78)59(77)79-49(39(10)18-2)58(76)66(15)48(37(7)8)53(71)62-43(33-40-26-21-19-22-27-40)54(72)64(13)45(34-41-28-23-20-24-29-41)56(74)68-31-25-30-44(68)51(69)63-46/h19-24,26-29,35-39,42-50,78H,17-18,25,30-34H2,1-16H3,(H,61,70)(H,62,71)(H,63,69)/t38-,39-,42-,43-,44+,45-,46-,47-,48-,49+,50+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Human MDR1 Pgp inhibitory activity by using standard calcein-AM efflux method with the human leukemia CEM cells. |
J Med Chem 43: 2547-56 (2000)
BindingDB Entry DOI: 10.7270/Q2PG1SFC |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50119419
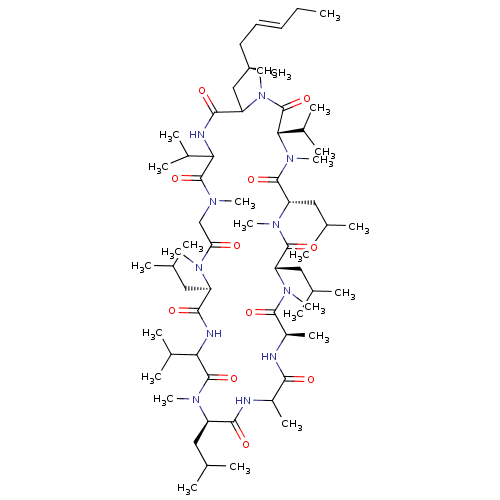
(6,9,18,24-Tetraisobutyl-3,21,30-triisopropyl-1,4,7...)Show SMILES CC\C=C\C[C@@H](C)CC1N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)C(C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)C(NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C(=O)C(NC1=O)C(C)C)C(C)C Show InChI InChI=1S/C64H115N11O11/c1-26-27-28-29-43(16)34-48-58(80)67-52(40(10)11)62(84)69(19)35-51(76)70(20)46(30-36(2)3)57(79)68-53(41(12)13)63(85)71(21)47(31-37(4)5)56(78)65-44(17)55(77)66-45(18)59(81)73(23)49(32-38(6)7)60(82)74(24)50(33-39(8)9)61(83)75(25)54(42(14)15)64(86)72(48)22/h27-28,36-50,52-54H,26,29-35H2,1-25H3,(H,65,78)(H,66,77)(H,67,80)(H,68,79)/b28-27+/t43-,44?,45-,46-,47-,48?,49+,50+,52?,53?,54+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human MDR1 P-Glycoprotein ABC Transporter using leukemia CEM cells |
J Med Chem 45: 4598-612 (2002)
BindingDB Entry DOI: 10.7270/Q29024HJ |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50369549
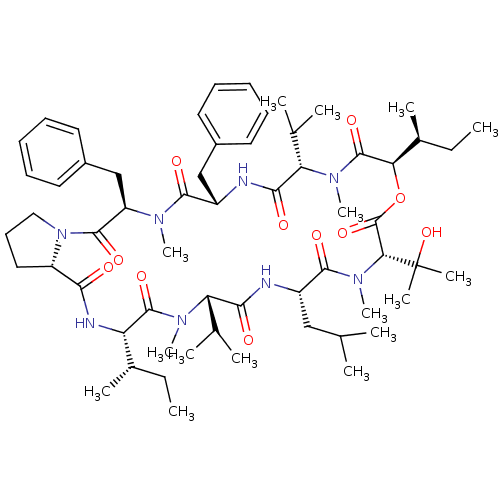
(CHEMBL1790693)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H](Cc2ccccc2)N(C)C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](OC(=O)[C@@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C1=O)C(C)(C)O)[C@@H](C)CC Show InChI InChI=1S/C60H92N8O11/c1-17-38(9)46-57(75)65(14)47(36(5)6)52(70)61-42(32-35(3)4)55(73)67(16)50(60(11,12)78)59(77)79-49(39(10)18-2)58(76)66(15)48(37(7)8)53(71)62-43(33-40-26-21-19-22-27-40)54(72)64(13)45(34-41-28-23-20-24-29-41)56(74)68-31-25-30-44(68)51(69)63-46/h19-24,26-29,35-39,42-50,78H,17-18,25,30-34H2,1-16H3,(H,61,70)(H,62,71)(H,63,69)/t38-,39-,42-,43-,44-,45+,46-,47-,48-,49+,50+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Human MDR1 Pgp inhibitory activity by using standard calcein-AM efflux method with the human leukemia CEM cells. |
J Med Chem 43: 2547-56 (2000)
BindingDB Entry DOI: 10.7270/Q2PG1SFC |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50369553
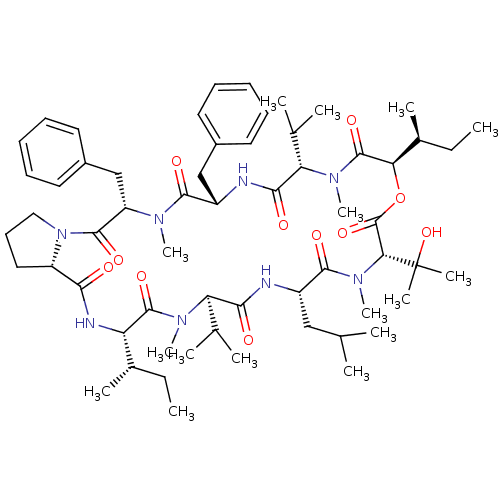
(CHEMBL1790694)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](OC(=O)[C@@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@@H](C(C)C)N(C)C1=O)C(C)(C)O)[C@@H](C)CC Show InChI InChI=1S/C60H92N8O11/c1-17-38(9)46-57(75)65(14)47(36(5)6)52(70)61-42(32-35(3)4)55(73)67(16)50(60(11,12)78)59(77)79-49(39(10)18-2)58(76)66(15)48(37(7)8)53(71)62-43(33-40-26-21-19-22-27-40)54(72)64(13)45(34-41-28-23-20-24-29-41)56(74)68-31-25-30-44(68)51(69)63-46/h19-24,26-29,35-39,42-50,78H,17-18,25,30-34H2,1-16H3,(H,61,70)(H,62,71)(H,63,69)/t38-,39-,42-,43-,44-,45-,46-,47+,48-,49+,50+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Human MDR1 Pgp inhibitory activity by using standard calcein-AM efflux method with the human leukemia CEM cells. |
J Med Chem 43: 2547-56 (2000)
BindingDB Entry DOI: 10.7270/Q2PG1SFC |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50369568
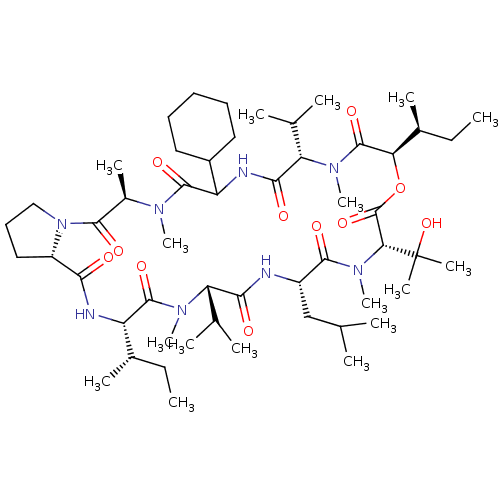
(CHEMBL1790688)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H](C)N(C)C(=O)C(NC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](OC(=O)[C@@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C1=O)C(C)(C)O)[C@@H](C)CC)C1CCCCC1 Show InChI InChI=1S/C53H92N8O11/c1-18-32(9)38-49(67)58(15)40(30(5)6)45(63)54-36(28-29(3)4)48(66)60(17)43(53(12,13)71)52(70)72-42(33(10)19-2)51(69)59(16)41(31(7)8)46(64)56-39(35-24-21-20-22-25-35)50(68)57(14)34(11)47(65)61-27-23-26-37(61)44(62)55-38/h29-43,71H,18-28H2,1-17H3,(H,54,63)(H,55,62)(H,56,64)/t32-,33-,34+,36-,37-,38-,39?,40-,41-,42+,43+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Human MDR1 Pgp inhibitory activity by using standard calcein-AM efflux method with the human leukemia CEM cells. |
J Med Chem 43: 2547-56 (2000)
BindingDB Entry DOI: 10.7270/Q2PG1SFC |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50026899

(CHEMBL2372468)Show SMILES [H][C@@]1([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C(=O)[C@@H](NC1=O)C(C)C)C(C)C |r| Show InChI InChI=1S/C62H111N11O12/c1-25-26-27-40(16)52(75)51-57(80)67-48(37(10)11)60(83)68(19)32-47(74)69(20)44(29-34(4)5)56(79)66-49(38(12)13)61(84)71(22)45(30-35(6)7)54(77)63-41(17)53(76)64-42(18)58(81)70(21)46(31-36(8)9)55(78)65-43(28-33(2)3)59(82)72(23)50(39(14)15)62(85)73(51)24/h25-26,33-46,48-52,75H,27-32H2,1-24H3,(H,63,77)(H,64,76)(H,65,78)(H,66,79)(H,67,80)/b26-25+/t40-,41+,42-,43+,44-,45-,46+,48+,49-,50+,51+,52-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against human formylpeptide receptor (FPR) of human leukemia HL-60 cells |
J Med Chem 45: 4613-28 (2002)
BindingDB Entry DOI: 10.7270/Q2571CQX |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50026936
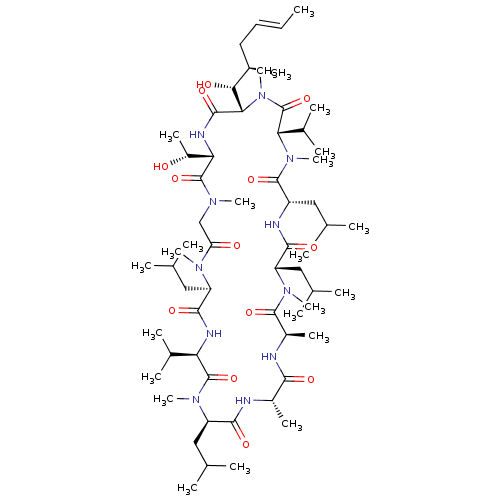
(CHEMBL2372492)Show SMILES [H][C@]1(NC(=O)[C@]([H])([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C)[C@@H](C)O |r| Show InChI InChI=1S/C61H109N11O13/c1-24-25-26-38(14)51(75)50-56(80)66-48(41(17)73)59(83)67(18)31-46(74)68(19)43(28-33(4)5)55(79)65-47(36(10)11)60(84)70(21)44(29-34(6)7)53(77)62-39(15)52(76)63-40(16)57(81)69(20)45(30-35(8)9)54(78)64-42(27-32(2)3)58(82)71(22)49(37(12)13)61(85)72(50)23/h24-25,32-45,47-51,73,75H,26-31H2,1-23H3,(H,62,77)(H,63,76)(H,64,78)(H,65,79)(H,66,80)/b25-24+/t38-,39+,40-,41-,42+,43-,44-,45+,47-,48+,49+,50+,51-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against human formylpeptide receptor (FPR) of human leukemia HL-60 cells |
J Med Chem 45: 4613-28 (2002)
BindingDB Entry DOI: 10.7270/Q2571CQX |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50119444
(33-(1-Hydroxy-2-methyl-hex-4-enyl)-6,9,18,24-tetra...)Show SMILES C\C=C\C[C@@H](C)[C@@H](O)C1N(C)C(=O)[C@H](C(C)C)N(C)C(=O)C(CC(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)C(C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C(=O)C(C)NC1=O)C(C)C Show InChI InChI=1S/C60H107N11O12/c1-24-25-26-38(14)50(73)49-55(78)63-40(16)56(79)66(18)31-46(72)67(19)43(28-33(4)5)54(77)65-47(36(10)11)59(82)69(21)44(29-34(6)7)52(75)61-39(15)51(74)62-41(17)57(80)68(20)45(30-35(8)9)53(76)64-42(27-32(2)3)58(81)70(22)48(37(12)13)60(83)71(49)23/h24-25,32-45,47-50,73H,26-31H2,1-23H3,(H,61,75)(H,62,74)(H,63,78)(H,64,76)(H,65,77)/b25-24+/t38-,39?,40?,41-,42?,43-,44-,45+,47-,48+,49?,50-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Human MDR1 P-Glycoprotein ABC Transporter using leukemia CEM cells |
J Med Chem 45: 4598-612 (2002)
BindingDB Entry DOI: 10.7270/Q29024HJ |
More data for this
Ligand-Target Pair | |
fMet-Leu-Phe receptor
(Homo sapiens (Human)) | BDBM50026905
(CHEMBL2372460)Show SMILES [H][C@@]1([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@H](NC(=O)[C@@H](CC(C)C)N(C)C(=O)CN(C)C(=O)[C@H](CCC)NC1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O12/c1-24-26-28-40(15)52(75)51-57(80)65-43(27-25-2)59(82)68(18)33-48(74)69(19)45(30-35(5)6)56(79)67-49(38(11)12)61(84)71(21)46(31-36(7)8)54(77)63-41(16)53(76)64-42(17)58(81)70(20)47(32-37(9)10)55(78)66-44(29-34(3)4)60(83)72(22)50(39(13)14)62(85)73(51)23/h24,26,34-47,49-52,75H,25,27-33H2,1-23H3,(H,63,77)(H,64,76)(H,65,80)(H,66,78)(H,67,79)/b26-24+/t40-,41+,42-,43+,44+,45-,46-,47+,49-,50+,51+,52-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Strasbourg 1 University
Curated by ChEMBL
| Assay Description
Inhibitory activity against human formylpeptide receptor (FPR) of human leukemia HL-60 cells |
J Med Chem 45: 4613-28 (2002)
BindingDB Entry DOI: 10.7270/Q2571CQX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































