 Found 260 hits with Last Name = 'whitesitt' and Initial = 'c'
Found 260 hits with Last Name = 'whitesitt' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85002
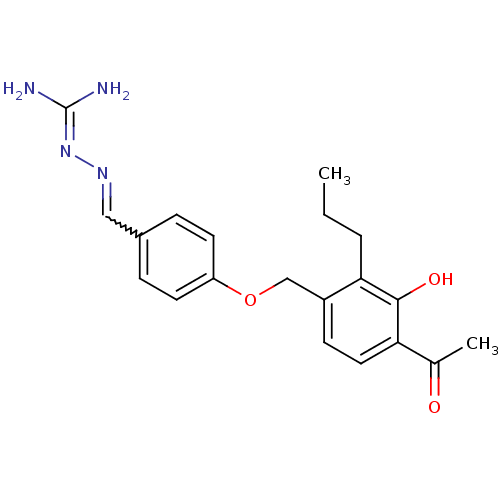
(LY 314228)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)ccc(-[#6](-[#6])=O)c1-[#8] |w:11.10| Show InChI InChI=1S/C20H24N4O3/c1-3-4-18-15(7-10-17(13(2)25)19(18)26)12-27-16-8-5-14(6-9-16)11-23-24-20(21)22/h5-11,26H,3-4,12H2,1-2H3,(H4,21,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85006
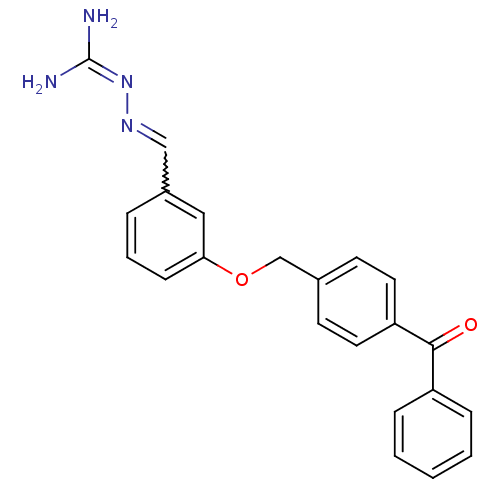
(LY 334362)Show SMILES [#7]\[#6](-[#7])=[#7]\[#7]=[#6]-c1cccc(-[#8]-[#6]-c2ccc(cc2)-[#6](=O)-c2ccccc2)c1 |w:5.5| Show InChI InChI=1S/C22H20N4O2/c23-22(24)26-25-14-17-5-4-8-20(13-17)28-15-16-9-11-19(12-10-16)21(27)18-6-2-1-3-7-18/h1-14H,15H2,(H4,23,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85007
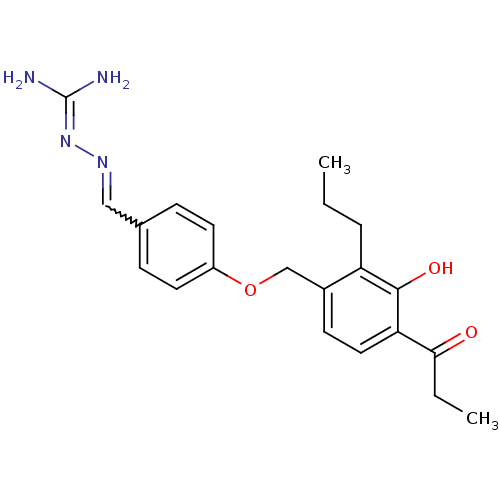
(LY 320954)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)ccc(-[#6](=O)-[#6]-[#6])c1-[#8] |w:11.10| Show InChI InChI=1S/C21H26N4O3/c1-3-5-17-15(8-11-18(20(17)27)19(26)4-2)13-28-16-9-6-14(7-10-16)12-24-25-21(22)23/h6-12,27H,3-5,13H2,1-2H3,(H4,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85000
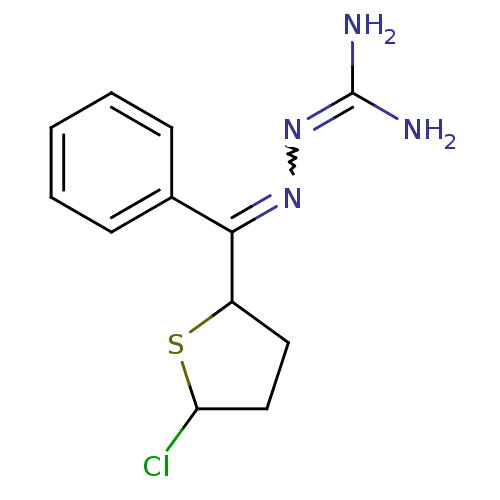
(LY 063518)Show SMILES [#7]\[#6](-[#7])=[#7]\[#7]=[#6](-[#6]-1-[#6]-[#6]-[#6](Cl)-[#16]-1)-c1ccccc1 |w:4.3| Show InChI InChI=1S/C12H15ClN4S/c13-10-7-6-9(18-10)11(16-17-12(14)15)8-4-2-1-3-5-8/h1-5,9-10H,6-7H2,(H4,14,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM21395

(3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85002

(LY 314228)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)ccc(-[#6](-[#6])=O)c1-[#8] |w:11.10| Show InChI InChI=1S/C20H24N4O3/c1-3-4-18-15(7-10-17(13(2)25)19(18)26)12-27-16-8-5-14(6-9-16)11-23-24-20(21)22/h5-11,26H,3-4,12H2,1-2H3,(H4,21,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 168 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85006

(LY 334362)Show SMILES [#7]\[#6](-[#7])=[#7]\[#7]=[#6]-c1cccc(-[#8]-[#6]-c2ccc(cc2)-[#6](=O)-c2ccccc2)c1 |w:5.5| Show InChI InChI=1S/C22H20N4O2/c23-22(24)26-25-14-17-5-4-8-20(13-17)28-15-16-9-11-19(12-10-16)21(27)18-6-2-1-3-7-18/h1-14H,15H2,(H4,23,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 179 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85004
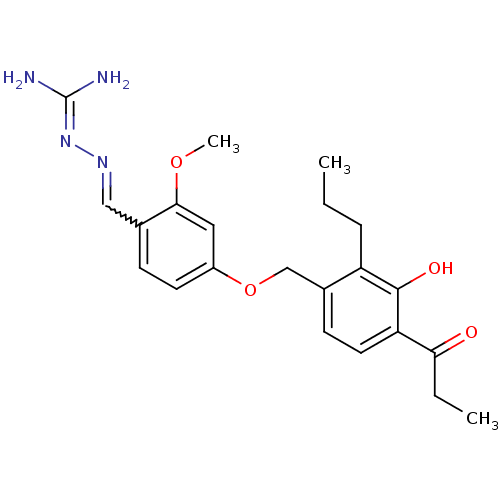
(LY 320950)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])c(-[#8]-[#6])c2)ccc(-[#6](=O)-[#6]-[#6])c1-[#8] |w:11.10| Show InChI InChI=1S/C22H28N4O4/c1-4-6-17-15(8-10-18(21(17)28)19(27)5-2)13-30-16-9-7-14(20(11-16)29-3)12-25-26-22(23)24/h7-12,28H,4-6,13H2,1-3H3,(H4,23,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85004

(LY 320950)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])c(-[#8]-[#6])c2)ccc(-[#6](=O)-[#6]-[#6])c1-[#8] |w:11.10| Show InChI InChI=1S/C22H28N4O4/c1-4-6-17-15(8-10-18(21(17)28)19(27)5-2)13-30-16-9-7-14(20(11-16)29-3)12-25-26-22(23)24/h7-12,28H,4-6,13H2,1-3H3,(H4,23,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 248 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85007

(LY 320954)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)ccc(-[#6](=O)-[#6]-[#6])c1-[#8] |w:11.10| Show InChI InChI=1S/C21H26N4O3/c1-3-5-17-15(8-11-18(20(17)27)19(26)4-2)13-28-16-9-6-14(7-10-16)12-24-25-21(22)23/h6-12,27H,3-5,13H2,1-2H3,(H4,22,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 257 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM21395

(3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85003
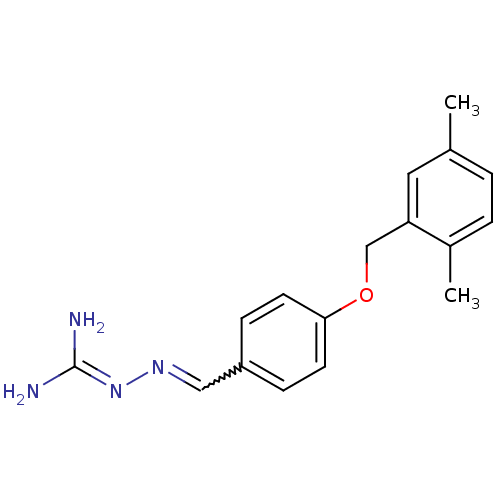
(LY 334359)Show SMILES [#6]-c1ccc(-[#6])c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)c1 |w:13.12| Show InChI InChI=1S/C17H20N4O/c1-12-3-4-13(2)15(9-12)11-22-16-7-5-14(6-8-16)10-20-21-17(18)19/h3-10H,11H2,1-2H3,(H4,18,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 388 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85003

(LY 334359)Show SMILES [#6]-c1ccc(-[#6])c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)c1 |w:13.12| Show InChI InChI=1S/C17H20N4O/c1-12-3-4-13(2)15(9-12)11-22-16-7-5-14(6-8-16)10-20-21-17(18)19/h3-10H,11H2,1-2H3,(H4,18,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 401 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85004

(LY 320950)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])c(-[#8]-[#6])c2)ccc(-[#6](=O)-[#6]-[#6])c1-[#8] |w:11.10| Show InChI InChI=1S/C22H28N4O4/c1-4-6-17-15(8-10-18(21(17)28)19(27)5-2)13-30-16-9-7-14(20(11-16)29-3)12-25-26-22(23)24/h7-12,28H,4-6,13H2,1-3H3,(H4,23,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 465 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85000

(LY 063518)Show SMILES [#7]\[#6](-[#7])=[#7]\[#7]=[#6](-[#6]-1-[#6]-[#6]-[#6](Cl)-[#16]-1)-c1ccccc1 |w:4.3| Show InChI InChI=1S/C12H15ClN4S/c13-10-7-6-9(18-10)11(16-17-12(14)15)8-4-2-1-3-5-8/h1-5,9-10H,6-7H2,(H4,14,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 472 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85006

(LY 334362)Show SMILES [#7]\[#6](-[#7])=[#7]\[#7]=[#6]-c1cccc(-[#8]-[#6]-c2ccc(cc2)-[#6](=O)-c2ccccc2)c1 |w:5.5| Show InChI InChI=1S/C22H20N4O2/c23-22(24)26-25-14-17-5-4-8-20(13-17)28-15-16-9-11-19(12-10-16)21(27)18-6-2-1-3-7-18/h1-14H,15H2,(H4,23,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 477 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85003

(LY 334359)Show SMILES [#6]-c1ccc(-[#6])c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)c1 |w:13.12| Show InChI InChI=1S/C17H20N4O/c1-12-3-4-13(2)15(9-12)11-22-16-7-5-14(6-8-16)10-20-21-17(18)19/h3-10H,11H2,1-2H3,(H4,18,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 569 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85001
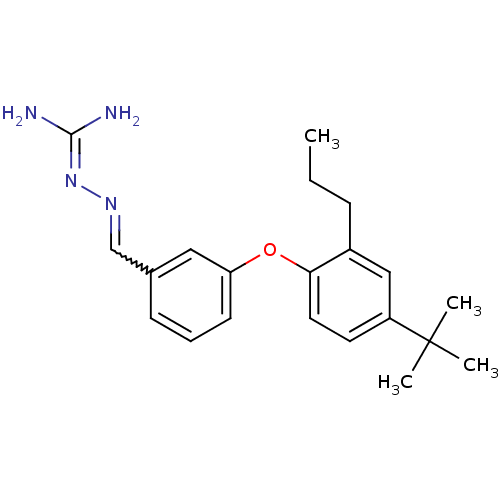
(LY 320951)Show SMILES [#6]-[#6]-[#6]-c1cc(ccc1-[#8]-c1cccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])c1)C([#6])([#6])[#6] |w:15.15| Show InChI InChI=1S/C21H28N4O/c1-5-7-16-13-17(21(2,3)4)10-11-19(16)26-18-9-6-8-15(12-18)14-24-25-20(22)23/h6,8-14H,5,7H2,1-4H3,(H4,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 839 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85001

(LY 320951)Show SMILES [#6]-[#6]-[#6]-c1cc(ccc1-[#8]-c1cccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])c1)C([#6])([#6])[#6] |w:15.15| Show InChI InChI=1S/C21H28N4O/c1-5-7-16-13-17(21(2,3)4)10-11-19(16)26-18-9-6-8-15(12-18)14-24-25-20(22)23/h6,8-14H,5,7H2,1-4H3,(H4,22,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85002

(LY 314228)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)ccc(-[#6](-[#6])=O)c1-[#8] |w:11.10| Show InChI InChI=1S/C20H24N4O3/c1-3-4-18-15(7-10-17(13(2)25)19(18)26)12-27-16-8-5-14(6-9-16)11-23-24-20(21)22/h5-11,26H,3-4,12H2,1-2H3,(H4,21,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85007

(LY 320954)Show SMILES [#6]-[#6]-[#6]-c1c(-[#6]-[#8]-c2ccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])cc2)ccc(-[#6](=O)-[#6]-[#6])c1-[#8] |w:11.10| Show InChI InChI=1S/C21H26N4O3/c1-3-5-17-15(8-11-18(20(17)27)19(26)4-2)13-28-16-9-6-14(7-10-16)12-24-25-21(22)23/h6-12,27H,3-5,13H2,1-2H3,(H4,22,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85000

(LY 063518)Show SMILES [#7]\[#6](-[#7])=[#7]\[#7]=[#6](-[#6]-1-[#6]-[#6]-[#6](Cl)-[#16]-1)-c1ccccc1 |w:4.3| Show InChI InChI=1S/C12H15ClN4S/c13-10-7-6-9(18-10)11(16-17-12(14)15)8-4-2-1-3-5-8/h1-5,9-10H,6-7H2,(H4,14,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85001

(LY 320951)Show SMILES [#6]-[#6]-[#6]-c1cc(ccc1-[#8]-c1cccc(-[#6]=[#7]\[#7]=[#6](\[#7])-[#7])c1)C([#6])([#6])[#6] |w:15.15| Show InChI InChI=1S/C21H28N4O/c1-5-7-16-13-17(21(2,3)4)10-11-19(16)26-18-9-6-8-15(12-18)14-24-25-20(22)23/h6,8-14H,5,7H2,1-4H3,(H4,22,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85005
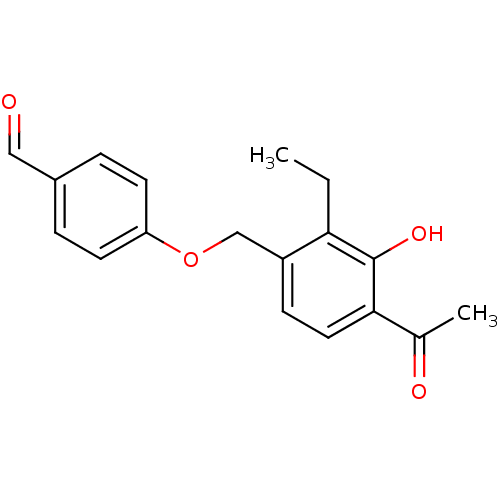
(LY 335102)Show InChI InChI=1S/C18H18O4/c1-3-16-14(6-9-17(12(2)20)18(16)21)11-22-15-7-4-13(10-19)5-8-15/h4-10,21H,3,11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85005

(LY 335102)Show InChI InChI=1S/C18H18O4/c1-3-16-14(6-9-17(12(2)20)18(16)21)11-22-15-7-4-13(10-19)5-8-15/h4-10,21H,3,11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
Life Sci 59: 1259-68 (1996)
Article DOI: 10.1016/0024-3205(96)00449-3
BindingDB Entry DOI: 10.7270/Q2VQ3179 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50062577
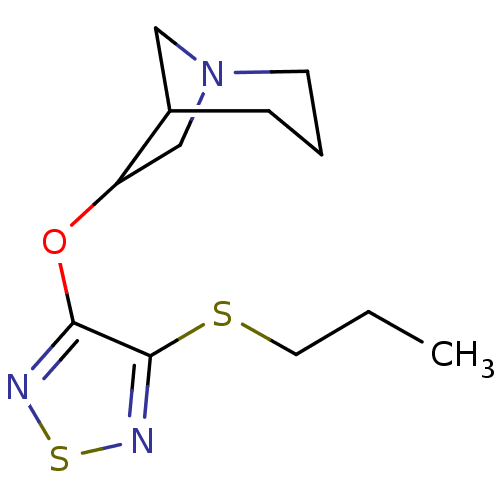
(6-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yloxy)-1-a...)Show SMILES CCCSc1nsnc1OC1CN2CC1CCC2 |TLB:9:10:13:17.15.16| Show InChI InChI=1S/C12H19N3OS2/c1-2-6-17-12-11(13-18-14-12)16-10-8-15-5-3-4-9(10)7-15/h9-10H,2-8H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-oxotremorine-M (Oxo-M) radioligand in rat hippocampus membranes |
J Med Chem 41: 379-92 (1998)
Article DOI: 10.1021/jm970125n
BindingDB Entry DOI: 10.7270/Q2SX6CBC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50062577

(6-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yloxy)-1-a...)Show SMILES CCCSc1nsnc1OC1CN2CC1CCC2 |TLB:9:10:13:17.15.16| Show InChI InChI=1S/C12H19N3OS2/c1-2-6-17-12-11(13-18-14-12)16-10-8-15-5-3-4-9(10)7-15/h9-10H,2-8H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-Pirenzepine (Pz) radioligand in rat hippocampus membranes. |
J Med Chem 41: 379-92 (1998)
Article DOI: 10.1021/jm970125n
BindingDB Entry DOI: 10.7270/Q2SX6CBC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50062577

(6-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yloxy)-1-a...)Show SMILES CCCSc1nsnc1OC1CN2CC1CCC2 |TLB:9:10:13:17.15.16| Show InChI InChI=1S/C12H19N3OS2/c1-2-6-17-12-11(13-18-14-12)16-10-8-15-5-3-4-9(10)7-15/h9-10H,2-8H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-Pirenzepine (Pz) radioligand in rat hippocampus membranes. |
J Med Chem 41: 379-92 (1998)
Article DOI: 10.1021/jm970125n
BindingDB Entry DOI: 10.7270/Q2SX6CBC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50062595
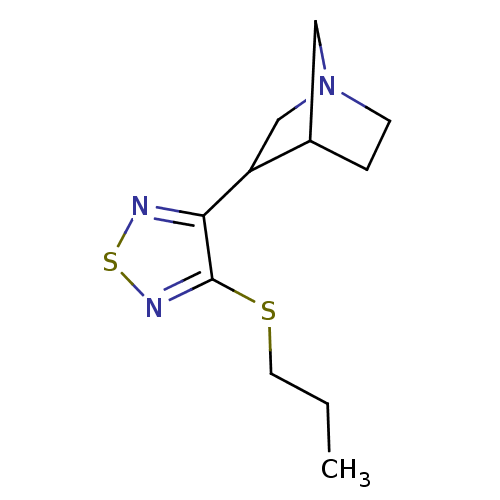
(3-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yl)-1-aza-...)Show InChI InChI=1S/C11H17N3S2/c1-2-5-15-11-10(12-16-13-11)9-7-14-4-3-8(9)6-14/h8-9H,2-7H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-oxotremorine-M (Oxo-M) radioligand in rat hippocampus membranes |
J Med Chem 41: 379-92 (1998)
Article DOI: 10.1021/jm970125n
BindingDB Entry DOI: 10.7270/Q2SX6CBC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50062595

(3-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yl)-1-aza-...)Show InChI InChI=1S/C11H17N3S2/c1-2-5-15-11-10(12-16-13-11)9-7-14-4-3-8(9)6-14/h8-9H,2-7H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-oxotremorine-M (Oxo-M) radioligand in rat hippocampus membranes |
J Med Chem 41: 379-92 (1998)
Article DOI: 10.1021/jm970125n
BindingDB Entry DOI: 10.7270/Q2SX6CBC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50070692
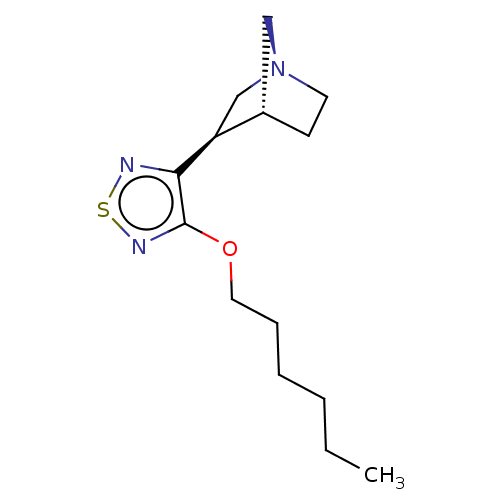
(CHEMBL99240)Show SMILES [H][C@@]12CC[N@@](C[C@@H]1c1nsnc1OCCCCCC)C2 Show InChI InChI=1S/C34H50N8O8/c1-3-20(2)28(42-30(46)24(12-7-8-16-35)39-29(45)23-19-22(43)14-15-27(23)44)32(48)41-26(18-21-10-5-4-6-11-21)31(47)40-25(33(49)50)13-9-17-38-34(36)37/h4-6,10-11,14-15,19-20,24-26,28,43-44H,3,7-9,12-13,16-18,35H2,1-2H3,(H,39,45)(H,40,47)(H,41,48)(H,42,46)(H,49,50)(H4,36,37,38)/t20?,24-,25-,26-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50062570
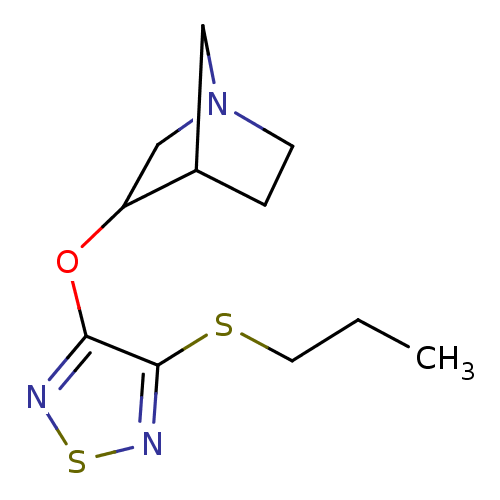
(3-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yloxy)-1-a...)Show SMILES CCCSc1nsnc1OC1CN2CCC1C2 |THB:9:10:16:14.13| Show InChI InChI=1S/C11H17N3OS2/c1-2-5-16-11-10(12-17-13-11)15-9-7-14-4-3-8(9)6-14/h8-9H,2-7H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-oxotremorine-M (Oxo-M) radioligand in rat hippocampus membranes |
J Med Chem 41: 379-92 (1998)
Article DOI: 10.1021/jm970125n
BindingDB Entry DOI: 10.7270/Q2SX6CBC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50072214
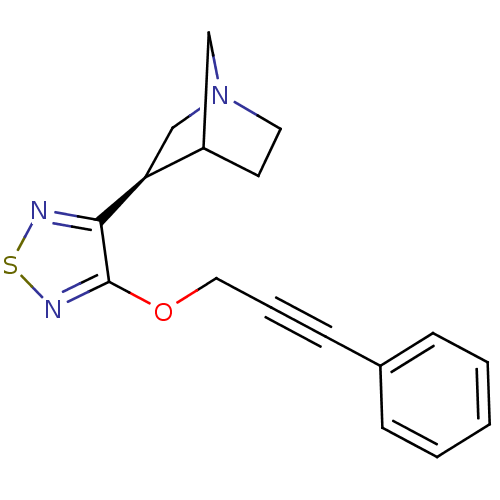
((R)-3-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]thiadiaz...)Show InChI InChI=1S/C17H17N3OS/c1-2-5-13(6-3-1)7-4-10-21-17-16(18-22-19-17)15-12-20-9-8-14(15)11-20/h1-3,5-6,14-15H,8-12H2/t14?,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50072227
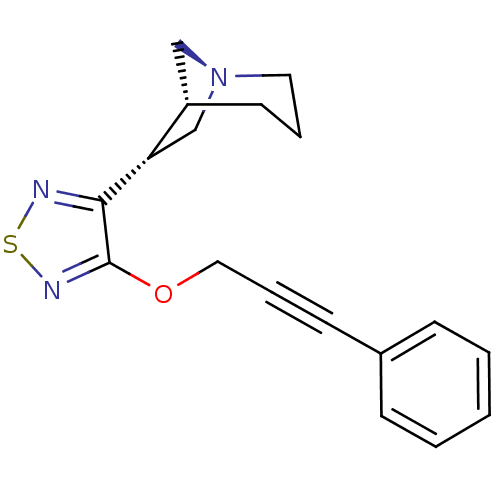
((1R,5R,6R)-6-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]t...)Show SMILES C(Oc1nsnc1[C@H]1C[N@]2C[C@@H]1CCC2)C#Cc1ccccc1 Show InChI InChI=1S/C18H19N3OS/c1-2-6-14(7-3-1)8-5-11-22-18-17(19-23-20-18)16-13-21-10-4-9-15(16)12-21/h1-3,6-7,15-16H,4,9-13H2/t15-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50070735
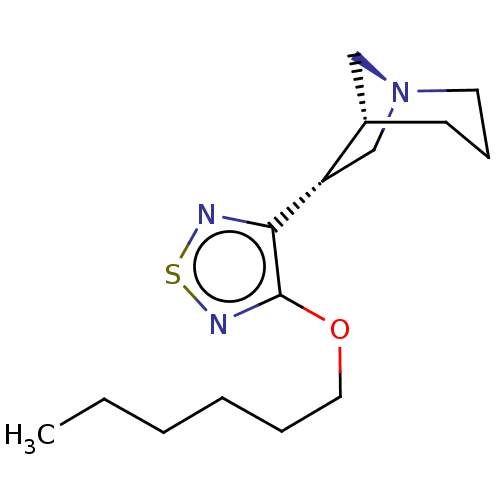
(CHEMBL317324)Show InChI InChI=1S/C29H48N8O8/c1-4-16(3)23(27(43)34-19(5-2)28(44)45)37-26(42)21(10-8-14-33-29(31)32)36-25(41)20(9-6-7-13-30)35-24(40)18-15-17(38)11-12-22(18)39/h11-12,15-16,19-21,23,38-39H,4-10,13-14,30H2,1-3H3,(H,34,43)(H,35,40)(H,36,41)(H,37,42)(H,44,45)(H4,31,32,33)/t16?,19?,20-,21-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50070647
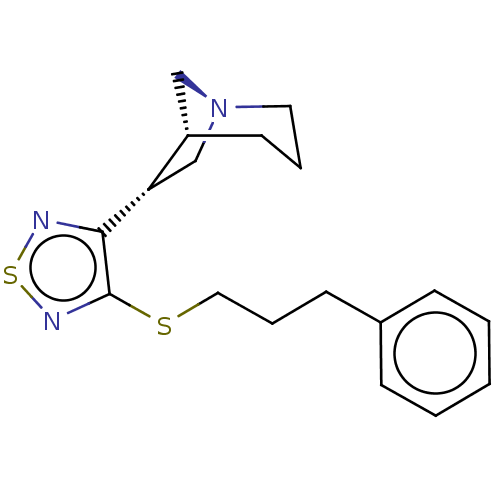
(CHEMBL329924)Show SMILES [H][C@]12C[N@](C[C@@H]1c1nsnc1SCCCc1ccccc1)CCC2 Show InChI InChI=1S/C18H23N5O/c1-3-6-13-7-4-5-8-14(13)9-15(12(2)24)23-11-22-16-17(19)20-10-21-18(16)23/h4-5,7-8,10-12,15,24H,3,6,9H2,1-2H3,(H2,19,20,21)/t12-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
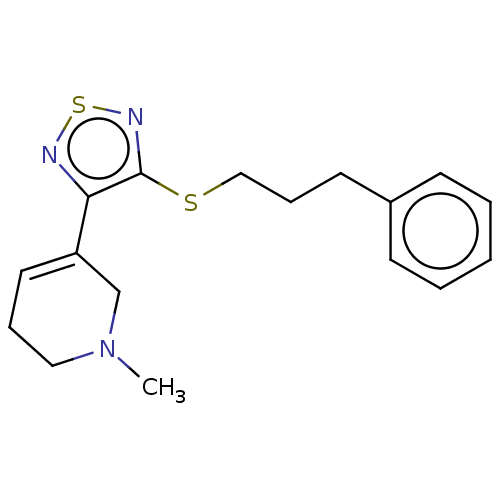
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50070725
(CHEMBL318403)Show InChI InChI=1S/C28H43N5O8/c1-4-16(3)23(26(38)30-19(5-2)28(40)41)32-25(37)21-10-8-14-33(21)27(39)20(9-6-7-13-29)31-24(36)18-15-17(34)11-12-22(18)35/h11-12,15-16,19-21,23,34-35H,4-10,13-14,29H2,1-3H3,(H,30,38)(H,31,36)(H,32,37)(H,40,41)/t16?,19?,20-,21-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50003351
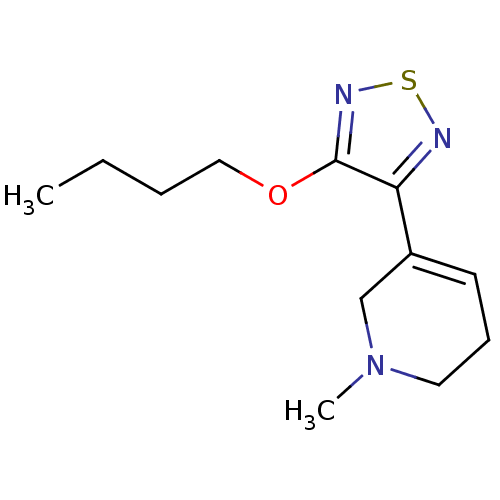
(3-(4-butoxy-1,2,5-thiadiazol-3-yl)-1-methyl-1,2,5,...)Show InChI InChI=1S/C12H19N3OS/c1-3-4-8-16-12-11(13-17-14-12)10-6-5-7-15(2)9-10/h6H,3-5,7-9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50003369
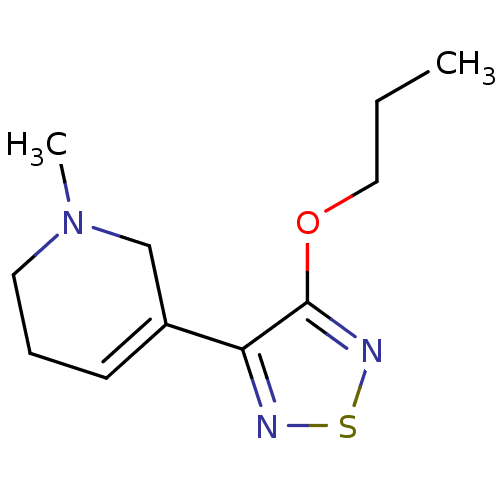
(1-Methyl-5-(4-propoxy-[1,2,5]thiadiazol-3-yl)-1,2,...)Show InChI InChI=1S/C11H17N3OS/c1-3-7-15-11-10(12-16-13-11)9-5-4-6-14(2)8-9/h5H,3-4,6-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50070684

(CHEMBL321073)Show SMILES [H][C@@]12CC[N@@](C[C@@H]1c1nsnc1SCCCc1ccccc1)C2 Show InChI InChI=1S/C28H43N7O8/c1-4-15(3)22(25(40)32-18(5-2)27(42)43)34-24(39)20-9-7-13-35(20)26(41)19(8-6-12-31-28(29)30)33-23(38)17-14-16(36)10-11-21(17)37/h10-11,14-15,18-20,22,36-37H,4-9,12-13H2,1-3H3,(H,32,40)(H,33,38)(H,34,39)(H,42,43)(H4,29,30,31)/t15?,18?,19-,20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50072225

((1R,5R,6R)-6-[4-(3-Phenyl-prop-2-ynylsulfanyl)-[1,...)Show SMILES C(Sc1nsnc1[C@H]1C[N@]2C[C@@H]1CCC2)C#Cc1ccccc1 Show InChI InChI=1S/C18H19N3S2/c1-2-6-14(7-3-1)8-5-11-22-18-17(19-23-20-18)16-13-21-10-4-9-15(16)12-21/h1-3,6-7,15-16H,4,9-13H2/t15-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50003359

(5-(4-Hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2...)Show InChI InChI=1S/C14H23N3OS/c1-3-4-5-6-10-18-14-13(15-19-16-14)12-8-7-9-17(2)11-12/h8H,3-7,9-11H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-oxotremorine-M (Oxo-M) radioligand in rat hippocampus membranes |
J Med Chem 41: 379-92 (1998)
Article DOI: 10.1021/jm970125n
BindingDB Entry DOI: 10.7270/Q2SX6CBC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50003366
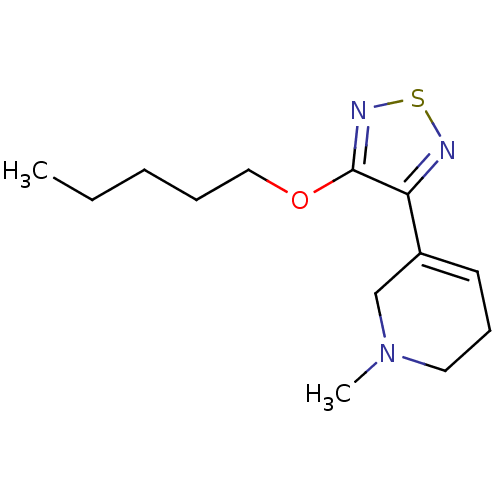
(1-Methyl-5-(4-pentyloxy-[1,2,5]thiadiazol-3-yl)-1,...)Show InChI InChI=1S/C13H21N3OS/c1-3-4-5-9-17-13-12(14-18-15-13)11-7-6-8-16(2)10-11/h7H,3-6,8-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50062589

(3-(4-Butylsulfanyl-[1,2,5]thiadiazol-3-yloxy)-1-az...)Show SMILES CCCCSc1nsnc1OC1CN2CCC1CC2 |(8.64,-14.44,;9.78,-13.42,;9.48,-11.91,;10.62,-10.88,;10.31,-9.38,;11.46,-8.36,;12.97,-8.68,;13.74,-7.33,;12.7,-6.19,;11.32,-6.82,;9.99,-6.05,;8.64,-6.82,;8.64,-8.36,;7.31,-9.12,;6,-8.36,;6,-6.82,;7.31,-6.04,;6.56,-7.38,;8.08,-7.78,)| Show InChI InChI=1S/C13H21N3OS2/c1-2-3-8-18-13-12(14-19-15-13)17-11-9-16-6-4-10(11)5-7-16/h10-11H,2-9H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-oxotremorine-M (Oxo-M) radioligand in rat hippocampus membranes |
J Med Chem 41: 379-92 (1998)
Article DOI: 10.1021/jm970125n
BindingDB Entry DOI: 10.7270/Q2SX6CBC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50062585

(3-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yloxy)-1-a...)Show SMILES CCCSc1nsnc1OC1CN2CCC1CC2 |(9.78,-13.42,;9.48,-11.91,;10.62,-10.88,;10.31,-9.38,;11.46,-8.36,;12.97,-8.68,;13.74,-7.33,;12.7,-6.19,;11.32,-6.82,;9.99,-6.05,;8.64,-6.82,;8.64,-8.36,;7.31,-9.12,;6,-8.36,;6,-6.82,;7.31,-6.04,;6.56,-7.38,;8.08,-7.78,)| Show InChI InChI=1S/C12H19N3OS2/c1-2-7-17-12-11(13-18-14-12)16-10-8-15-5-3-9(10)4-6-15/h9-10H,2-8H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-oxotremorine-M (Oxo-M) radioligand in rat hippocampus membranes |
J Med Chem 41: 379-92 (1998)
Article DOI: 10.1021/jm970125n
BindingDB Entry DOI: 10.7270/Q2SX6CBC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50072227

((1R,5R,6R)-6-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]t...)Show SMILES C(Oc1nsnc1[C@H]1C[N@]2C[C@@H]1CCC2)C#Cc1ccccc1 Show InChI InChI=1S/C18H19N3OS/c1-2-6-14(7-3-1)8-5-11-22-18-17(19-23-20-18)16-13-21-10-4-9-15(16)12-21/h1-3,6-7,15-16H,4,9-13H2/t15-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of cAMP in CHO cells expressing human m2 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50213839
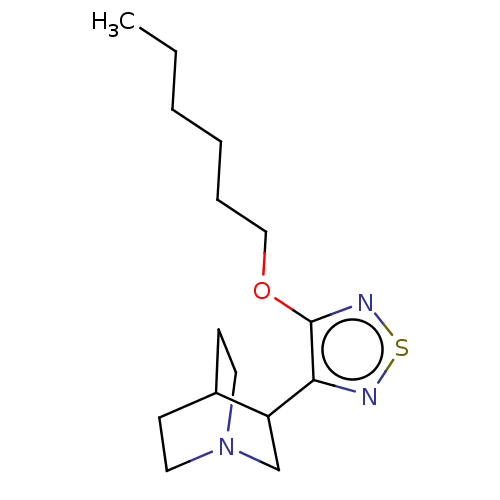
(CHEMBL98529)Show SMILES CCCCCCOc1nsnc1C1CN2CCC1CC2 |(14.95,-12.38,;14.22,-11.01,;12.68,-10.94,;11.97,-9.56,;10.43,-9.5,;9.72,-8.13,;8.18,-8.07,;7.36,-9.37,;7.92,-10.78,;6.73,-11.78,;5.43,-10.94,;5.8,-9.46,;4.83,-8.26,;3.54,-9.08,;2.17,-8.39,;3.19,-7.15,;3.83,-7.92,;4.77,-6.73,;3.41,-6.02,;2.13,-7.08,)| Show InChI InChI=1S/C15H25N3OS/c1-2-3-4-5-10-19-15-14(16-20-17-15)13-11-18-8-6-12(13)7-9-18/h12-13H,2-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50062570

(3-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yloxy)-1-a...)Show SMILES CCCSc1nsnc1OC1CN2CCC1C2 |THB:9:10:16:14.13| Show InChI InChI=1S/C11H17N3OS2/c1-2-5-16-11-10(12-17-13-11)15-9-7-14-4-3-8(9)6-14/h8-9H,2-7H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-Pirenzepine (Pz) radioligand in rat hippocampus membranes. |
J Med Chem 41: 379-92 (1998)
Article DOI: 10.1021/jm970125n
BindingDB Entry DOI: 10.7270/Q2SX6CBC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50062579
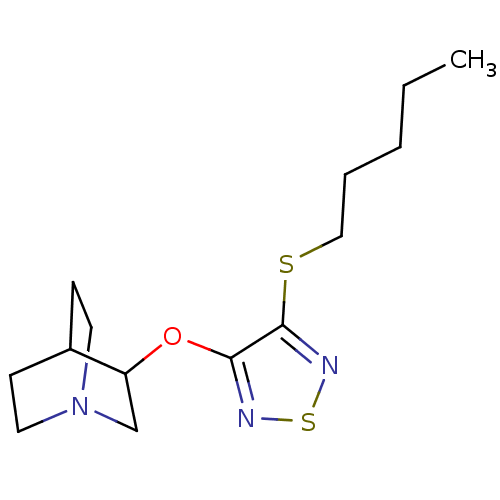
(3-(4-Pentylsulfanyl-[1,2,5]thiadiazol-3-yloxy)-1-a...)Show SMILES CCCCCSc1nsnc1OC1CN2CCC1CC2 |(8.94,-15.94,;8.64,-14.44,;9.78,-13.42,;9.48,-11.91,;10.62,-10.88,;10.31,-9.38,;11.46,-8.36,;12.97,-8.68,;13.74,-7.33,;12.7,-6.19,;11.32,-6.82,;9.99,-6.05,;8.64,-6.82,;8.64,-8.36,;7.31,-9.12,;6,-8.36,;6,-6.82,;7.31,-6.04,;6.56,-7.38,;8.08,-7.78,)| Show InChI InChI=1S/C14H23N3OS2/c1-2-3-4-9-19-14-13(15-20-16-14)18-12-10-17-7-5-11(12)6-8-17/h11-12H,2-10H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-oxotremorine-M (Oxo-M) radioligand in rat hippocampus membranes |
J Med Chem 41: 379-92 (1998)
Article DOI: 10.1021/jm970125n
BindingDB Entry DOI: 10.7270/Q2SX6CBC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50062570

(3-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yloxy)-1-a...)Show SMILES CCCSc1nsnc1OC1CN2CCC1C2 |THB:9:10:16:14.13| Show InChI InChI=1S/C11H17N3OS2/c1-2-5-16-11-10(12-17-13-11)15-9-7-14-4-3-8(9)6-14/h8-9H,2-7H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for muscarinic acetylcholine receptor M1 using [3H]-Pirenzepine (Pz) radioligand in rat hippocampus membranes. |
J Med Chem 41: 379-92 (1998)
Article DOI: 10.1021/jm970125n
BindingDB Entry DOI: 10.7270/Q2SX6CBC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data