 Found 643 hits with Last Name = 'wolf' and Initial = 'g'
Found 643 hits with Last Name = 'wolf' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50370575
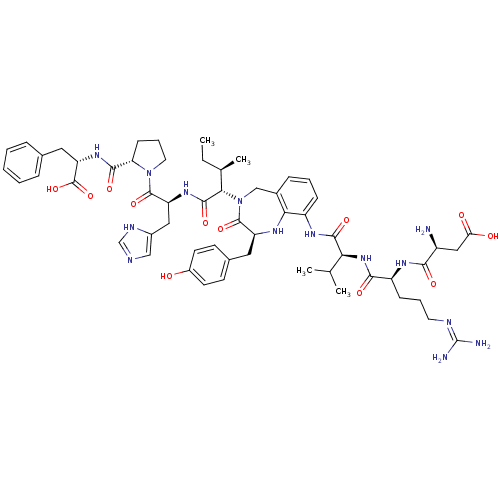
(CHEMBL1791308)Show SMILES CC[C@@H](C)[C@H](N1Cc2cccc(NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)c2N[C@@H](Cc2ccc(O)cc2)C1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:68.73,15.15,55.57,41.42,30.30,2.2,wD:4.4,19.26,72.76,(20.86,-11.16,;20.86,-9.62,;19.5,-8.85,;18.13,-9.62,;19.5,-7.31,;18.13,-6.53,;17.29,-7.83,;15.7,-8,;15.29,-9.47,;13.78,-9.86,;12.67,-8.77,;13.1,-7.3,;11.74,-6.61,;10.41,-7.27,;10.41,-8.62,;9.06,-6.59,;7.72,-7.27,;6.37,-6.59,;6.4,-5.24,;5.03,-7.24,;5.03,-8.61,;6.22,-9.28,;6.2,-10.63,;7.41,-11.31,;7.4,-12.65,;6.2,-13.32,;8.58,-13.35,;3.69,-6.58,;2.35,-7.24,;2.35,-8.58,;.99,-6.58,;-.35,-7.21,;1.02,-5.24,;-.33,-4.54,;-1.69,-5.21,;-.32,-3.2,;9.07,-5.22,;10.45,-4.54,;7.71,-4.51,;14.61,-6.9,;14.8,-5.36,;16.14,-4.57,;15.99,-3.04,;16.77,-1.69,;15.97,-.38,;16.74,.97,;18.32,.97,;19.09,2.32,;19.13,-.34,;18.35,-1.69,;17.63,-5.09,;18.76,-3.99,;20.86,-6.53,;20.86,-5,;22.25,-7.3,;23.67,-6.69,;23.73,-5.14,;24.52,-3.79,;26.07,-3.63,;26.4,-2.11,;25.03,-1.35,;23.84,-2.38,;25,-7.41,;24.92,-8.77,;26.39,-6.67,;25.88,-5.19,;27.18,-4.28,;28.45,-5.19,;27.97,-6.67,;29.35,-7.43,;29.34,-8.98,;30.71,-6.67,;32.02,-7.41,;31.94,-8.77,;33.27,-9.48,;34.67,-8.88,;35.98,-9.63,;35.9,-10.95,;34.48,-11.61,;33.19,-10.87,;33.44,-6.8,;34.77,-7.53,;33.52,-5.43,)| Show InChI InChI=1S/C57H76N14O12/c1-5-32(4)48(53(79)67-42(26-36-28-61-30-63-36)54(80)70-23-11-17-44(70)51(77)68-43(56(82)83)25-33-12-7-6-8-13-33)71-29-35-14-9-15-39(47(35)64-41(55(71)81)24-34-18-20-37(72)21-19-34)65-52(78)46(31(2)3)69-50(76)40(16-10-22-62-57(59)60)66-49(75)38(58)27-45(73)74/h6-9,12-15,18-21,28,30-32,38,40-44,46,48,64,72H,5,10-11,16-17,22-27,29,58H2,1-4H3,(H,61,63)(H,65,78)(H,66,75)(H,67,79)(H,68,77)(H,69,76)(H,73,74)(H,82,83)(H4,59,60,62)/t32-,38+,40+,41+,42+,43+,44+,46+,48+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from angiotensin II receptor type 2 in pig uterus myometrium |
J Med Chem 48: 4009-24 (2005)
Article DOI: 10.1021/jm0491492
BindingDB Entry DOI: 10.7270/Q2BR8SZD |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50370575

(CHEMBL1791308)Show SMILES CC[C@@H](C)[C@H](N1Cc2cccc(NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)c2N[C@@H](Cc2ccc(O)cc2)C1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:68.73,15.15,55.57,41.42,30.30,2.2,wD:4.4,19.26,72.76,(20.86,-11.16,;20.86,-9.62,;19.5,-8.85,;18.13,-9.62,;19.5,-7.31,;18.13,-6.53,;17.29,-7.83,;15.7,-8,;15.29,-9.47,;13.78,-9.86,;12.67,-8.77,;13.1,-7.3,;11.74,-6.61,;10.41,-7.27,;10.41,-8.62,;9.06,-6.59,;7.72,-7.27,;6.37,-6.59,;6.4,-5.24,;5.03,-7.24,;5.03,-8.61,;6.22,-9.28,;6.2,-10.63,;7.41,-11.31,;7.4,-12.65,;6.2,-13.32,;8.58,-13.35,;3.69,-6.58,;2.35,-7.24,;2.35,-8.58,;.99,-6.58,;-.35,-7.21,;1.02,-5.24,;-.33,-4.54,;-1.69,-5.21,;-.32,-3.2,;9.07,-5.22,;10.45,-4.54,;7.71,-4.51,;14.61,-6.9,;14.8,-5.36,;16.14,-4.57,;15.99,-3.04,;16.77,-1.69,;15.97,-.38,;16.74,.97,;18.32,.97,;19.09,2.32,;19.13,-.34,;18.35,-1.69,;17.63,-5.09,;18.76,-3.99,;20.86,-6.53,;20.86,-5,;22.25,-7.3,;23.67,-6.69,;23.73,-5.14,;24.52,-3.79,;26.07,-3.63,;26.4,-2.11,;25.03,-1.35,;23.84,-2.38,;25,-7.41,;24.92,-8.77,;26.39,-6.67,;25.88,-5.19,;27.18,-4.28,;28.45,-5.19,;27.97,-6.67,;29.35,-7.43,;29.34,-8.98,;30.71,-6.67,;32.02,-7.41,;31.94,-8.77,;33.27,-9.48,;34.67,-8.88,;35.98,-9.63,;35.9,-10.95,;34.48,-11.61,;33.19,-10.87,;33.44,-6.8,;34.77,-7.53,;33.52,-5.43,)| Show InChI InChI=1S/C57H76N14O12/c1-5-32(4)48(53(79)67-42(26-36-28-61-30-63-36)54(80)70-23-11-17-44(70)51(77)68-43(56(82)83)25-33-12-7-6-8-13-33)71-29-35-14-9-15-39(47(35)64-41(55(71)81)24-34-18-20-37(72)21-19-34)65-52(78)46(31(2)3)69-50(76)40(16-10-22-62-57(59)60)66-49(75)38(58)27-45(73)74/h6-9,12-15,18-21,28,30-32,38,40-44,46,48,64,72H,5,10-11,16-17,22-27,29,58H2,1-4H3,(H,61,63)(H,65,78)(H,66,75)(H,67,79)(H,68,77)(H,69,76)(H,73,74)(H,82,83)(H4,59,60,62)/t32-,38+,40+,41+,42+,43+,44+,46+,48+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from angiotensin II receptor type 2 in pig uterus myometrium |
J Med Chem 48: 4009-24 (2005)
Article DOI: 10.1021/jm0491492
BindingDB Entry DOI: 10.7270/Q2BR8SZD |
More data for this
Ligand-Target Pair | |
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50527135
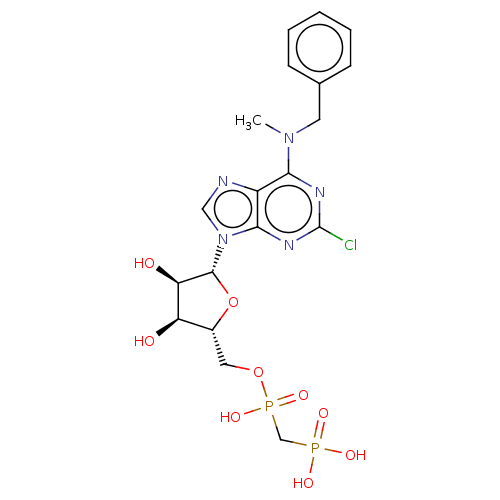
(CHEMBL4452072)Show SMILES CN(Cc1ccccc1)c1nc(Cl)nc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C19H24ClN5O9P2/c1-24(7-11-5-3-2-4-6-11)16-13-17(23-19(20)22-16)25(9-21-13)18-15(27)14(26)12(34-18)8-33-36(31,32)10-35(28,29)30/h2-6,9,12,14-15,18,26-27H,7-8,10H2,1H3,(H,31,32)(H2,28,29,30)/t12-,14-,15-,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.381 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal His-tagged CD73 (27 to 549 residues) expressed in Sf9 cells using [2,8-3H]-AMP as substrate incubated for ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00391
BindingDB Entry DOI: 10.7270/Q2CF9TTM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5'-nucleotidase
(Rattus norvegicus (Rat)) | BDBM50527135

(CHEMBL4452072)Show SMILES CN(Cc1ccccc1)c1nc(Cl)nc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C19H24ClN5O9P2/c1-24(7-11-5-3-2-4-6-11)16-13-17(23-19(20)22-16)25(9-21-13)18-15(27)14(26)12(34-18)8-33-36(31,32)10-35(28,29)30/h2-6,9,12,14-15,18,26-27H,7-8,10H2,1H3,(H,31,32)(H2,28,29,30)/t12-,14-,15-,18-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.746 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant rat CD73 expressed in Sf9 cells using [2,8-3H]-AMP as substrate incubated for 25 mins by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00391
BindingDB Entry DOI: 10.7270/Q2CF9TTM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50168428
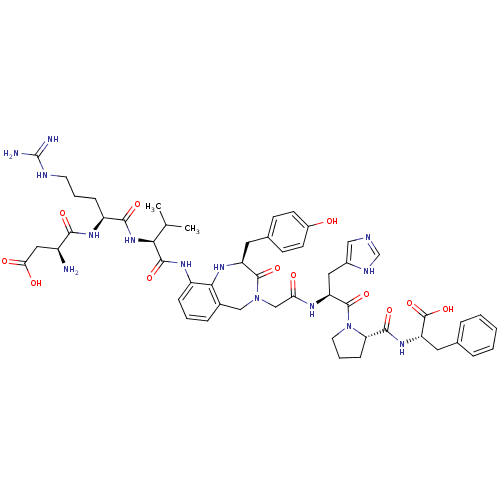
((3S)-3-amino-3-{[(1S)-1-{[(1S)-1-{[(2S)-4-({[(2S)-...)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CC(O)=O)C(=O)Nc1cccc2CN(CC(=O)N[C@@H](Cc3cnc[nH]3)C(=O)N3CCC[C@H]3C(=O)N[C@@H](Cc3ccccc3)C(O)=O)C(=O)[C@H](Cc3ccc(O)cc3)Nc12 Show InChI InChI=1S/C53H68N14O12/c1-29(2)44(65-47(73)37(13-7-19-58-53(55)56)63-46(72)35(54)24-43(70)71)49(75)62-36-12-6-11-32-26-66(50(76)38(61-45(32)36)21-31-15-17-34(68)18-16-31)27-42(69)60-39(23-33-25-57-28-59-33)51(77)67-20-8-14-41(67)48(74)64-40(52(78)79)22-30-9-4-3-5-10-30/h3-6,9-12,15-18,25,28-29,35,37-41,44,61,68H,7-8,13-14,19-24,26-27,54H2,1-2H3,(H,57,59)(H,60,69)(H,62,75)(H,63,72)(H,64,74)(H,65,73)(H,70,71)(H,78,79)(H4,55,56,58)/t35-,37-,38-,39-,40-,41-,44-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from angiotensin II receptor type 2 in pig uterus myometrium |
J Med Chem 48: 4009-24 (2005)
Article DOI: 10.1021/jm0491492
BindingDB Entry DOI: 10.7270/Q2BR8SZD |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50370375

(CHEMBL268815)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#7])cc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](-[#8])=O Show InChI InChI=1S/C53H74N12O12/c1-5-30(4)44(50(74)61-39(26-32-15-19-34(54)20-16-32)51(75)65-24-10-14-41(65)48(72)62-40(52(76)77)27-31-11-7-6-8-12-31)64-47(71)38(25-33-17-21-35(66)22-18-33)60-49(73)43(29(2)3)63-46(70)37(13-9-23-58-53(56)57)59-45(69)36(55)28-42(67)68/h6-8,11-12,15-22,29-30,36-41,43-44,66H,5,9-10,13-14,23-28,54-55H2,1-4H3,(H,59,69)(H,60,73)(H,61,74)(H,62,72)(H,63,70)(H,64,71)(H,67,68)(H,76,77)(H4,56,57,58)/t30-,36+,37+,38+,39+,40+,41+,43+,44+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from angiotensin II receptor type 2 in pig uterus myometrium |
J Med Chem 48: 4009-24 (2005)
Article DOI: 10.1021/jm0491492
BindingDB Entry DOI: 10.7270/Q2BR8SZD |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50370576

(CHEMBL1791309)Show SMILES CC[C@@H](C)[C@H](N1Cc2cccc(NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)c2N[C@@H](Cc2ccc(O)cc2)C1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:61.66,48.50,15.22,34.35,2.2,wD:4.4,65.69,26.26,(17.04,-11.58,;17.04,-10.04,;15.66,-9.27,;14.31,-10.04,;15.66,-7.73,;14.31,-6.96,;13.45,-8.27,;11.88,-8.43,;11.49,-9.89,;9.96,-10.28,;8.85,-9.2,;9.27,-7.73,;7.91,-6.94,;6.57,-7.6,;6.59,-8.95,;5.22,-6.94,;5.22,-5.59,;3.89,-4.92,;3.91,-3.59,;2.54,-2.91,;2.56,-1.57,;1.23,-.89,;3.92,-.92,;3.91,-7.62,;2.54,-6.94,;2.54,-5.61,;1.2,-7.62,;-.15,-6.96,;1.2,-8.95,;-.12,-9.64,;-1.47,-8.98,;-.11,-10.97,;10.79,-7.33,;10.97,-5.81,;12.32,-5.01,;12.16,-3.47,;12.94,-2.15,;14.52,-2.12,;15.29,-.81,;14.5,.52,;15.27,1.86,;12.91,.49,;12.14,-.84,;13.8,-5.53,;14.93,-4.46,;17.04,-6.96,;17.04,-5.43,;18.42,-7.73,;19.82,-7.11,;19.89,-5.56,;20.67,-4.25,;22.22,-4.06,;22.54,-2.56,;21.18,-1.8,;20.01,-2.83,;21.14,-7.82,;21.05,-9.2,;22.54,-7.1,;22.04,-5.64,;23.33,-4.73,;24.61,-5.64,;24.12,-7.1,;25.48,-7.86,;25.48,-9.4,;26.86,-7.11,;28.17,-7.85,;28.07,-9.22,;29.42,-9.91,;29.32,-11.29,;30.62,-12.02,;32.03,-11.39,;32.13,-10.05,;30.8,-9.29,;29.59,-7.23,;30.9,-7.96,;29.66,-5.86,)| Show InChI InChI=1S/C52H67N13O11/c1-3-29(2)44(48(72)62-39(24-33-26-56-28-58-33)49(73)64-21-9-15-41(64)47(71)63-40(51(75)76)23-30-10-5-4-6-11-30)65-27-32-12-7-13-36(43(32)59-38(50(65)74)22-31-16-18-34(66)19-17-31)60-46(70)37(14-8-20-57-52(54)55)61-45(69)35(53)25-42(67)68/h4-7,10-13,16-19,26,28-29,35,37-41,44,59,66H,3,8-9,14-15,20-25,27,53H2,1-2H3,(H,56,58)(H,60,70)(H,61,69)(H,62,72)(H,63,71)(H,67,68)(H,75,76)(H4,54,55,57)/t29-,35+,37+,38+,39+,40+,41+,44+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from angiotensin II receptor type 2 in pig uterus myometrium |
J Med Chem 48: 4009-24 (2005)
Article DOI: 10.1021/jm0491492
BindingDB Entry DOI: 10.7270/Q2BR8SZD |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50370576

(CHEMBL1791309)Show SMILES CC[C@@H](C)[C@H](N1Cc2cccc(NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)c2N[C@@H](Cc2ccc(O)cc2)C1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:61.66,48.50,15.22,34.35,2.2,wD:4.4,65.69,26.26,(17.04,-11.58,;17.04,-10.04,;15.66,-9.27,;14.31,-10.04,;15.66,-7.73,;14.31,-6.96,;13.45,-8.27,;11.88,-8.43,;11.49,-9.89,;9.96,-10.28,;8.85,-9.2,;9.27,-7.73,;7.91,-6.94,;6.57,-7.6,;6.59,-8.95,;5.22,-6.94,;5.22,-5.59,;3.89,-4.92,;3.91,-3.59,;2.54,-2.91,;2.56,-1.57,;1.23,-.89,;3.92,-.92,;3.91,-7.62,;2.54,-6.94,;2.54,-5.61,;1.2,-7.62,;-.15,-6.96,;1.2,-8.95,;-.12,-9.64,;-1.47,-8.98,;-.11,-10.97,;10.79,-7.33,;10.97,-5.81,;12.32,-5.01,;12.16,-3.47,;12.94,-2.15,;14.52,-2.12,;15.29,-.81,;14.5,.52,;15.27,1.86,;12.91,.49,;12.14,-.84,;13.8,-5.53,;14.93,-4.46,;17.04,-6.96,;17.04,-5.43,;18.42,-7.73,;19.82,-7.11,;19.89,-5.56,;20.67,-4.25,;22.22,-4.06,;22.54,-2.56,;21.18,-1.8,;20.01,-2.83,;21.14,-7.82,;21.05,-9.2,;22.54,-7.1,;22.04,-5.64,;23.33,-4.73,;24.61,-5.64,;24.12,-7.1,;25.48,-7.86,;25.48,-9.4,;26.86,-7.11,;28.17,-7.85,;28.07,-9.22,;29.42,-9.91,;29.32,-11.29,;30.62,-12.02,;32.03,-11.39,;32.13,-10.05,;30.8,-9.29,;29.59,-7.23,;30.9,-7.96,;29.66,-5.86,)| Show InChI InChI=1S/C52H67N13O11/c1-3-29(2)44(48(72)62-39(24-33-26-56-28-58-33)49(73)64-21-9-15-41(64)47(71)63-40(51(75)76)23-30-10-5-4-6-11-30)65-27-32-12-7-13-36(43(32)59-38(50(65)74)22-31-16-18-34(66)19-17-31)60-46(70)37(14-8-20-57-52(54)55)61-45(69)35(53)25-42(67)68/h4-7,10-13,16-19,26,28-29,35,37-41,44,59,66H,3,8-9,14-15,20-25,27,53H2,1-2H3,(H,56,58)(H,60,70)(H,61,69)(H,62,72)(H,63,71)(H,67,68)(H,75,76)(H4,54,55,57)/t29-,35+,37+,38+,39+,40+,41+,44+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from angiotensin II receptor type 2 in pig uterus myometrium |
J Med Chem 48: 4009-24 (2005)
Article DOI: 10.1021/jm0491492
BindingDB Entry DOI: 10.7270/Q2BR8SZD |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50168424
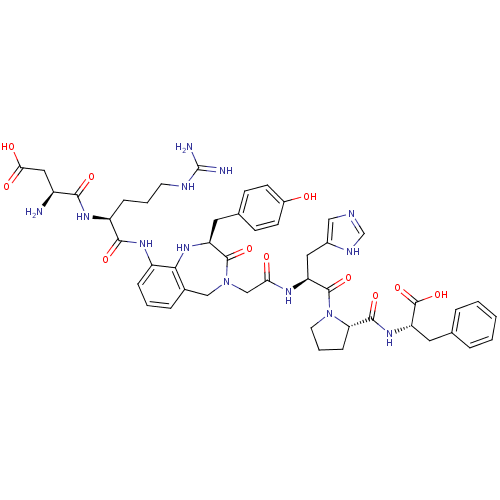
((3S)-3-amino-3-{[(1S)-1-{[(2S)-4-({[(2S)-1-[(2S)-2...)Show SMILES N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)Nc1cccc2CN(CC(=O)N[C@@H](Cc3cnc[nH]3)C(=O)N3CCC[C@H]3C(=O)N[C@@H](Cc3ccccc3)C(O)=O)C(=O)[C@H](Cc3ccc(O)cc3)Nc12 Show InChI InChI=1S/C48H59N13O11/c49-32(22-40(64)65)42(66)58-34(11-5-17-53-48(50)51)43(67)57-33-10-4-9-29-24-60(45(69)35(56-41(29)33)19-28-13-15-31(62)16-14-28)25-39(63)55-36(21-30-23-52-26-54-30)46(70)61-18-6-12-38(61)44(68)59-37(47(71)72)20-27-7-2-1-3-8-27/h1-4,7-10,13-16,23,26,32,34-38,56,62H,5-6,11-12,17-22,24-25,49H2,(H,52,54)(H,55,63)(H,57,67)(H,58,66)(H,59,68)(H,64,65)(H,71,72)(H4,50,51,53)/t32-,34-,35-,36-,37-,38-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from angiotensin II receptor type 2 in pig uterus myometrium |
J Med Chem 48: 4009-24 (2005)
Article DOI: 10.1021/jm0491492
BindingDB Entry DOI: 10.7270/Q2BR8SZD |
More data for this
Ligand-Target Pair | |
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50561892
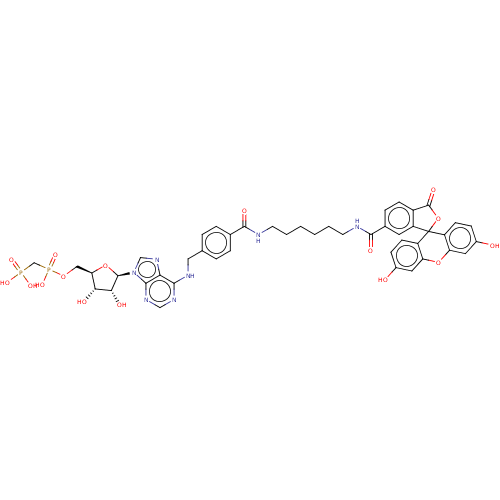
(CHEMBL4795486)Show SMILES O[C@@H]1[C@@H](COP(O)(=O)CP(O)(O)=O)O[C@H]([C@@H]1O)n1cnc2c(NCc3ccc(cc3)C(=O)NCCCCCCNC(=O)c3ccc4C(=O)OC5(c4c3)c3ccc(O)cc3Oc3cc(O)ccc53)ncnc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal His-tagged CD73 (27 to 549 residues) expressed in Sf9 cells using [2,8-3H]-AMP as substrate incubated for ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00391
BindingDB Entry DOI: 10.7270/Q2CF9TTM |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50139787
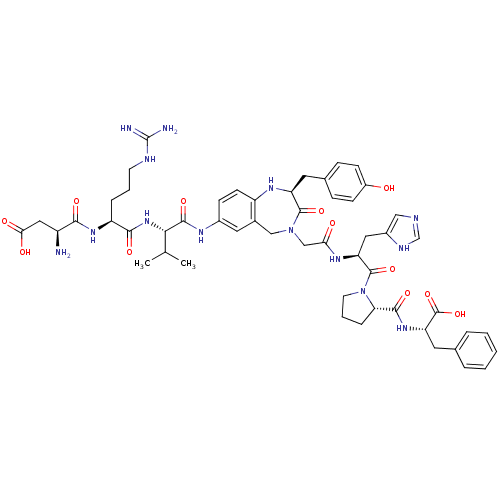
((3S)-3-amino-3-{[(1S)-1-{[(1S)-1-{[(2S)-4-({[(2S)-...)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CC(O)=O)C(=O)Nc1ccc2N[C@@H](Cc3ccc(O)cc3)C(=O)N(CC(=O)N[C@@H](Cc3cnc[nH]3)C(=O)N3CCC[C@H]3C(=O)N[C@@H](Cc3ccccc3)C(O)=O)Cc2c1 Show InChI InChI=1S/C53H68N14O12/c1-29(2)45(65-47(73)38(10-6-18-58-53(55)56)63-46(72)36(54)24-44(70)71)49(75)60-33-14-17-37-32(22-33)26-66(50(76)39(61-37)20-31-12-15-35(68)16-13-31)27-43(69)62-40(23-34-25-57-28-59-34)51(77)67-19-7-11-42(67)48(74)64-41(52(78)79)21-30-8-4-3-5-9-30/h3-5,8-9,12-17,22,25,28-29,36,38-42,45,61,68H,6-7,10-11,18-21,23-24,26-27,54H2,1-2H3,(H,57,59)(H,60,75)(H,62,69)(H,63,72)(H,64,74)(H,65,73)(H,70,71)(H,78,79)(H4,55,56,58)/t36-,38-,39-,40-,41-,42-,45-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from angiotensin II receptor type 2 in pig uterus myometrium |
J Med Chem 48: 4009-24 (2005)
Article DOI: 10.1021/jm0491492
BindingDB Entry DOI: 10.7270/Q2BR8SZD |
More data for this
Ligand-Target Pair | |
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50561892

(CHEMBL4795486)Show SMILES O[C@@H]1[C@@H](COP(O)(=O)CP(O)(O)=O)O[C@H]([C@@H]1O)n1cnc2c(NCc3ccc(cc3)C(=O)NCCCCCCNC(=O)c3ccc4C(=O)OC5(c4c3)c3ccc(O)cc3Oc3cc(O)ccc53)ncnc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CD73 in human MDA-MB-231 cells using [2,8-3H]-AMP as substrate incubated for 25 mins by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00391
BindingDB Entry DOI: 10.7270/Q2CF9TTM |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50168423

((3S)-3-amino-3-{[(1S)-1-{[(1S)-1-{[(2S)-4-({[(2S)-...)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](N)CC(O)=O)C(=O)Nc1cccc2CN(CC(=O)N[C@@H](Cc3cnc[nH]3)C(=O)N3CCC[C@H]3C(=O)N[C@@H](Cc3ccccc3)C(O)=O)C(=O)[C@H](Cc3ccc(O)cc3)Nc12 Show InChI InChI=1S/C50H61N11O12/c1-27(2)42(59-44(66)28(3)54-45(67)34(51)22-41(64)65)47(69)57-35-12-7-11-31-24-60(48(70)36(56-43(31)35)19-30-14-16-33(62)17-15-30)25-40(63)55-37(21-32-23-52-26-53-32)49(71)61-18-8-13-39(61)46(68)58-38(50(72)73)20-29-9-5-4-6-10-29/h4-7,9-12,14-17,23,26-28,34,36-39,42,56,62H,8,13,18-22,24-25,51H2,1-3H3,(H,52,53)(H,54,67)(H,55,63)(H,57,69)(H,58,68)(H,59,66)(H,64,65)(H,72,73)/t28-,34-,36-,37-,38-,39-,42-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from angiotensin II receptor type 2 in pig uterus myometrium |
J Med Chem 48: 4009-24 (2005)
Article DOI: 10.1021/jm0491492
BindingDB Entry DOI: 10.7270/Q2BR8SZD |
More data for this
Ligand-Target Pair | |
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50561893
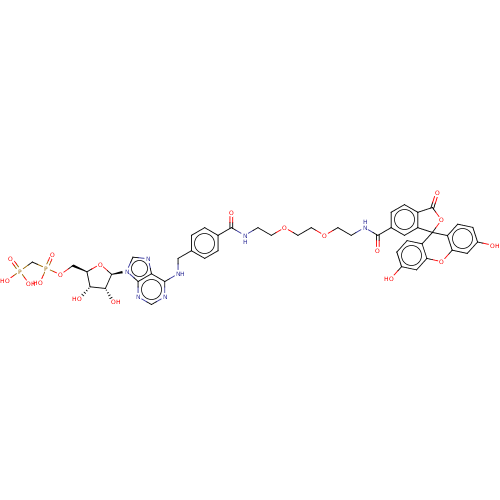
(CHEMBL4761798)Show SMILES O[C@@H]1[C@@H](COP(O)(=O)CP(O)(O)=O)O[C@H]([C@@H]1O)n1cnc2c(NCc3ccc(cc3)C(=O)NCCOCCOCCNC(=O)c3ccc4C(=O)OC5(c4c3)c3ccc(O)cc3Oc3cc(O)ccc53)ncnc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal His-tagged CD73 (27 to 549 residues) expressed in Sf9 cells using [2,8-3H]-AMP as substrate incubated for ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00391
BindingDB Entry DOI: 10.7270/Q2CF9TTM |
More data for this
Ligand-Target Pair | |
5'-nucleotidase
(Rattus norvegicus (Rat)) | BDBM50561892

(CHEMBL4795486)Show SMILES O[C@@H]1[C@@H](COP(O)(=O)CP(O)(O)=O)O[C@H]([C@@H]1O)n1cnc2c(NCc3ccc(cc3)C(=O)NCCCCCCNC(=O)c3ccc4C(=O)OC5(c4c3)c3ccc(O)cc3Oc3cc(O)ccc53)ncnc12 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant rat CD73 expressed in Sf9 cells using [2,8-3H]-AMP as substrate incubated for 25 mins by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00391
BindingDB Entry DOI: 10.7270/Q2CF9TTM |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50168425
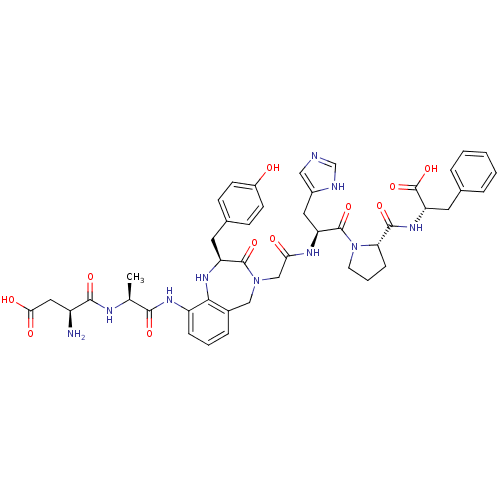
((3S)-3-amino-3-{[(1S)-1-{[(2S)-4-({[(2S)-1-[(2S)-2...)Show SMILES C[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)Nc1cccc2CN(CC(=O)N[C@@H](Cc3cnc[nH]3)C(=O)N3CCC[C@H]3C(=O)N[C@@H](Cc3ccccc3)C(O)=O)C(=O)[C@H](Cc3ccc(O)cc3)Nc12 Show InChI InChI=1S/C45H52N10O11/c1-25(49-41(61)31(46)20-38(58)59)40(60)52-32-10-5-9-28-22-54(43(63)33(51-39(28)32)17-27-12-14-30(56)15-13-27)23-37(57)50-34(19-29-21-47-24-48-29)44(64)55-16-6-11-36(55)42(62)53-35(45(65)66)18-26-7-3-2-4-8-26/h2-5,7-10,12-15,21,24-25,31,33-36,51,56H,6,11,16-20,22-23,46H2,1H3,(H,47,48)(H,49,61)(H,50,57)(H,52,60)(H,53,62)(H,58,59)(H,65,66)/t25-,31-,33-,34-,35-,36-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ang II from angiotensin II receptor type 2 in pig uterus myometrium |
J Med Chem 48: 4009-24 (2005)
Article DOI: 10.1021/jm0491492
BindingDB Entry DOI: 10.7270/Q2BR8SZD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM113220

(US8633206, 139)Show SMILES COCCOC1CCCN(C1)S(=O)(=O)C[C@H]1CC[C@@H](CC1)N(C)c1ncnc2[nH]ccc12 |r,wU:18.22,wD:15.15,(8,-2.69,;6.67,-1.92,;6.67,-.38,;5.33,.39,;5.33,1.93,;4,2.69,;4,4.23,;2.67,5,;1.33,4.23,;1.33,2.69,;2.67,1.93,;,1.93,;-.77,.59,;.77,.59,;-1.33,2.69,;-2.67,1.93,;-4,2.69,;-5.33,1.93,;-5.33,.38,;-4,-.38,;-2.67,.38,;-6.67,-.38,;-8,.38,;-6.67,-1.93,;-8,-2.69,;-8,-4.23,;-6.67,-5,;-5.33,-4.23,;-3.87,-4.71,;-2.96,-3.47,;-3.87,-2.22,;-5.33,-2.69,)| Show InChI InChI=1S/C22H35N5O4S/c1-26(22-20-9-10-23-21(20)24-16-25-22)18-7-5-17(6-8-18)15-32(28,29)27-11-3-4-19(14-27)31-13-12-30-2/h9-10,16-19H,3-8,11-15H2,1-2H3,(H,23,24,25)/t17-,18-,19? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.00125 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... |
US Patent US8633206 (2014)
BindingDB Entry DOI: 10.7270/Q2ZS2V44 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM113219
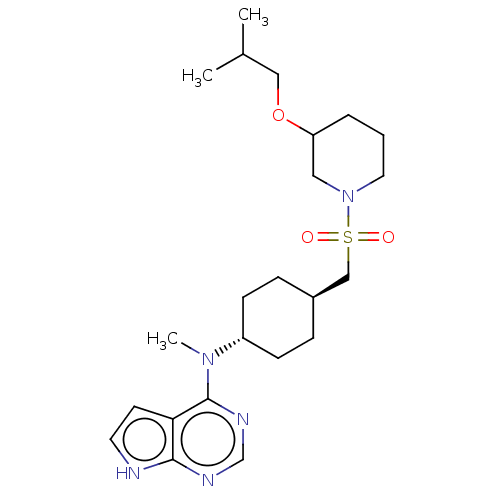
(US8633206, 138)Show SMILES CC(C)COC1CCCN(C1)S(=O)(=O)C[C@H]1CC[C@@H](CC1)N(C)c1ncnc2[nH]ccc12 |r,wU:18.22,wD:15.15,(7.27,-1.5,;6.87,-.01,;7.96,1.08,;5.38,.39,;5.38,1.93,;4.05,2.69,;4.05,4.23,;2.71,5,;1.38,4.23,;1.38,2.69,;2.71,1.93,;.05,1.93,;-.72,.59,;.82,.59,;-1.29,2.69,;-2.62,1.93,;-3.96,2.69,;-5.29,1.93,;-5.29,.38,;-3.96,-.38,;-2.62,.38,;-6.62,-.38,;-7.96,.38,;-6.62,-1.93,;-7.96,-2.69,;-7.96,-4.23,;-6.62,-5,;-5.29,-4.23,;-3.82,-4.71,;-2.92,-3.47,;-3.82,-2.22,;-5.29,-2.69,)| Show InChI InChI=1S/C23H37N5O3S/c1-17(2)14-31-20-5-4-12-28(13-20)32(29,30)15-18-6-8-19(9-7-18)27(3)23-21-10-11-24-22(21)25-16-26-23/h10-11,16-20H,4-9,12-15H2,1-3H3,(H,24,25,26)/t18-,19-,20? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.00133 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... |
US Patent US8633206 (2014)
BindingDB Entry DOI: 10.7270/Q2ZS2V44 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM113216
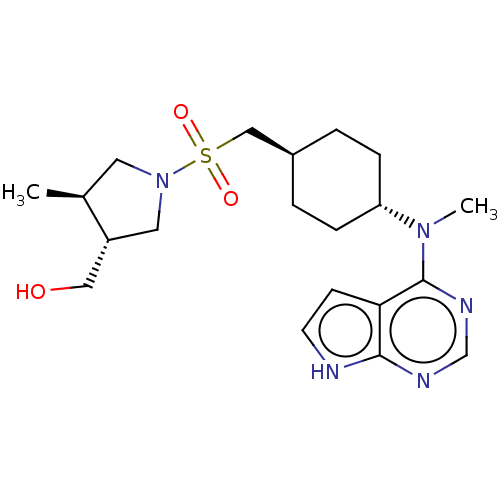
(US8633206, 135)Show SMILES C[C@H]1CN(C[C@@H]1CO)S(=O)(=O)C[C@H]1CC[C@@H](CC1)N(C)c1ncnc2[nH]ccc12 |r,wU:15.19,5.6,wD:12.12,1.0,(4.56,5.52,;3.79,4.19,;2.33,3.72,;2.33,2.18,;3.79,1.7,;4.7,2.95,;6.24,2.95,;7.01,1.61,;.99,1.41,;.22,.07,;1.76,.07,;-.34,2.18,;-1.67,1.41,;-3.01,2.18,;-4.34,1.41,;-4.34,-.13,;-3.01,-.9,;-1.67,-.13,;-5.67,-.9,;-7.01,-.13,;-5.67,-2.44,;-7.01,-3.21,;-7.01,-4.75,;-5.67,-5.52,;-4.34,-4.75,;-2.88,-5.23,;-1.97,-3.98,;-2.88,-2.74,;-4.34,-3.21,)| Show InChI InChI=1S/C20H31N5O3S/c1-14-9-25(10-16(14)11-26)29(27,28)12-15-3-5-17(6-4-15)24(2)20-18-7-8-21-19(18)22-13-23-20/h7-8,13-17,26H,3-6,9-12H2,1-2H3,(H,21,22,23)/t14-,15-,16+,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.00199 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... |
US Patent US8633206 (2014)
BindingDB Entry DOI: 10.7270/Q2ZS2V44 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM113220

(US8633206, 139)Show SMILES COCCOC1CCCN(C1)S(=O)(=O)C[C@H]1CC[C@@H](CC1)N(C)c1ncnc2[nH]ccc12 |r,wU:18.22,wD:15.15,(8,-2.69,;6.67,-1.92,;6.67,-.38,;5.33,.39,;5.33,1.93,;4,2.69,;4,4.23,;2.67,5,;1.33,4.23,;1.33,2.69,;2.67,1.93,;,1.93,;-.77,.59,;.77,.59,;-1.33,2.69,;-2.67,1.93,;-4,2.69,;-5.33,1.93,;-5.33,.38,;-4,-.38,;-2.67,.38,;-6.67,-.38,;-8,.38,;-6.67,-1.93,;-8,-2.69,;-8,-4.23,;-6.67,-5,;-5.33,-4.23,;-3.87,-4.71,;-2.96,-3.47,;-3.87,-2.22,;-5.33,-2.69,)| Show InChI InChI=1S/C22H35N5O4S/c1-26(22-20-9-10-23-21(20)24-16-25-22)18-7-5-17(6-8-18)15-32(28,29)27-11-3-4-19(14-27)31-13-12-30-2/h9-10,16-19H,3-8,11-15H2,1-2H3,(H,23,24,25)/t17-,18-,19? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.00405 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... |
US Patent US8633206 (2014)
BindingDB Entry DOI: 10.7270/Q2ZS2V44 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM113214
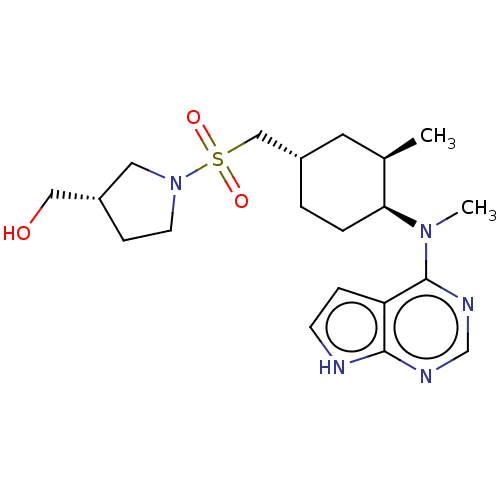
(US8633206, 133)Show SMILES C[C@@H]1C[C@@H](CS(=O)(=O)N2CC[C@H](CO)C2)CC[C@@H]1N(C)c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C20H33N5O3S/c1-14-9-15(12-29(27,28)25-8-6-16(10-25)11-26)3-4-18(14)24(2)20-17-5-7-21-19(17)22-13-23-20/h5,7,13-16,18,26,29H,3-4,6,8-12H2,1-2H3,(H,27,28)(H,21,22,23)/t14-,15+,16+,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.00508 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... |
US Patent US8633206 (2014)
BindingDB Entry DOI: 10.7270/Q2ZS2V44 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM113219

(US8633206, 138)Show SMILES CC(C)COC1CCCN(C1)S(=O)(=O)C[C@H]1CC[C@@H](CC1)N(C)c1ncnc2[nH]ccc12 |r,wU:18.22,wD:15.15,(7.27,-1.5,;6.87,-.01,;7.96,1.08,;5.38,.39,;5.38,1.93,;4.05,2.69,;4.05,4.23,;2.71,5,;1.38,4.23,;1.38,2.69,;2.71,1.93,;.05,1.93,;-.72,.59,;.82,.59,;-1.29,2.69,;-2.62,1.93,;-3.96,2.69,;-5.29,1.93,;-5.29,.38,;-3.96,-.38,;-2.62,.38,;-6.62,-.38,;-7.96,.38,;-6.62,-1.93,;-7.96,-2.69,;-7.96,-4.23,;-6.62,-5,;-5.29,-4.23,;-3.82,-4.71,;-2.92,-3.47,;-3.82,-2.22,;-5.29,-2.69,)| Show InChI InChI=1S/C23H37N5O3S/c1-17(2)14-31-20-5-4-12-28(13-20)32(29,30)15-18-6-8-19(9-7-18)27(3)23-21-10-11-24-22(21)25-16-26-23/h10-11,16-20H,4-9,12-15H2,1-3H3,(H,24,25,26)/t18-,19-,20? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.00767 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... |
US Patent US8633206 (2014)
BindingDB Entry DOI: 10.7270/Q2ZS2V44 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM113218

(US8633206, 137)Show SMILES CN([C@H]1CC[C@H](CS(=O)(=O)N2CCC(C)(O)C2)CC1)c1ncnc2[nH]ccc12 |r,wU:2.1,wD:5.5,(-6.62,.49,;-5.29,-.28,;-3.96,.49,;-3.96,2.03,;-2.62,2.8,;-1.29,2.03,;.05,2.8,;1.38,2.03,;.61,.69,;2.15,.69,;2.71,2.8,;2.71,4.34,;4.18,4.81,;5.08,3.57,;5.85,4.9,;6.62,3.57,;4.18,2.32,;-1.29,.49,;-2.62,-.28,;-5.29,-1.82,;-6.62,-2.59,;-6.62,-4.13,;-5.29,-4.9,;-3.96,-4.13,;-2.49,-4.61,;-1.59,-3.36,;-2.49,-2.12,;-3.96,-2.59,)| Show InChI InChI=1S/C19H29N5O3S/c1-19(25)8-10-24(12-19)28(26,27)11-14-3-5-15(6-4-14)23(2)18-16-7-9-20-17(16)21-13-22-18/h7,9,13-15,25H,3-6,8,10-12H2,1-2H3,(H,20,21,22)/t14-,15-,19? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.00777 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... |
US Patent US8633206 (2014)
BindingDB Entry DOI: 10.7270/Q2ZS2V44 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM113216

(US8633206, 135)Show SMILES C[C@H]1CN(C[C@@H]1CO)S(=O)(=O)C[C@H]1CC[C@@H](CC1)N(C)c1ncnc2[nH]ccc12 |r,wU:15.19,5.6,wD:12.12,1.0,(4.56,5.52,;3.79,4.19,;2.33,3.72,;2.33,2.18,;3.79,1.7,;4.7,2.95,;6.24,2.95,;7.01,1.61,;.99,1.41,;.22,.07,;1.76,.07,;-.34,2.18,;-1.67,1.41,;-3.01,2.18,;-4.34,1.41,;-4.34,-.13,;-3.01,-.9,;-1.67,-.13,;-5.67,-.9,;-7.01,-.13,;-5.67,-2.44,;-7.01,-3.21,;-7.01,-4.75,;-5.67,-5.52,;-4.34,-4.75,;-2.88,-5.23,;-1.97,-3.98,;-2.88,-2.74,;-4.34,-3.21,)| Show InChI InChI=1S/C20H31N5O3S/c1-14-9-25(10-16(14)11-26)29(27,28)12-15-3-5-17(6-4-15)24(2)20-18-7-8-21-19(18)22-13-23-20/h7-8,13-17,26H,3-6,9-12H2,1-2H3,(H,21,22,23)/t14-,15-,16+,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0165 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... |
US Patent US8633206 (2014)
BindingDB Entry DOI: 10.7270/Q2ZS2V44 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM113214

(US8633206, 133)Show SMILES C[C@@H]1C[C@@H](CS(=O)(=O)N2CC[C@H](CO)C2)CC[C@@H]1N(C)c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C20H33N5O3S/c1-14-9-15(12-29(27,28)25-8-6-16(10-25)11-26)3-4-18(14)24(2)20-17-5-7-21-19(17)22-13-23-20/h5,7,13-16,18,26,29H,3-4,6,8-12H2,1-2H3,(H,27,28)(H,21,22,23)/t14-,15+,16+,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0199 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... |
US Patent US8633206 (2014)
BindingDB Entry DOI: 10.7270/Q2ZS2V44 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM113215
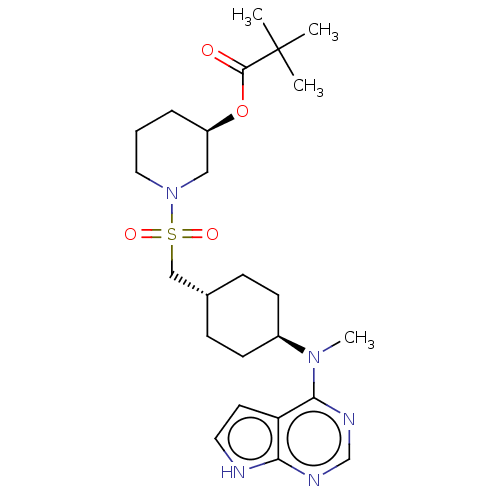
(US8633206, 134)Show SMILES CN([C@H]1CC[C@H](CS(=O)(=O)N2CCC[C@H](C2)OC(=O)C(C)(C)C)CC1)c1ncnc2[nH]ccc12 |r,wU:2.1,14.16,wD:5.5,(-8.67,.38,;-7.34,-.38,;-6,.38,;-6,1.93,;-4.67,2.69,;-3.33,1.93,;-2,2.69,;-.67,1.93,;-2,1.15,;-.67,.38,;.67,2.69,;.67,4.23,;2,5,;3.33,4.23,;3.33,2.69,;2,1.93,;4.67,1.93,;6,2.69,;6,4.23,;7.34,1.93,;8.67,2.69,;7.34,.38,;8.67,1.15,;-3.33,.38,;-4.67,-.38,;-7.34,-1.93,;-8.67,-2.69,;-8.67,-4.23,;-7.34,-5,;-6,-4.23,;-4.54,-4.71,;-3.63,-3.47,;-4.54,-2.22,;-6,-2.69,)| Show InChI InChI=1S/C24H37N5O4S/c1-24(2,3)23(30)33-19-6-5-13-29(14-19)34(31,32)15-17-7-9-18(10-8-17)28(4)22-20-11-12-25-21(20)26-16-27-22/h11-12,16-19H,5-10,13-15H2,1-4H3,(H,25,26,27)/t17-,18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0227 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... |
US Patent US8633206 (2014)
BindingDB Entry DOI: 10.7270/Q2ZS2V44 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM113221
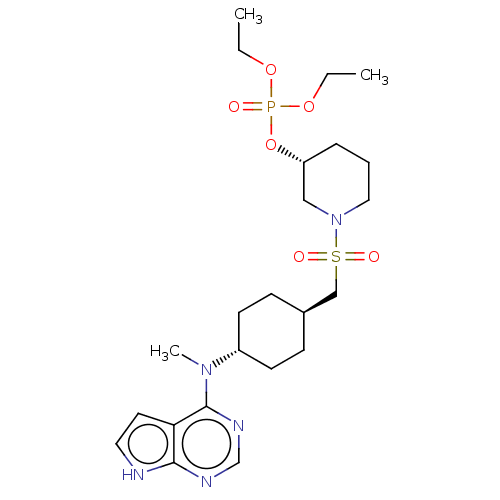
(US8633206, 140)Show SMILES CCOP(=O)(OCC)O[C@@H]1CCCN(C1)S(=O)(=O)C[C@H]1CC[C@@H](CC1)N(C)c1ncnc2[nH]ccc12 |r,wU:22.26,9.8,wD:19.19,(9.44,2.7,;8.11,1.93,;6.77,2.7,;5.23,2.7,;5.23,4.24,;6,1.36,;7.34,.59,;8.67,1.36,;3.9,1.93,;2.56,2.69,;2.56,4.23,;1.23,5,;-.1,4.23,;-.1,2.69,;1.23,1.93,;-1.44,1.93,;-2.21,.59,;-.67,.59,;-2.77,2.69,;-4.1,1.93,;-5.44,2.69,;-6.77,1.93,;-6.77,.38,;-5.44,-.38,;-4.1,.38,;-8.11,-.38,;-9.44,.38,;-8.11,-1.93,;-9.44,-2.69,;-9.44,-4.23,;-8.11,-5,;-6.77,-4.23,;-5.31,-4.71,;-4.4,-3.47,;-5.31,-2.22,;-6.77,-2.69,)| Show InChI InChI=1S/C23H38N5O6PS/c1-4-32-35(29,33-5-2)34-20-7-6-14-28(15-20)36(30,31)16-18-8-10-19(11-9-18)27(3)23-21-12-13-24-22(21)25-17-26-23/h12-13,17-20H,4-11,14-16H2,1-3H3,(H,24,25,26)/t18-,19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0235 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... |
US Patent US8633206 (2014)
BindingDB Entry DOI: 10.7270/Q2ZS2V44 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM113217
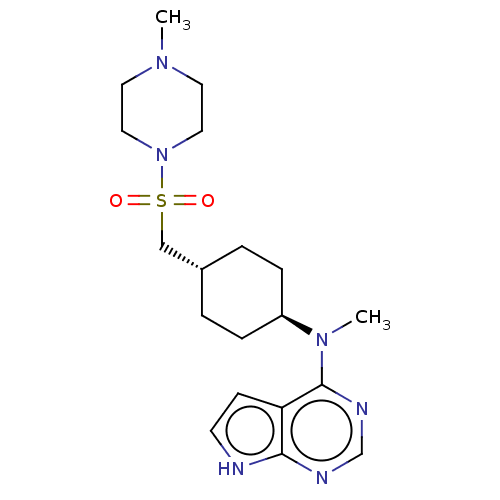
(US8633206, 136)Show SMILES CN([C@H]1CC[C@H](CS(=O)(=O)N2CCN(C)CC2)CC1)c1ncnc2[nH]ccc12 |r,wU:2.1,wD:5.5,(-6.67,.38,;-5.33,-.38,;-4,.38,;-4,1.93,;-2.67,2.69,;-1.33,1.93,;,2.69,;1.33,1.93,;.56,.59,;2.1,.59,;2.67,2.69,;4,1.93,;5.33,2.69,;5.33,4.23,;6.67,5,;4,5,;2.67,4.23,;-1.33,.38,;-2.67,-.38,;-5.33,-1.93,;-6.67,-2.69,;-6.67,-4.23,;-5.33,-5,;-4,-4.23,;-2.54,-4.71,;-1.63,-3.47,;-2.54,-2.22,;-4,-2.69,)| Show InChI InChI=1S/C19H30N6O2S/c1-23-9-11-25(12-10-23)28(26,27)13-15-3-5-16(6-4-15)24(2)19-17-7-8-20-18(17)21-14-22-19/h7-8,14-16H,3-6,9-13H2,1-2H3,(H,20,21,22)/t15-,16- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0245 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... |
US Patent US8633206 (2014)
BindingDB Entry DOI: 10.7270/Q2ZS2V44 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM113222
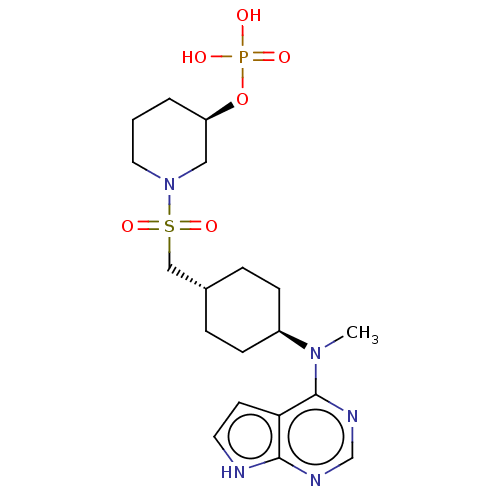
(US8633206, 141)Show SMILES CN([C@H]1CC[C@H](CS(=O)(=O)N2CCC[C@H](C2)OP(O)(O)=O)CC1)c1ncnc2[nH]ccc12 |r,wU:2.1,14.16,wD:5.5,(-8.11,.38,;-6.77,-.38,;-5.44,.38,;-5.44,1.93,;-4.1,2.69,;-2.77,1.93,;-1.44,2.69,;-.1,1.93,;-.87,.59,;.67,.59,;1.23,2.69,;1.23,4.23,;2.56,5,;3.9,4.23,;3.9,2.69,;2.56,1.93,;5.23,1.93,;6.57,2.7,;8.11,2.7,;7.34,1.36,;6.57,4.24,;-2.77,.38,;-4.1,-.38,;-6.77,-1.93,;-8.11,-2.69,;-8.11,-4.23,;-6.77,-5,;-5.44,-4.23,;-3.97,-4.71,;-3.07,-3.47,;-3.97,-2.22,;-5.44,-2.69,)| Show InChI InChI=1S/C19H30N5O6PS/c1-23(19-17-8-9-20-18(17)21-13-22-19)15-6-4-14(5-7-15)12-32(28,29)24-10-2-3-16(11-24)30-31(25,26)27/h8-9,13-16H,2-7,10-12H2,1H3,(H,20,21,22)(H2,25,26,27)/t14-,15-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0263 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... |
US Patent US8633206 (2014)
BindingDB Entry DOI: 10.7270/Q2ZS2V44 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM113218

(US8633206, 137)Show SMILES CN([C@H]1CC[C@H](CS(=O)(=O)N2CCC(C)(O)C2)CC1)c1ncnc2[nH]ccc12 |r,wU:2.1,wD:5.5,(-6.62,.49,;-5.29,-.28,;-3.96,.49,;-3.96,2.03,;-2.62,2.8,;-1.29,2.03,;.05,2.8,;1.38,2.03,;.61,.69,;2.15,.69,;2.71,2.8,;2.71,4.34,;4.18,4.81,;5.08,3.57,;5.85,4.9,;6.62,3.57,;4.18,2.32,;-1.29,.49,;-2.62,-.28,;-5.29,-1.82,;-6.62,-2.59,;-6.62,-4.13,;-5.29,-4.9,;-3.96,-4.13,;-2.49,-4.61,;-1.59,-3.36,;-2.49,-2.12,;-3.96,-2.59,)| Show InChI InChI=1S/C19H29N5O3S/c1-19(25)8-10-24(12-19)28(26,27)11-14-3-5-15(6-4-14)23(2)18-16-7-9-20-17(16)21-13-22-18/h7,9,13-15,25H,3-6,8,10-12H2,1-2H3,(H,20,21,22)/t14-,15-,19? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0366 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... |
US Patent US8633206 (2014)
BindingDB Entry DOI: 10.7270/Q2ZS2V44 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM113220

(US8633206, 139)Show SMILES COCCOC1CCCN(C1)S(=O)(=O)C[C@H]1CC[C@@H](CC1)N(C)c1ncnc2[nH]ccc12 |r,wU:18.22,wD:15.15,(8,-2.69,;6.67,-1.92,;6.67,-.38,;5.33,.39,;5.33,1.93,;4,2.69,;4,4.23,;2.67,5,;1.33,4.23,;1.33,2.69,;2.67,1.93,;,1.93,;-.77,.59,;.77,.59,;-1.33,2.69,;-2.67,1.93,;-4,2.69,;-5.33,1.93,;-5.33,.38,;-4,-.38,;-2.67,.38,;-6.67,-.38,;-8,.38,;-6.67,-1.93,;-8,-2.69,;-8,-4.23,;-6.67,-5,;-5.33,-4.23,;-3.87,-4.71,;-2.96,-3.47,;-3.87,-2.22,;-5.33,-2.69,)| Show InChI InChI=1S/C22H35N5O4S/c1-26(22-20-9-10-23-21(20)24-16-25-22)18-7-5-17(6-8-18)15-32(28,29)27-11-3-4-19(14-27)31-13-12-30-2/h9-10,16-19H,3-8,11-15H2,1-2H3,(H,23,24,25)/t17-,18-,19? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0368 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... |
US Patent US8633206 (2014)
BindingDB Entry DOI: 10.7270/Q2ZS2V44 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM113214

(US8633206, 133)Show SMILES C[C@@H]1C[C@@H](CS(=O)(=O)N2CC[C@H](CO)C2)CC[C@@H]1N(C)c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C20H33N5O3S/c1-14-9-15(12-29(27,28)25-8-6-16(10-25)11-26)3-4-18(14)24(2)20-17-5-7-21-19(17)22-13-23-20/h5,7,13-16,18,26,29H,3-4,6,8-12H2,1-2H3,(H,27,28)(H,21,22,23)/t14-,15+,16+,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0421 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... |
US Patent US8633206 (2014)
BindingDB Entry DOI: 10.7270/Q2ZS2V44 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM113222

(US8633206, 141)Show SMILES CN([C@H]1CC[C@H](CS(=O)(=O)N2CCC[C@H](C2)OP(O)(O)=O)CC1)c1ncnc2[nH]ccc12 |r,wU:2.1,14.16,wD:5.5,(-8.11,.38,;-6.77,-.38,;-5.44,.38,;-5.44,1.93,;-4.1,2.69,;-2.77,1.93,;-1.44,2.69,;-.1,1.93,;-.87,.59,;.67,.59,;1.23,2.69,;1.23,4.23,;2.56,5,;3.9,4.23,;3.9,2.69,;2.56,1.93,;5.23,1.93,;6.57,2.7,;8.11,2.7,;7.34,1.36,;6.57,4.24,;-2.77,.38,;-4.1,-.38,;-6.77,-1.93,;-8.11,-2.69,;-8.11,-4.23,;-6.77,-5,;-5.44,-4.23,;-3.97,-4.71,;-3.07,-3.47,;-3.97,-2.22,;-5.44,-2.69,)| Show InChI InChI=1S/C19H30N5O6PS/c1-23(19-17-8-9-20-18(17)21-13-22-19)15-6-4-14(5-7-15)12-32(28,29)24-10-2-3-16(11-24)30-31(25,26)27/h8-9,13-16H,2-7,10-12H2,1H3,(H,20,21,22)(H2,25,26,27)/t14-,15-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.106 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... |
US Patent US8633206 (2014)
BindingDB Entry DOI: 10.7270/Q2ZS2V44 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM113217

(US8633206, 136)Show SMILES CN([C@H]1CC[C@H](CS(=O)(=O)N2CCN(C)CC2)CC1)c1ncnc2[nH]ccc12 |r,wU:2.1,wD:5.5,(-6.67,.38,;-5.33,-.38,;-4,.38,;-4,1.93,;-2.67,2.69,;-1.33,1.93,;,2.69,;1.33,1.93,;.56,.59,;2.1,.59,;2.67,2.69,;4,1.93,;5.33,2.69,;5.33,4.23,;6.67,5,;4,5,;2.67,4.23,;-1.33,.38,;-2.67,-.38,;-5.33,-1.93,;-6.67,-2.69,;-6.67,-4.23,;-5.33,-5,;-4,-4.23,;-2.54,-4.71,;-1.63,-3.47,;-2.54,-2.22,;-4,-2.69,)| Show InChI InChI=1S/C19H30N6O2S/c1-23-9-11-25(12-10-23)28(26,27)13-15-3-5-16(6-4-15)24(2)19-17-7-8-20-18(17)21-14-22-19/h7-8,14-16H,3-6,9-13H2,1-2H3,(H,20,21,22)/t15-,16- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.109 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... |
US Patent US8633206 (2014)
BindingDB Entry DOI: 10.7270/Q2ZS2V44 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM113221

(US8633206, 140)Show SMILES CCOP(=O)(OCC)O[C@@H]1CCCN(C1)S(=O)(=O)C[C@H]1CC[C@@H](CC1)N(C)c1ncnc2[nH]ccc12 |r,wU:22.26,9.8,wD:19.19,(9.44,2.7,;8.11,1.93,;6.77,2.7,;5.23,2.7,;5.23,4.24,;6,1.36,;7.34,.59,;8.67,1.36,;3.9,1.93,;2.56,2.69,;2.56,4.23,;1.23,5,;-.1,4.23,;-.1,2.69,;1.23,1.93,;-1.44,1.93,;-2.21,.59,;-.67,.59,;-2.77,2.69,;-4.1,1.93,;-5.44,2.69,;-6.77,1.93,;-6.77,.38,;-5.44,-.38,;-4.1,.38,;-8.11,-.38,;-9.44,.38,;-8.11,-1.93,;-9.44,-2.69,;-9.44,-4.23,;-8.11,-5,;-6.77,-4.23,;-5.31,-4.71,;-4.4,-3.47,;-5.31,-2.22,;-6.77,-2.69,)| Show InChI InChI=1S/C23H38N5O6PS/c1-4-32-35(29,33-5-2)34-20-7-6-14-28(15-20)36(30,31)16-18-8-10-19(11-9-18)27(3)23-21-12-13-24-22(21)25-17-26-23/h12-13,17-20H,4-11,14-16H2,1-3H3,(H,24,25,26)/t18-,19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.116 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... |
US Patent US8633206 (2014)
BindingDB Entry DOI: 10.7270/Q2ZS2V44 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM113215

(US8633206, 134)Show SMILES CN([C@H]1CC[C@H](CS(=O)(=O)N2CCC[C@H](C2)OC(=O)C(C)(C)C)CC1)c1ncnc2[nH]ccc12 |r,wU:2.1,14.16,wD:5.5,(-8.67,.38,;-7.34,-.38,;-6,.38,;-6,1.93,;-4.67,2.69,;-3.33,1.93,;-2,2.69,;-.67,1.93,;-2,1.15,;-.67,.38,;.67,2.69,;.67,4.23,;2,5,;3.33,4.23,;3.33,2.69,;2,1.93,;4.67,1.93,;6,2.69,;6,4.23,;7.34,1.93,;8.67,2.69,;7.34,.38,;8.67,1.15,;-3.33,.38,;-4.67,-.38,;-7.34,-1.93,;-8.67,-2.69,;-8.67,-4.23,;-7.34,-5,;-6,-4.23,;-4.54,-4.71,;-3.63,-3.47,;-4.54,-2.22,;-6,-2.69,)| Show InChI InChI=1S/C24H37N5O4S/c1-24(2,3)23(30)33-19-6-5-13-29(14-19)34(31,32)15-17-7-9-18(10-8-17)28(4)22-20-11-12-25-21(20)26-16-27-22/h11-12,16-19H,5-10,13-15H2,1-4H3,(H,25,26,27)/t17-,18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.129 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... |
US Patent US8633206 (2014)
BindingDB Entry DOI: 10.7270/Q2ZS2V44 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM113219

(US8633206, 138)Show SMILES CC(C)COC1CCCN(C1)S(=O)(=O)C[C@H]1CC[C@@H](CC1)N(C)c1ncnc2[nH]ccc12 |r,wU:18.22,wD:15.15,(7.27,-1.5,;6.87,-.01,;7.96,1.08,;5.38,.39,;5.38,1.93,;4.05,2.69,;4.05,4.23,;2.71,5,;1.38,4.23,;1.38,2.69,;2.71,1.93,;.05,1.93,;-.72,.59,;.82,.59,;-1.29,2.69,;-2.62,1.93,;-3.96,2.69,;-5.29,1.93,;-5.29,.38,;-3.96,-.38,;-2.62,.38,;-6.62,-.38,;-7.96,.38,;-6.62,-1.93,;-7.96,-2.69,;-7.96,-4.23,;-6.62,-5,;-5.29,-4.23,;-3.82,-4.71,;-2.92,-3.47,;-3.82,-2.22,;-5.29,-2.69,)| Show InChI InChI=1S/C23H37N5O3S/c1-17(2)14-31-20-5-4-12-28(13-20)32(29,30)15-18-6-8-19(9-7-18)27(3)23-21-10-11-24-22(21)25-16-26-23/h10-11,16-20H,4-9,12-15H2,1-3H3,(H,24,25,26)/t18-,19-,20? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.132 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... |
US Patent US8633206 (2014)
BindingDB Entry DOI: 10.7270/Q2ZS2V44 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM113216

(US8633206, 135)Show SMILES C[C@H]1CN(C[C@@H]1CO)S(=O)(=O)C[C@H]1CC[C@@H](CC1)N(C)c1ncnc2[nH]ccc12 |r,wU:15.19,5.6,wD:12.12,1.0,(4.56,5.52,;3.79,4.19,;2.33,3.72,;2.33,2.18,;3.79,1.7,;4.7,2.95,;6.24,2.95,;7.01,1.61,;.99,1.41,;.22,.07,;1.76,.07,;-.34,2.18,;-1.67,1.41,;-3.01,2.18,;-4.34,1.41,;-4.34,-.13,;-3.01,-.9,;-1.67,-.13,;-5.67,-.9,;-7.01,-.13,;-5.67,-2.44,;-7.01,-3.21,;-7.01,-4.75,;-5.67,-5.52,;-4.34,-4.75,;-2.88,-5.23,;-1.97,-3.98,;-2.88,-2.74,;-4.34,-3.21,)| Show InChI InChI=1S/C20H31N5O3S/c1-14-9-25(10-16(14)11-26)29(27,28)12-15-3-5-17(6-4-15)24(2)20-18-7-8-21-19(18)22-13-23-20/h7-8,13-17,26H,3-6,9-12H2,1-2H3,(H,21,22,23)/t14-,15-,16+,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.198 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... |
US Patent US8633206 (2014)
BindingDB Entry DOI: 10.7270/Q2ZS2V44 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM113218

(US8633206, 137)Show SMILES CN([C@H]1CC[C@H](CS(=O)(=O)N2CCC(C)(O)C2)CC1)c1ncnc2[nH]ccc12 |r,wU:2.1,wD:5.5,(-6.62,.49,;-5.29,-.28,;-3.96,.49,;-3.96,2.03,;-2.62,2.8,;-1.29,2.03,;.05,2.8,;1.38,2.03,;.61,.69,;2.15,.69,;2.71,2.8,;2.71,4.34,;4.18,4.81,;5.08,3.57,;5.85,4.9,;6.62,3.57,;4.18,2.32,;-1.29,.49,;-2.62,-.28,;-5.29,-1.82,;-6.62,-2.59,;-6.62,-4.13,;-5.29,-4.9,;-3.96,-4.13,;-2.49,-4.61,;-1.59,-3.36,;-2.49,-2.12,;-3.96,-2.59,)| Show InChI InChI=1S/C19H29N5O3S/c1-19(25)8-10-24(12-19)28(26,27)11-14-3-5-15(6-4-14)23(2)18-16-7-9-20-17(16)21-13-22-18/h7,9,13-15,25H,3-6,8,10-12H2,1-2H3,(H,20,21,22)/t14-,15-,19? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.302 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... |
US Patent US8633206 (2014)
BindingDB Entry DOI: 10.7270/Q2ZS2V44 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50336163
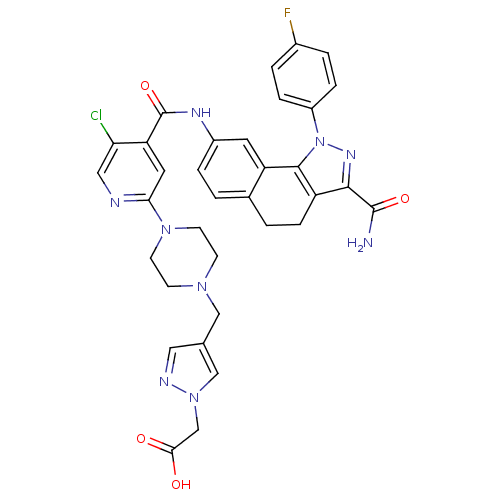
(8-[(5-Chloro-2-{4-[(1-methyl-1H-pyrazol-4-yl)methy...)Show SMILES NC(=O)c1nn(c-2c1CCc1ccc(NC(=O)c3cc(ncc3Cl)N3CCN(Cc4cnn(CC(O)=O)c4)CC3)cc-21)-c1ccc(F)cc1 Show InChI InChI=1S/C34H31ClFN9O4/c35-28-16-38-29(43-11-9-42(10-12-43)17-20-15-39-44(18-20)19-30(46)47)14-27(28)34(49)40-23-5-1-21-2-8-25-31(33(37)48)41-45(32(25)26(21)13-23)24-6-3-22(36)4-7-24/h1,3-7,13-16,18H,2,8-12,17,19H2,(H2,37,48)(H,40,49)(H,46,47) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IKK2 in human PBMC |
Bioorg Med Chem 19: 1242-55 (2011)
Article DOI: 10.1016/j.bmc.2010.12.027
BindingDB Entry DOI: 10.7270/Q2F76CTN |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50336181
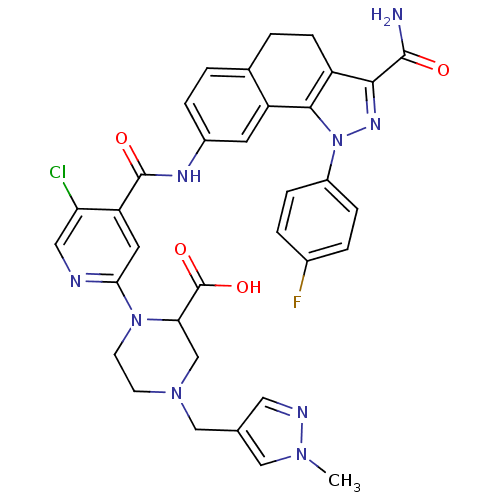
(1-(4-{[3-Carbamoyl-1-(4-fluorophenyl)-4,5-dihydro-...)Show SMILES Cn1cc(CN2CCN(C(C2)C(O)=O)c2cc(C(=O)Nc3ccc4CCc5c(nn(c5-c4c3)-c3ccc(F)cc3)C(N)=O)c(Cl)cn2)cn1 Show InChI InChI=1S/C34H31ClFN9O4/c1-42-16-19(14-39-42)17-43-10-11-44(28(18-43)34(48)49)29-13-26(27(35)15-38-29)33(47)40-22-6-2-20-3-9-24-30(32(37)46)41-45(31(24)25(20)12-22)23-7-4-21(36)5-8-23/h2,4-8,12-16,28H,3,9-11,17-18H2,1H3,(H2,37,46)(H,40,47)(H,48,49) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IKK2 in human PBMC |
Bioorg Med Chem 19: 1242-55 (2011)
Article DOI: 10.1016/j.bmc.2010.12.027
BindingDB Entry DOI: 10.7270/Q2F76CTN |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
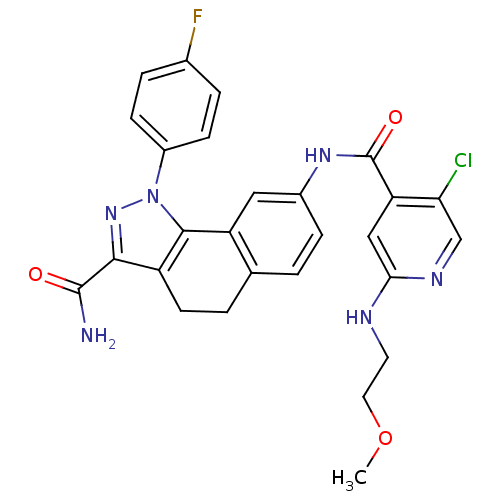
(Homo sapiens (Human)) | BDBM50336173
(8-(5-chloro-2-(2-methoxyethylamino)isonicotinamido...)Show SMILES COCCNc1cc(C(=O)Nc2ccc3CCc4c(nn(c4-c3c2)-c2ccc(F)cc2)C(N)=O)c(Cl)cn1 Show InChI InChI=1S/C27H24ClFN6O3/c1-38-11-10-31-23-13-21(22(28)14-32-23)27(37)33-17-6-2-15-3-9-19-24(26(30)36)34-35(25(19)20(15)12-17)18-7-4-16(29)5-8-18/h2,4-8,12-14H,3,9-11H2,1H3,(H2,30,36)(H,31,32)(H,33,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IKK2 in human PBMC |
Bioorg Med Chem 19: 1242-55 (2011)
Article DOI: 10.1016/j.bmc.2010.12.027
BindingDB Entry DOI: 10.7270/Q2F76CTN |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
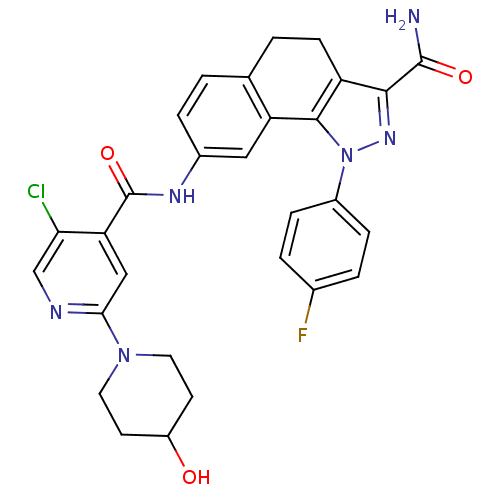
(Homo sapiens (Human)) | BDBM50336172
(8-(5-chloro-2-(4-hydroxypiperidin-1-yl)isonicotina...)Show SMILES NC(=O)c1nn(c-2c1CCc1ccc(NC(=O)c3cc(ncc3Cl)N3CCC(O)CC3)cc-21)-c1ccc(F)cc1 Show InChI InChI=1S/C29H26ClFN6O3/c30-24-15-33-25(36-11-9-20(38)10-12-36)14-23(24)29(40)34-18-5-1-16-2-8-21-26(28(32)39)35-37(27(21)22(16)13-18)19-6-3-17(31)4-7-19/h1,3-7,13-15,20,38H,2,8-12H2,(H2,32,39)(H,34,40) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IKK2 in human PBMC |
Bioorg Med Chem 19: 1242-55 (2011)
Article DOI: 10.1016/j.bmc.2010.12.027
BindingDB Entry DOI: 10.7270/Q2F76CTN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM113222

(US8633206, 141)Show SMILES CN([C@H]1CC[C@H](CS(=O)(=O)N2CCC[C@H](C2)OP(O)(O)=O)CC1)c1ncnc2[nH]ccc12 |r,wU:2.1,14.16,wD:5.5,(-8.11,.38,;-6.77,-.38,;-5.44,.38,;-5.44,1.93,;-4.1,2.69,;-2.77,1.93,;-1.44,2.69,;-.1,1.93,;-.87,.59,;.67,.59,;1.23,2.69,;1.23,4.23,;2.56,5,;3.9,4.23,;3.9,2.69,;2.56,1.93,;5.23,1.93,;6.57,2.7,;8.11,2.7,;7.34,1.36,;6.57,4.24,;-2.77,.38,;-4.1,-.38,;-6.77,-1.93,;-8.11,-2.69,;-8.11,-4.23,;-6.77,-5,;-5.44,-4.23,;-3.97,-4.71,;-3.07,-3.47,;-3.97,-2.22,;-5.44,-2.69,)| Show InChI InChI=1S/C19H30N5O6PS/c1-23(19-17-8-9-20-18(17)21-13-22-19)15-6-4-14(5-7-15)12-32(28,29)24-10-2-3-16(11-24)30-31(25,26)27/h8-9,13-16H,2-7,10-12H2,1H3,(H,20,21,22)(H2,25,26,27)/t14-,15-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.768 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... |
US Patent US8633206 (2014)
BindingDB Entry DOI: 10.7270/Q2ZS2V44 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
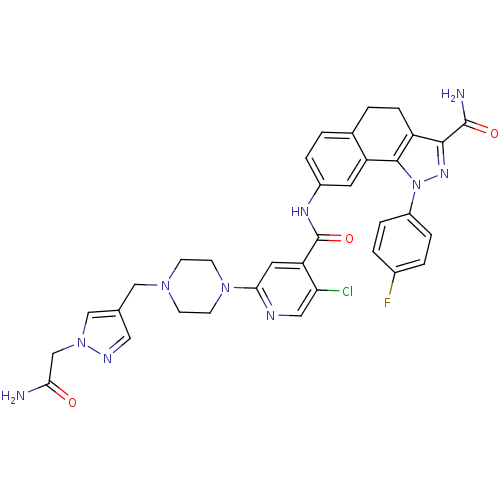
(Homo sapiens (Human)) | BDBM50336193
(8-{[2-(4-{[1-(2-Amino-2-oxoethyl)-1H-pyrazol-4-yl]...)Show SMILES NC(=O)Cn1cc(CN2CCN(CC2)c2cc(C(=O)Nc3ccc4CCc5c(nn(c5-c4c3)-c3ccc(F)cc3)C(N)=O)c(Cl)cn2)cn1 Show InChI InChI=1S/C34H32ClFN10O3/c35-28-16-39-30(44-11-9-43(10-12-44)17-20-15-40-45(18-20)19-29(37)47)14-27(28)34(49)41-23-5-1-21-2-8-25-31(33(38)48)42-46(32(25)26(21)13-23)24-6-3-22(36)4-7-24/h1,3-7,13-16,18H,2,8-12,17,19H2,(H2,37,47)(H2,38,48)(H,41,49) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IKK2 in human PBMC |
Bioorg Med Chem 19: 1242-55 (2011)
Article DOI: 10.1016/j.bmc.2010.12.027
BindingDB Entry DOI: 10.7270/Q2F76CTN |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50336176
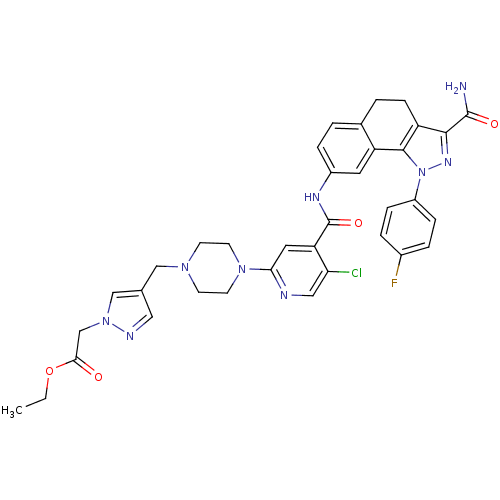
(CHEMBL1669565 | Ethyl [4-({4-[4-({[3-(aminocarbony...)Show SMILES CCOC(=O)Cn1cc(CN2CCN(CC2)c2cc(C(=O)Nc3ccc4CCc5c(nn(c5-c4c3)-c3ccc(F)cc3)C(N)=O)c(Cl)cn2)cn1 Show InChI InChI=1S/C36H35ClFN9O4/c1-2-51-32(48)21-46-20-22(17-41-46)19-44-11-13-45(14-12-44)31-16-29(30(37)18-40-31)36(50)42-25-7-3-23-4-10-27-33(35(39)49)43-47(34(27)28(23)15-25)26-8-5-24(38)6-9-26/h3,5-9,15-18,20H,2,4,10-14,19,21H2,1H3,(H2,39,49)(H,42,50) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IKK2 in human PBMC |
Bioorg Med Chem 19: 1242-55 (2011)
Article DOI: 10.1016/j.bmc.2010.12.027
BindingDB Entry DOI: 10.7270/Q2F76CTN |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50336199
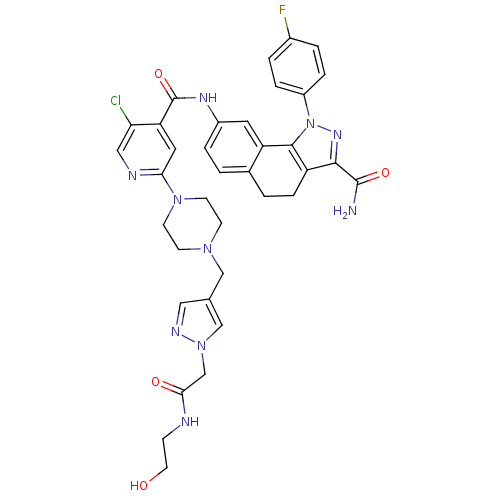
(8-[(5-Chloro-2-{4-[(1-{2-[(2-hydroxyethyl)amino]-2...)Show SMILES NC(=O)c1nn(c-2c1CCc1ccc(NC(=O)c3cc(ncc3Cl)N3CCN(Cc4cnn(CC(=O)NCCO)c4)CC3)cc-21)-c1ccc(F)cc1 Show InChI InChI=1S/C36H36ClFN10O4/c37-30-18-41-31(46-12-10-45(11-13-46)19-22-17-42-47(20-22)21-32(50)40-9-14-49)16-29(30)36(52)43-25-5-1-23-2-8-27-33(35(39)51)44-48(34(27)28(23)15-25)26-6-3-24(38)4-7-26/h1,3-7,15-18,20,49H,2,8-14,19,21H2,(H2,39,51)(H,40,50)(H,43,52) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IKK2 in human PBMC |
Bioorg Med Chem 19: 1242-55 (2011)
Article DOI: 10.1016/j.bmc.2010.12.027
BindingDB Entry DOI: 10.7270/Q2F76CTN |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50336190
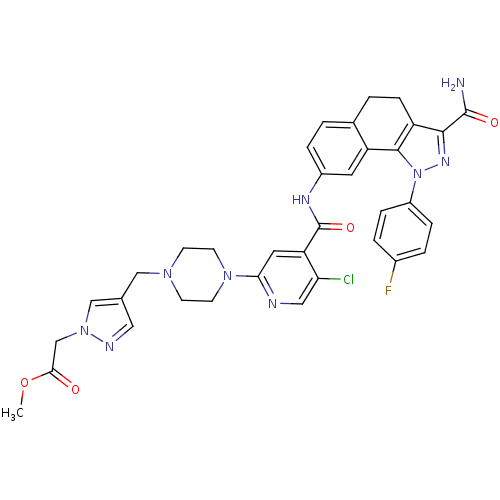
(CHEMBL1669596 | Methyl [4-({4-[4-({[3-(aminocarbon...)Show SMILES COC(=O)Cn1cc(CN2CCN(CC2)c2cc(C(=O)Nc3ccc4CCc5c(nn(c5-c4c3)-c3ccc(F)cc3)C(N)=O)c(Cl)cn2)cn1 Show InChI InChI=1S/C35H33ClFN9O4/c1-50-31(47)20-45-19-21(16-40-45)18-43-10-12-44(13-11-43)30-15-28(29(36)17-39-30)35(49)41-24-6-2-22-3-9-26-32(34(38)48)42-46(33(26)27(22)14-24)25-7-4-23(37)5-8-25/h2,4-8,14-17,19H,3,9-13,18,20H2,1H3,(H2,38,48)(H,41,49) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IKK2 in human PBMC |
Bioorg Med Chem 19: 1242-55 (2011)
Article DOI: 10.1016/j.bmc.2010.12.027
BindingDB Entry DOI: 10.7270/Q2F76CTN |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50336170
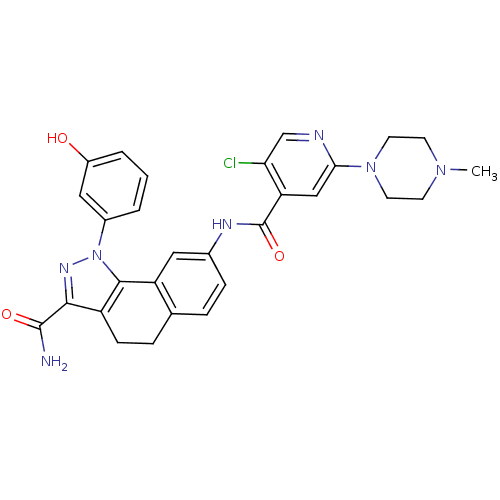
(8-(5-chloro-2-(4-methylpiperazin-1-yl)isonicotinam...)Show SMILES CN1CCN(CC1)c1cc(C(=O)Nc2ccc3CCc4c(nn(c4-c3c2)-c2cccc(O)c2)C(N)=O)c(Cl)cn1 Show InChI InChI=1S/C29H28ClN7O3/c1-35-9-11-36(12-10-35)25-15-23(24(30)16-32-25)29(40)33-18-7-5-17-6-8-21-26(28(31)39)34-37(27(21)22(17)13-18)19-3-2-4-20(38)14-19/h2-5,7,13-16,38H,6,8-12H2,1H3,(H2,31,39)(H,33,40) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IKK2 in human PBMC |
Bioorg Med Chem 19: 1242-55 (2011)
Article DOI: 10.1016/j.bmc.2010.12.027
BindingDB Entry DOI: 10.7270/Q2F76CTN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM113085
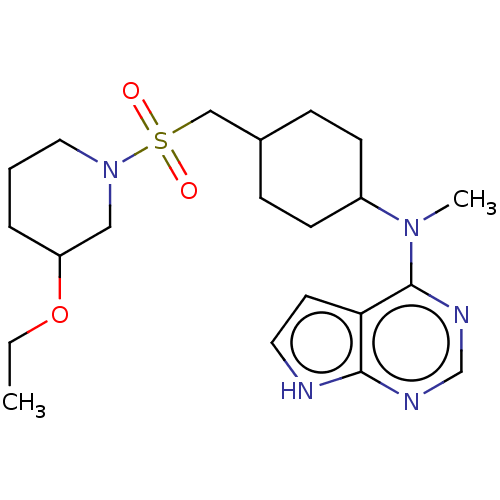
(US8633206, 3)Show SMILES CCOC1CCCN(C1)S(=O)(=O)CC1CCC(CC1)N(C)c1ncnc2[nH]ccc12 |(7.34,-.38,;6,.39,;6,1.93,;4.67,2.7,;4.67,4.24,;3.33,5.01,;2,4.24,;2,2.7,;3.33,1.93,;.67,1.93,;-.1,.59,;1.44,.59,;-.67,2.7,;-2,1.92,;-2,.38,;-3.33,-.38,;-4.67,.38,;-4.67,1.92,;-3.33,2.69,;-6,-.39,;-7.34,.38,;-6,-1.93,;-7.34,-2.7,;-7.34,-4.24,;-6,-5.01,;-4.67,-4.24,;-3.2,-4.71,;-2.3,-3.47,;-3.2,-2.22,;-4.67,-2.7,)| Show InChI InChI=1S/C21H33N5O3S/c1-3-29-18-5-4-12-26(13-18)30(27,28)14-16-6-8-17(9-7-16)25(2)21-19-10-11-22-20(19)23-15-24-21/h10-11,15-18H,3-9,12-14H2,1-2H3,(H,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Inc.
US Patent
| Assay Description
A peptide mobility shift assay was used to quantify the phosphorylation of the JAKtide (JAK2 and JAK3) or the IRS-1 peptide (JAK1 and Tyk2). Reaction... |
US Patent US8633206 (2014)
BindingDB Entry DOI: 10.7270/Q2ZS2V44 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data



















































