

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50144204 (CHEMBL63989 | [4-(2-Chloro-4,6-dimethoxy-phenyl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc Curated by ChEMBL | Assay Description Binding affinity towards human Corticotropin releasing factor receptor 1 by the displacement of [125I]-CRF from CHO cells | Bioorg Med Chem Lett 14: 2083-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.053 BindingDB Entry DOI: 10.7270/Q2JH3KKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50144191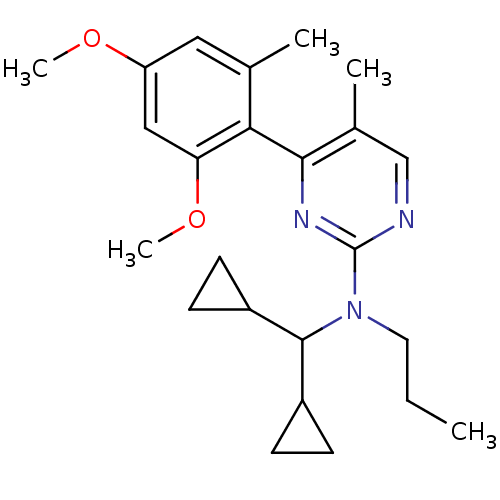 (CHEMBL61954 | Dicyclopropylmethyl-[4-(2,4-dimethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc Curated by ChEMBL | Assay Description Binding affinity towards human Corticotropin releasing factor receptor 1 by the displacement of [125I]-CRF from CHO cells | Bioorg Med Chem Lett 14: 2083-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.053 BindingDB Entry DOI: 10.7270/Q2JH3KKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50144193 (CHEMBL65484 | Dicyclopropylmethyl-propyl-[4-(2,4,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc Curated by ChEMBL | Assay Description Binding affinity towards human Corticotropin releasing factor receptor 1 by the displacement of [125I]-CRF from CHO cells | Bioorg Med Chem Lett 14: 2083-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.053 BindingDB Entry DOI: 10.7270/Q2JH3KKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50144194 (CHEMBL293627 | Dicyclopropylmethyl-[4-(2,4-dimetho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc Curated by ChEMBL | Assay Description Binding affinity towards human Corticotropin releasing factor receptor 1 by the displacement of [125I]-CRF from CHO cells | Bioorg Med Chem Lett 14: 2083-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.053 BindingDB Entry DOI: 10.7270/Q2JH3KKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50144196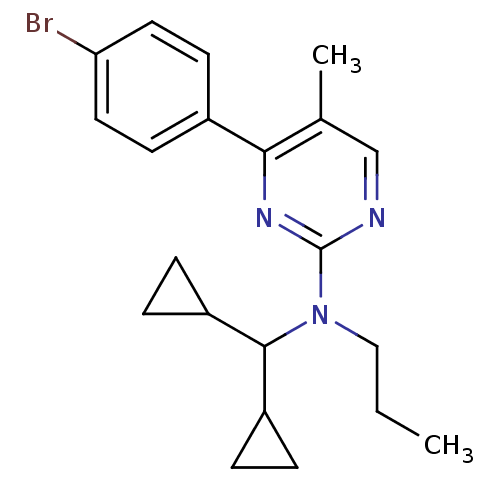 (CHEMBL63029 | [4-(4-Bromo-phenyl)-5-methyl-pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc Curated by ChEMBL | Assay Description Binding affinity towards human Corticotropin releasing factor receptor 1 by the displacement of [125I]-CRF from CHO cells | Bioorg Med Chem Lett 14: 2083-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.053 BindingDB Entry DOI: 10.7270/Q2JH3KKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50144200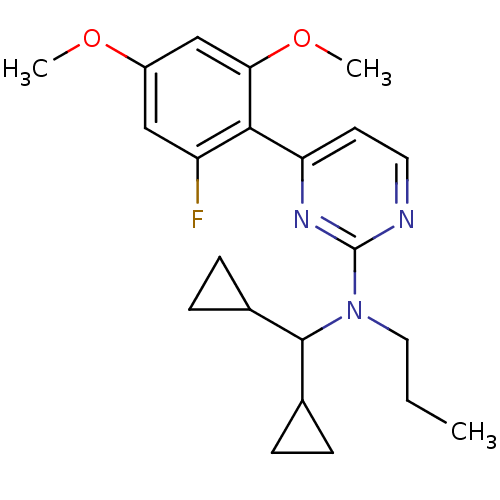 (CHEMBL63457 | Dicyclopropylmethyl-[4-(2-fluoro-4,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc Curated by ChEMBL | Assay Description Binding affinity towards human Corticotropin releasing factor receptor 1 by the displacement of [125I]-CRF from CHO cells | Bioorg Med Chem Lett 14: 2083-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.053 BindingDB Entry DOI: 10.7270/Q2JH3KKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50144197 (CHEMBL66249 | [4-(4-Bromo-2-methyl-phenyl)-5-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc Curated by ChEMBL | Assay Description Binding affinity towards human Corticotropin releasing factor receptor 1 by the displacement of [125I]-CRF from CHO cells | Bioorg Med Chem Lett 14: 2083-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.053 BindingDB Entry DOI: 10.7270/Q2JH3KKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50144203 (Allyl-dicyclopropylmethyl-[4-(2,4,6-trimethoxy-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc Curated by ChEMBL | Assay Description Binding affinity towards human Corticotropin releasing factor receptor 1 by the displacement of [125I]-CRF from CHO cells | Bioorg Med Chem Lett 14: 2083-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.053 BindingDB Entry DOI: 10.7270/Q2JH3KKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50144202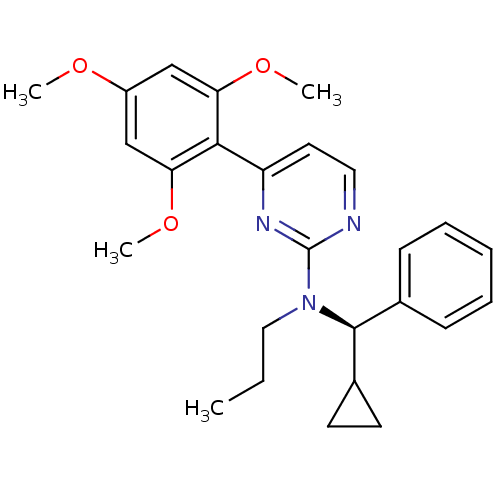 (((R)-Cyclopropyl-phenyl-methyl)-propyl-[4-(2,4,6-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc Curated by ChEMBL | Assay Description Binding affinity towards human Corticotropin releasing factor receptor 1 by the displacement of [125I]-CRF from CHO cells | Bioorg Med Chem Lett 14: 2083-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.053 BindingDB Entry DOI: 10.7270/Q2JH3KKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50144192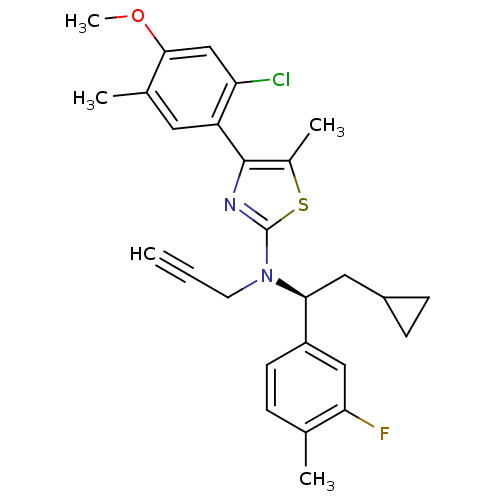 (CHEMBL291657 | [4-(4-Bromo-phenyl)-5,6-dimethyl-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc Curated by ChEMBL | Assay Description Binding affinity towards human Corticotropin releasing factor receptor 1 by the displacement of [125I]-CRF from CHO cells | Bioorg Med Chem Lett 14: 2083-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.053 BindingDB Entry DOI: 10.7270/Q2JH3KKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50144190 (CHEMBL302396 | Propyl-(1-thiophen-2-yl-cyclopropyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc Curated by ChEMBL | Assay Description Binding affinity towards human Corticotropin releasing factor receptor 1 by the displacement of [125I]-CRF from CHO cells | Bioorg Med Chem Lett 14: 2083-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.053 BindingDB Entry DOI: 10.7270/Q2JH3KKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50144201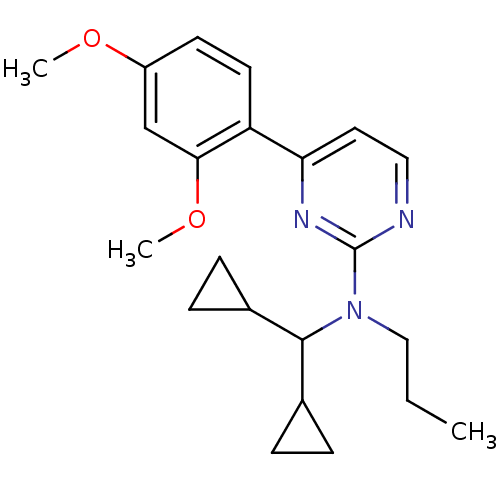 (CHEMBL305354 | Dicyclopropylmethyl-[4-(2,4-dimetho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc Curated by ChEMBL | Assay Description Binding affinity towards human Corticotropin releasing factor receptor 1 by the displacement of [125I]-CRF from CHO cells | Bioorg Med Chem Lett 14: 2083-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.053 BindingDB Entry DOI: 10.7270/Q2JH3KKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50144199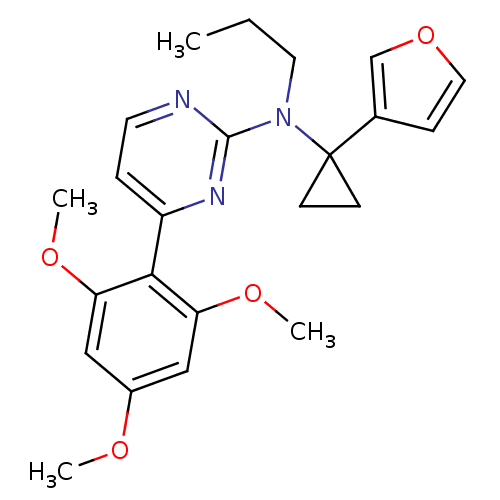 ((1-Furan-3-yl-cyclopropyl)-propyl-[4-(2,4,6-trimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc Curated by ChEMBL | Assay Description Binding affinity towards human Corticotropin releasing factor receptor 1 by the displacement of [125I]-CRF from CHO cells | Bioorg Med Chem Lett 14: 2083-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.053 BindingDB Entry DOI: 10.7270/Q2JH3KKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50144195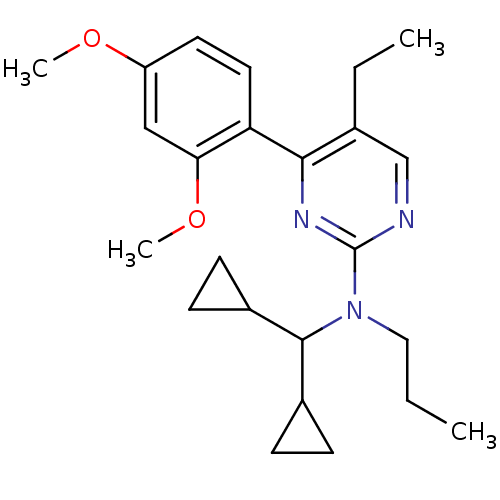 (CHEMBL63585 | Dicyclopropylmethyl-[4-(2,4-dimethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc Curated by ChEMBL | Assay Description Binding affinity towards human Corticotropin releasing factor receptor 1 by the displacement of [125I]-CRF from CHO cells | Bioorg Med Chem Lett 14: 2083-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.053 BindingDB Entry DOI: 10.7270/Q2JH3KKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50144198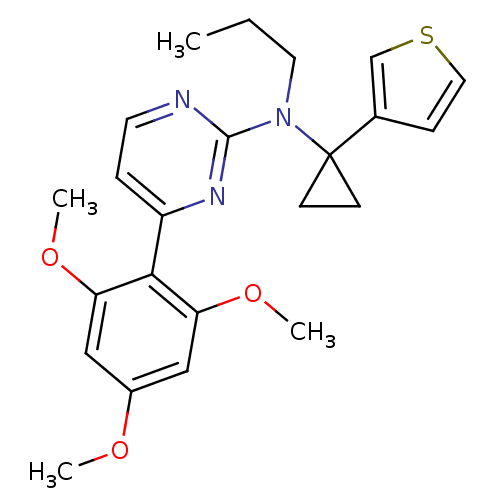 (CHEMBL305583 | Propyl-(1-thiophen-3-yl-cyclopropyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc Curated by ChEMBL | Assay Description Binding affinity towards human Corticotropin releasing factor receptor 1 by the displacement of [125I]-CRF from CHO cells | Bioorg Med Chem Lett 14: 2083-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.053 BindingDB Entry DOI: 10.7270/Q2JH3KKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50144189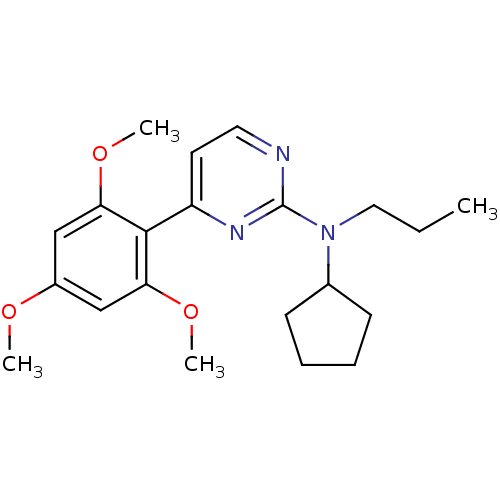 (CHEMBL65979 | Cyclopentyl-propyl-[4-(2,4,6-trimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc Curated by ChEMBL | Assay Description Binding affinity towards human Corticotropin releasing factor receptor 1 by the displacement of [125I]-CRF from CHO cells | Bioorg Med Chem Lett 14: 2083-6 (2004) Article DOI: 10.1016/j.bmcl.2004.02.053 BindingDB Entry DOI: 10.7270/Q2JH3KKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531233 (CHEMBL4540346) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531233 (CHEMBL4540346) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531235 (CHEBI:22 | CHEMBL484848) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531235 (CHEBI:22 | CHEMBL484848) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531228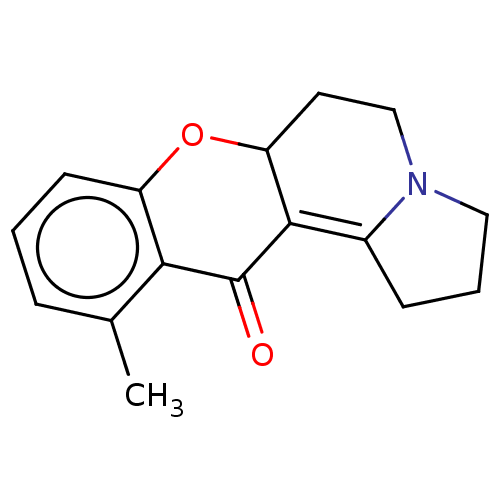 (CHEMBL4468856) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531228 (CHEMBL4468856) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531229 (CHEMBL4534725) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531230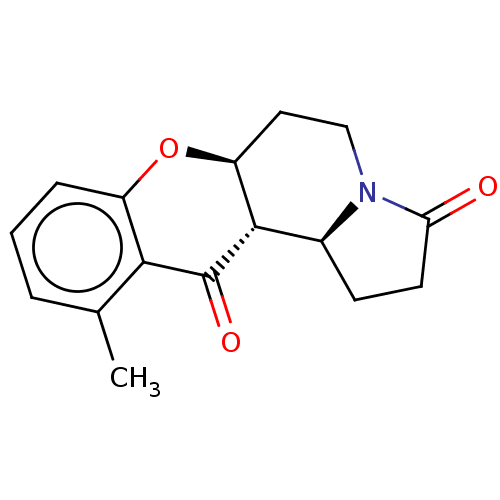 (CHEMBL4548238) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531231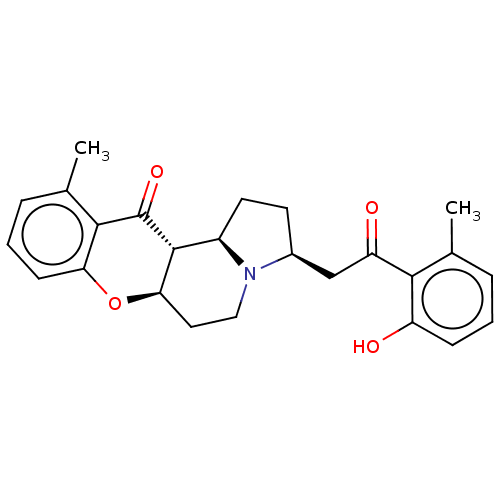 (CHEMBL4440797) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531232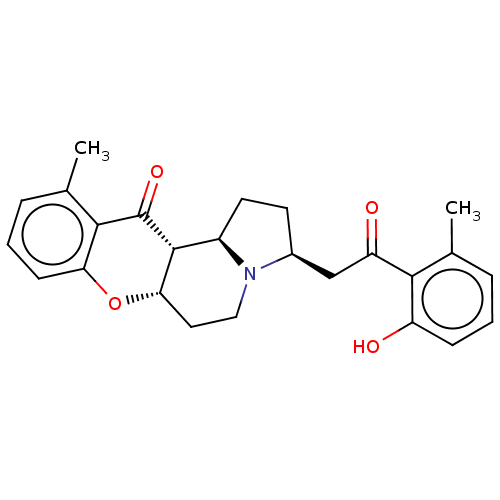 (CHEMBL4435030) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531234 (CHEMBL4440414) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531236 (CHEMBL4591389) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531237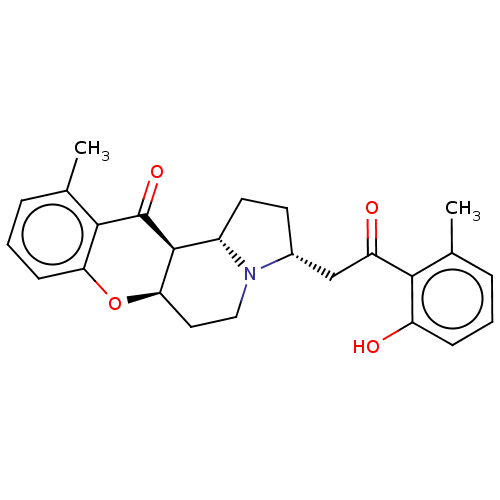 (CHEMBL4552158) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531237 (CHEMBL4552158) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531238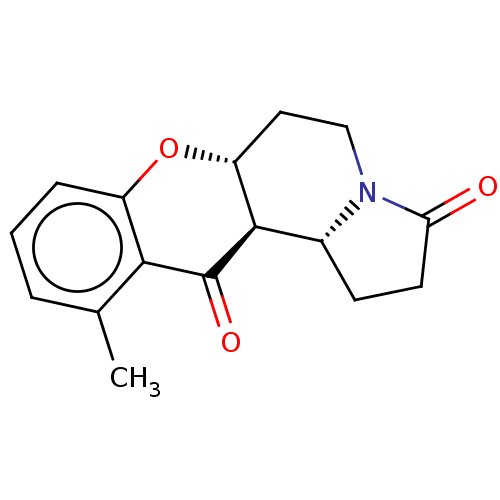 (CHEMBL4584910) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531231 (CHEMBL4440797) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531229 (CHEMBL4534725) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531234 (CHEMBL4440414) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531239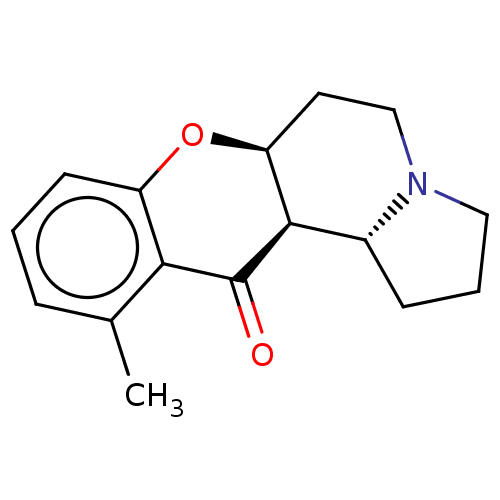 (Isoelaeocarpine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531240 (CHEMBL4533104) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531241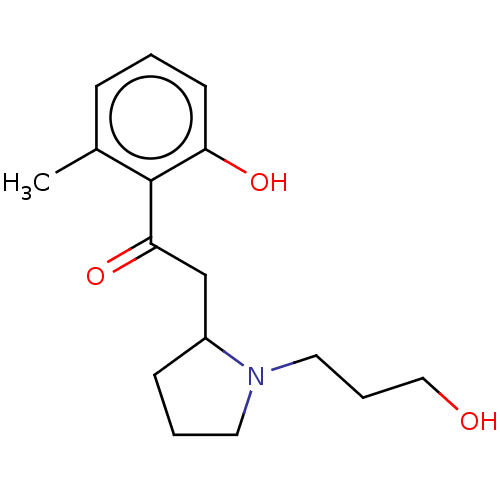 (CHEMBL4534205) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531238 (CHEMBL4584910) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531241 (CHEMBL4534205) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531232 (CHEMBL4435030) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531240 (CHEMBL4533104) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531239 (Isoelaeocarpine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531230 (CHEMBL4548238) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50531236 (CHEMBL4591389) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human erythrocyte BuChE using S-butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and meas... | J Nat Prod 82: 3221-3226 (2019) Article DOI: 10.1021/acs.jnatprod.8b01027 BindingDB Entry DOI: 10.7270/Q2T72MXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50006930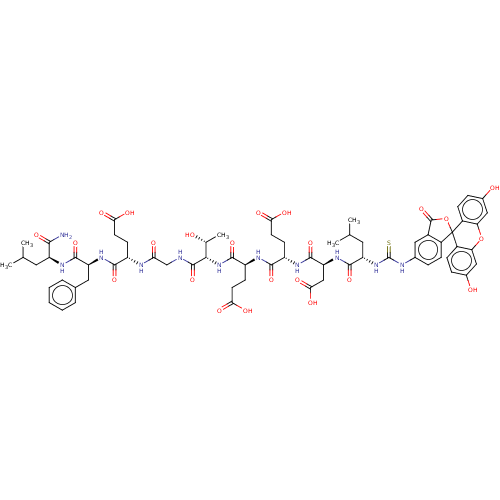 (CHEMBL3237244) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Binding affinity to biotinylated Keap1 DC domain (unknown origin) by biolayer interferometry assay | J Med Chem 57: 2736-45 (2014) Article DOI: 10.1021/jm5000529 BindingDB Entry DOI: 10.7270/Q2X3500X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear factor erythroid 2-related factor 2 (Homo sapiens (Human)) | BDBM50493520 (CHEMBL2431315) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Induction of Nrf2-mediated ARE activity in human HepG2-ARE-C8 cells after 12 hrs by luciferase reporter gene assay | J Med Chem 56: 7925-38 (2013) Article DOI: 10.1021/jm400944k BindingDB Entry DOI: 10.7270/Q2Z322KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50006932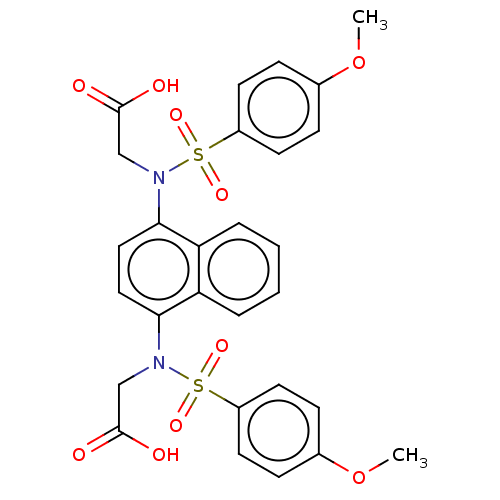 (CHEMBL3237245) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Binding affinity to biotinylated Keap1 DC domain (unknown origin) by biolayer interferometry assay | J Med Chem 57: 2736-45 (2014) Article DOI: 10.1021/jm5000529 BindingDB Entry DOI: 10.7270/Q2X3500X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50006930 (CHEMBL3237244) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Binding affinity to recombinant human Keap1 Kelch domain (321 to 609) expressed in Escherichia coli BL21(DE3)pLysS cells after 30 mins by fluorescenc... | J Med Chem 57: 2736-45 (2014) Article DOI: 10.1021/jm5000529 BindingDB Entry DOI: 10.7270/Q2X3500X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 100 total ) | Next | Last >> |