 Found 63 hits with Last Name = 'xiao' and Initial = 'a'
Found 63 hits with Last Name = 'xiao' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Diphosphomevalonate decarboxylase
(Homo sapiens (Human)) | BDBM50287714
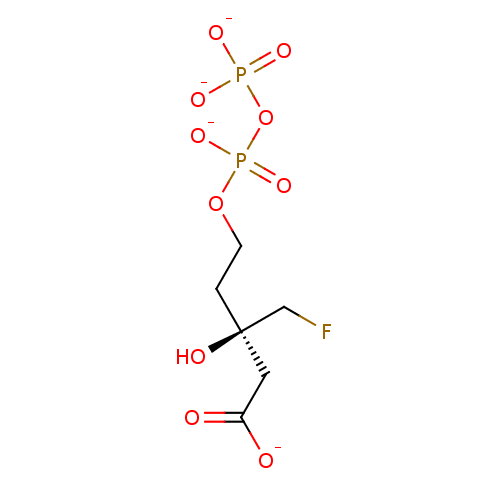
((3R)-3-(fluoromethyl)-3-hydroxy-5-{[(phosphonatoox...)Show SMILES O[C@](CF)(CCOP([O-])(=O)OP([O-])([O-])=O)CC([O-])=O Show InChI InChI=1S/C6H13FO10P2/c7-4-6(10,3-5(8)9)1-2-16-19(14,15)17-18(11,12)13/h10H,1-4H2,(H,8,9)(H,14,15)(H2,11,12,13)/p-4/t6-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against Mevalonate 5-pyrophosphate decarboxylase |
Bioorg Med Chem Lett 6: 2091-2096 (1996)
Article DOI: 10.1016/0960-894X(96)00374-5
BindingDB Entry DOI: 10.7270/Q2930TPB |
More data for this
Ligand-Target Pair | |
Diphosphomevalonate decarboxylase
(Homo sapiens (Human)) | BDBM50287713
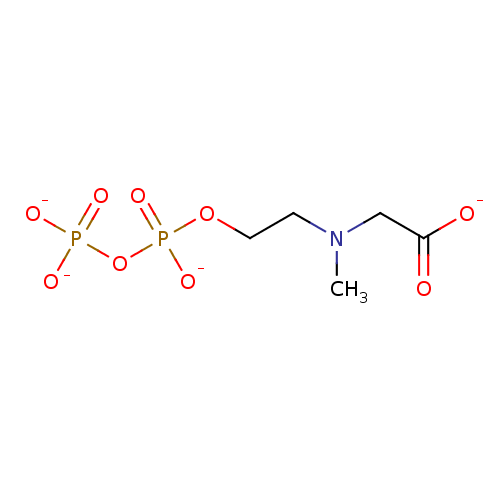
([methyl(2-{[(phosphonatooxy)phosphinato]oxy}ethyl)...)Show SMILES CN(CCOP([O-])(=O)OP([O-])([O-])=O)CC([O-])=O Show InChI InChI=1S/C5H13NO9P2/c1-6(4-5(7)8)2-3-14-17(12,13)15-16(9,10)11/h2-4H2,1H3,(H,7,8)(H,12,13)(H2,9,10,11)/p-4 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
| 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against Mevalonate 5-pyrophosphate decarboxylase |
Bioorg Med Chem Lett 6: 2091-2096 (1996)
Article DOI: 10.1016/0960-894X(96)00374-5
BindingDB Entry DOI: 10.7270/Q2930TPB |
More data for this
Ligand-Target Pair | |
Diphosphomevalonate decarboxylase
(Homo sapiens (Human)) | BDBM50287710

((2S)-1-({[(phosphonatooxy)phosphinato]oxy}acetyl)p...)Show SMILES [O-]C(=O)[C@@H]1CCCN1C(=O)COP([O-])(=O)OP([O-])([O-])=O Show InChI InChI=1S/C7H13NO10P2/c9-6(8-3-1-2-5(8)7(10)11)4-17-20(15,16)18-19(12,13)14/h5H,1-4H2,(H,10,11)(H,15,16)(H2,12,13,14)/p-4/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against Mevalonate 5-pyrophosphate decarboxylase |
Bioorg Med Chem Lett 6: 2091-2096 (1996)
Article DOI: 10.1016/0960-894X(96)00374-5
BindingDB Entry DOI: 10.7270/Q2930TPB |
More data for this
Ligand-Target Pair | |
Diphosphomevalonate decarboxylase
(Homo sapiens (Human)) | BDBM50287715

((2R)-1-({[(phosphonatooxy)phosphinato]oxy}acetyl)p...)Show SMILES [O-]C(=O)[C@H]1CCCN1C(=O)COP([O-])(=O)OP([O-])([O-])=O Show InChI InChI=1S/C7H13NO10P2/c9-6(8-3-1-2-5(8)7(10)11)4-17-20(15,16)18-19(12,13)14/h5H,1-4H2,(H,10,11)(H,15,16)(H2,12,13,14)/p-4/t5-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against Mevalonate 5-pyrophosphate decarboxylase |
Bioorg Med Chem Lett 6: 2091-2096 (1996)
Article DOI: 10.1016/0960-894X(96)00374-5
BindingDB Entry DOI: 10.7270/Q2930TPB |
More data for this
Ligand-Target Pair | |
Diphosphomevalonate decarboxylase
(Homo sapiens (Human)) | BDBM50287715

((2R)-1-({[(phosphonatooxy)phosphinato]oxy}acetyl)p...)Show SMILES [O-]C(=O)[C@H]1CCCN1C(=O)COP([O-])(=O)OP([O-])([O-])=O Show InChI InChI=1S/C7H13NO10P2/c9-6(8-3-1-2-5(8)7(10)11)4-17-20(15,16)18-19(12,13)14/h5H,1-4H2,(H,10,11)(H,15,16)(H2,12,13,14)/p-4/t5-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against MevPP decarboxylase |
Bioorg Med Chem Lett 6: 2091-2096 (1996)
Article DOI: 10.1016/0960-894X(96)00374-5
BindingDB Entry DOI: 10.7270/Q2930TPB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50016496
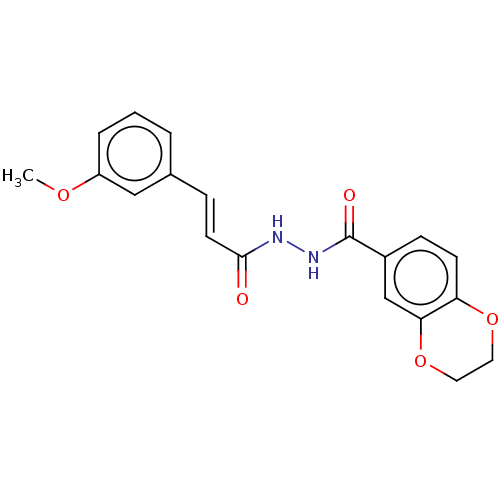
(CHEMBL3260241)Show SMILES COc1cccc(\C=C\C(=O)NNC(=O)c2ccc3OCCOc3c2)c1 Show InChI InChI=1S/C19H18N2O5/c1-24-15-4-2-3-13(11-15)5-8-18(22)20-21-19(23)14-6-7-16-17(12-14)26-10-9-25-16/h2-8,11-12H,9-10H2,1H3,(H,20,22)(H,21,23)/b8-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) using RRRDELMEASFADQEAKV as substrate after 20 mins by liquid scintillation counting analysis |
Eur J Med Chem 81: 420-6 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.026
BindingDB Entry DOI: 10.7270/Q2ZP47PK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50016508
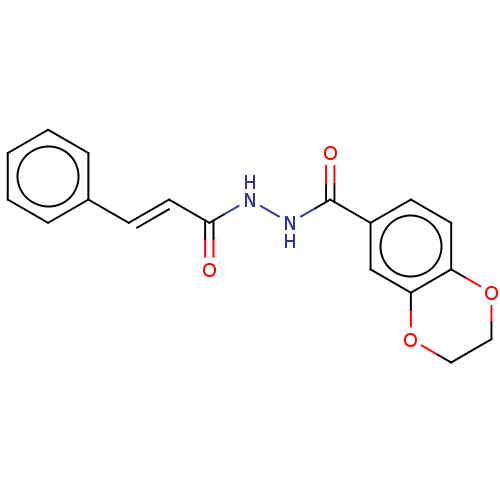
(CHEMBL3260249)Show InChI InChI=1S/C18H16N2O4/c21-17(9-6-13-4-2-1-3-5-13)19-20-18(22)14-7-8-15-16(12-14)24-11-10-23-15/h1-9,12H,10-11H2,(H,19,21)(H,20,22)/b9-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) using RRRDELMEASFADQEAKV as substrate after 20 mins by liquid scintillation counting analysis |
Eur J Med Chem 81: 420-6 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.026
BindingDB Entry DOI: 10.7270/Q2ZP47PK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50016502
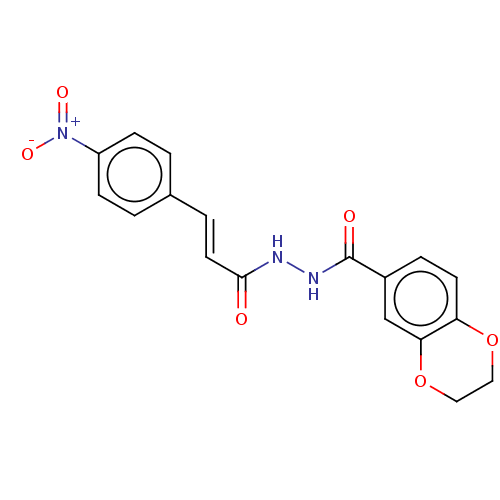
(CHEMBL3260248)Show SMILES [O-][N+](=O)c1ccc(\C=C\C(=O)NNC(=O)c2ccc3OCCOc3c2)cc1 Show InChI InChI=1S/C18H15N3O6/c22-17(8-3-12-1-5-14(6-2-12)21(24)25)19-20-18(23)13-4-7-15-16(11-13)27-10-9-26-15/h1-8,11H,9-10H2,(H,19,22)(H,20,23)/b8-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) using RRRDELMEASFADQEAKV as substrate after 20 mins by liquid scintillation counting analysis |
Eur J Med Chem 81: 420-6 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.026
BindingDB Entry DOI: 10.7270/Q2ZP47PK |
More data for this
Ligand-Target Pair | |
Diphosphomevalonate decarboxylase
(Homo sapiens (Human)) | BDBM50287712
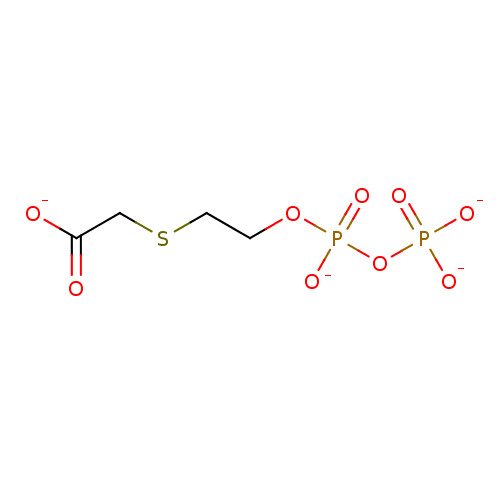
([(2-{[(phosphonatooxy)phosphinato]oxy}ethyl)thio]a...)Show InChI InChI=1S/C4H10O9P2S/c5-4(6)3-16-2-1-12-15(10,11)13-14(7,8)9/h1-3H2,(H,5,6)(H,10,11)(H2,7,8,9)/p-4 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against Mevalonate 5-pyrophosphate decarboxylase |
Bioorg Med Chem Lett 6: 2091-2096 (1996)
Article DOI: 10.1016/0960-894X(96)00374-5
BindingDB Entry DOI: 10.7270/Q2930TPB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50016495
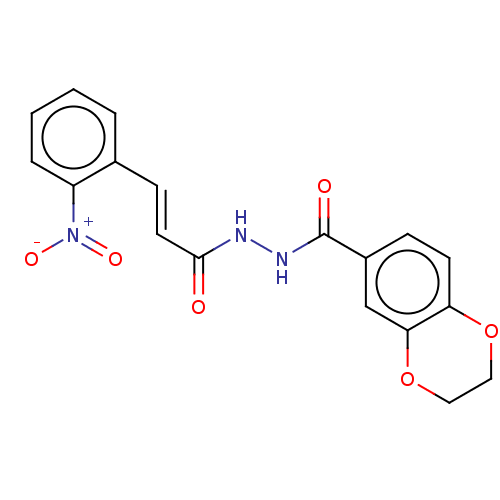
(CHEMBL3260236)Show SMILES [O-][N+](=O)c1ccccc1\C=C\C(=O)NNC(=O)c1ccc2OCCOc2c1 Show InChI InChI=1S/C18H15N3O6/c22-17(8-6-12-3-1-2-4-14(12)21(24)25)19-20-18(23)13-5-7-15-16(11-13)27-10-9-26-15/h1-8,11H,9-10H2,(H,19,22)(H,20,23)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) using RRRDELMEASFADQEAKV as substrate after 20 mins by liquid scintillation counting analysis |
Eur J Med Chem 81: 420-6 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.026
BindingDB Entry DOI: 10.7270/Q2ZP47PK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50016501
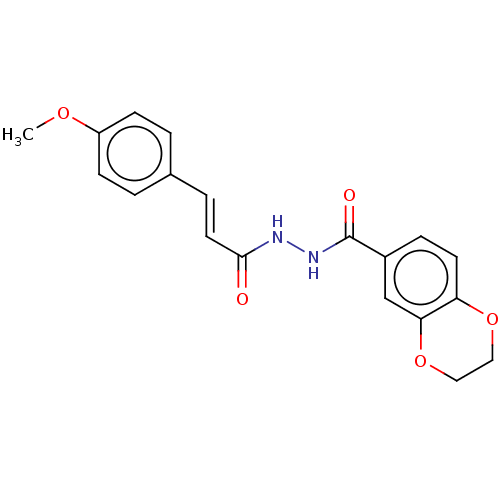
(CHEMBL3260247)Show SMILES COc1ccc(\C=C\C(=O)NNC(=O)c2ccc3OCCOc3c2)cc1 Show InChI InChI=1S/C19H18N2O5/c1-24-15-6-2-13(3-7-15)4-9-18(22)20-21-19(23)14-5-8-16-17(12-14)26-11-10-25-16/h2-9,12H,10-11H2,1H3,(H,20,22)(H,21,23)/b9-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) using RRRDELMEASFADQEAKV as substrate after 20 mins by liquid scintillation counting analysis |
Eur J Med Chem 81: 420-6 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.026
BindingDB Entry DOI: 10.7270/Q2ZP47PK |
More data for this
Ligand-Target Pair | |
Diphosphomevalonate decarboxylase
(Homo sapiens (Human)) | BDBM50287711
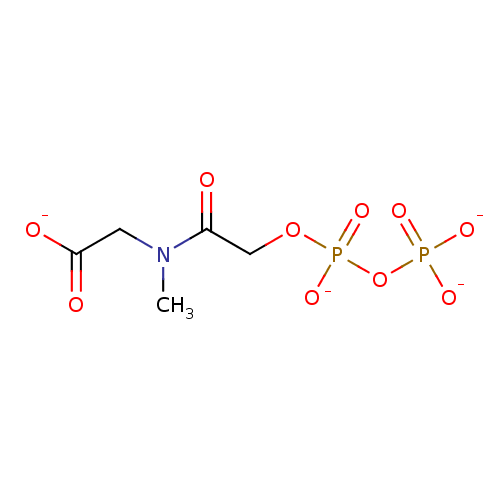
([methyl({[(phosphonatooxy)phosphinato]oxy}acetyl)a...)Show SMILES CN(CC([O-])=O)C(=O)COP([O-])(=O)OP([O-])([O-])=O Show InChI InChI=1S/C5H11NO10P2/c1-6(2-5(8)9)4(7)3-15-18(13,14)16-17(10,11)12/h2-3H2,1H3,(H,8,9)(H,13,14)(H2,10,11,12)/p-4 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against MevPP decarboxylase |
Bioorg Med Chem Lett 6: 2091-2096 (1996)
Article DOI: 10.1016/0960-894X(96)00374-5
BindingDB Entry DOI: 10.7270/Q2930TPB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50016498

(CHEMBL3260243)Show InChI InChI=1S/C18H15FN2O4/c19-14-5-1-12(2-6-14)3-8-17(22)20-21-18(23)13-4-7-15-16(11-13)25-10-9-24-15/h1-8,11H,9-10H2,(H,20,22)(H,21,23)/b8-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) using RRRDELMEASFADQEAKV as substrate after 20 mins by liquid scintillation counting analysis |
Eur J Med Chem 81: 420-6 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.026
BindingDB Entry DOI: 10.7270/Q2ZP47PK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50016500
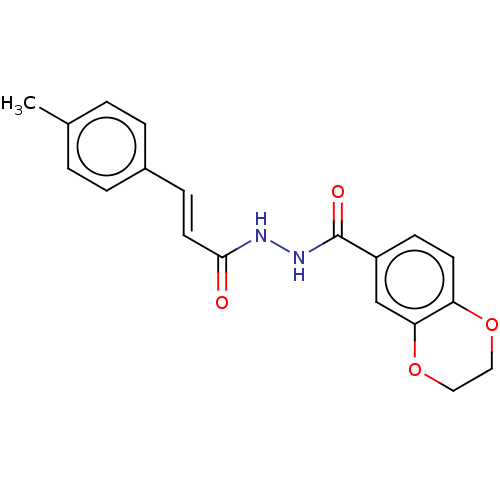
(CHEMBL3260246)Show InChI InChI=1S/C19H18N2O4/c1-13-2-4-14(5-3-13)6-9-18(22)20-21-19(23)15-7-8-16-17(12-15)25-11-10-24-16/h2-9,12H,10-11H2,1H3,(H,20,22)(H,21,23)/b9-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) using RRRDELMEASFADQEAKV as substrate after 20 mins by liquid scintillation counting analysis |
Eur J Med Chem 81: 420-6 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.026
BindingDB Entry DOI: 10.7270/Q2ZP47PK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50016499
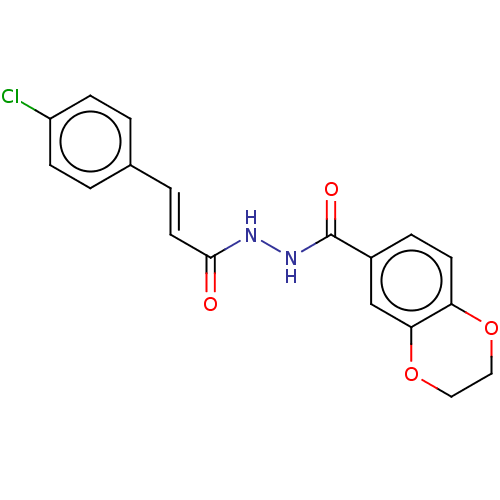
(CHEMBL3260244)Show SMILES Clc1ccc(\C=C\C(=O)NNC(=O)c2ccc3OCCOc3c2)cc1 Show InChI InChI=1S/C18H15ClN2O4/c19-14-5-1-12(2-6-14)3-8-17(22)20-21-18(23)13-4-7-15-16(11-13)25-10-9-24-15/h1-8,11H,9-10H2,(H,20,22)(H,21,23)/b8-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) using RRRDELMEASFADQEAKV as substrate after 20 mins by liquid scintillation counting analysis |
Eur J Med Chem 81: 420-6 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.026
BindingDB Entry DOI: 10.7270/Q2ZP47PK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50016497
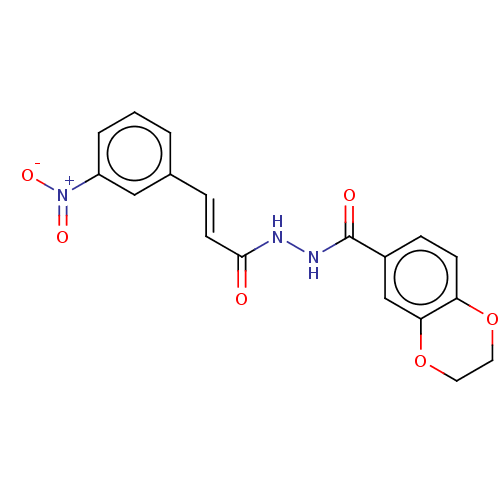
(CHEMBL3260242)Show SMILES [O-][N+](=O)c1cccc(\C=C\C(=O)NNC(=O)c2ccc3OCCOc3c2)c1 Show InChI InChI=1S/C18H15N3O6/c22-17(7-4-12-2-1-3-14(10-12)21(24)25)19-20-18(23)13-5-6-15-16(11-13)27-9-8-26-15/h1-7,10-11H,8-9H2,(H,19,22)(H,20,23)/b7-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) using RRRDELMEASFADQEAKV as substrate after 20 mins by liquid scintillation counting analysis |
Eur J Med Chem 81: 420-6 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.026
BindingDB Entry DOI: 10.7270/Q2ZP47PK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50016509
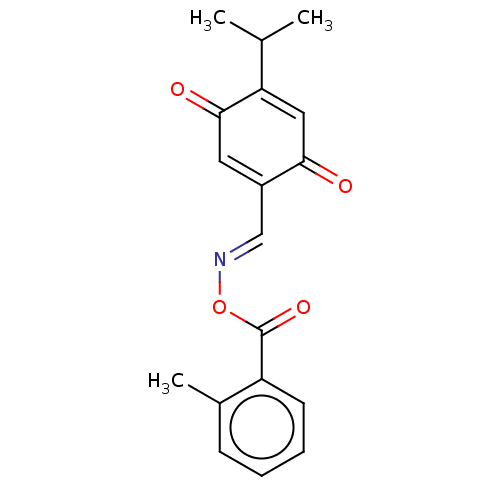
(CHEMBL3260250)Show SMILES CC(C)C1=CC(=O)C(\C=N\OC(=O)c2ccccc2C)=CC1=O |c:20,t:3| Show InChI InChI=1S/C18H17NO4/c1-11(2)15-9-16(20)13(8-17(15)21)10-19-23-18(22)14-7-5-4-6-12(14)3/h4-11H,1-3H3/b19-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) using RRRDELMEASFADQEAKV as substrate after 20 mins by liquid scintillation counting analysis |
Eur J Med Chem 81: 420-6 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.026
BindingDB Entry DOI: 10.7270/Q2ZP47PK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423984
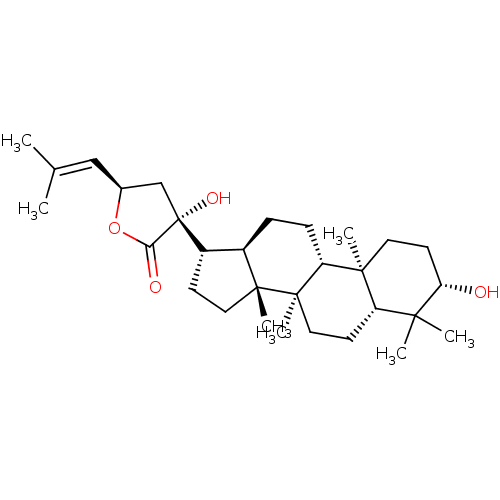
(CHEMBL2313419)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6@H]-1-[#6][C@@]([#8])([#6@H]-2-[#6]-[#6][C@]3([#6])[#6@@H]-2-[#6]-[#6]-[#6@@H]2[C@@]4([#6])[#6]-[#6]-[#6@H](-[#8])C([#6])([#6])[#6@@H]4-[#6]-[#6][C@@]32[#6])[#6](=O)-[#8]1 |r| Show InChI InChI=1S/C30H48O4/c1-18(2)16-19-17-30(33,25(32)34-19)21-10-14-28(6)20(21)8-9-23-27(5)13-12-24(31)26(3,4)22(27)11-15-29(23,28)7/h16,19-24,31,33H,8-15,17H2,1-7H3/t19-,20+,21-,22-,23+,24-,27-,28+,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Sialidase
(Vibrio cholerae) | BDBM4706

((2R,3R,4S)-3-acetamido-4-hydroxy-2-[(1R,2R)-1,2,3-...)Show SMILES CC(=O)N[C@@H]1[C@@H](O)C=C(O[C@H]1[C@H](O)[C@H](O)CO)C(O)=O |r,c:7| Show InChI InChI=1S/C11H17NO8/c1-4(14)12-8-5(15)2-7(11(18)19)20-10(8)9(17)6(16)3-13/h2,5-6,8-10,13,15-17H,3H2,1H3,(H,12,14)(H,18,19)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of Vibrio cholerae sialidase using Neu5Acalpha2-3GalbetapNP as substrate after 30 mins by colorimetric assay |
Bioorg Med Chem 26: 5751-5757 (2018)
Article DOI: 10.1016/j.bmc.2018.10.028
BindingDB Entry DOI: 10.7270/Q2BK1G2N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sialidase A
(Streptococcus pneumoniae) | BDBM4706

((2R,3R,4S)-3-acetamido-4-hydroxy-2-[(1R,2R)-1,2,3-...)Show SMILES CC(=O)N[C@@H]1[C@@H](O)C=C(O[C@H]1[C@H](O)[C@H](O)CO)C(O)=O |r,c:7| Show InChI InChI=1S/C11H17NO8/c1-4(14)12-8-5(15)2-7(11(18)19)20-10(8)9(17)6(16)3-13/h2,5-6,8-10,13,15-17H,3H2,1H3,(H,12,14)(H,18,19)/t5-,6+,8+,9+,10+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae NanA using Neu5Acalpha2-3GalbetapNP as substrate after 30 mins by colorimetric assay |
Bioorg Med Chem 26: 5751-5757 (2018)
Article DOI: 10.1016/j.bmc.2018.10.028
BindingDB Entry DOI: 10.7270/Q2BK1G2N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sialidase
(Vibrio cholerae) | BDBM50466472

(CHEMBL4289476)Show SMILES [H][C@]1(OC(=C[C@H](O)[C@H]1NC(C)=O)C(O)=O)[C@H](O)[C@H](O)Cn1cc(C[C@H](N)C(=O)N[C@H](CCC(O)=O)C(N)=O)nn1 |r,c:3| Show InChI InChI=1S/C21H31N7O11/c1-8(29)24-16-12(30)5-14(21(37)38)39-18(16)17(34)13(31)7-28-6-9(26-27-28)4-10(22)20(36)25-11(19(23)35)2-3-15(32)33/h5-6,10-13,16-18,30-31,34H,2-4,7,22H2,1H3,(H2,23,35)(H,24,29)(H,25,36)(H,32,33)(H,37,38)/t10-,11+,12-,13+,16+,17+,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of Vibrio cholerae sialidase using Neu5Acalpha2-3GalbetapNP as substrate after 30 mins by colorimetric assay |
Bioorg Med Chem 26: 5751-5757 (2018)
Article DOI: 10.1016/j.bmc.2018.10.028
BindingDB Entry DOI: 10.7270/Q2BK1G2N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423983

(CHEMBL2313420)Show SMILES C[C@@]12CC[C@@H]([C@H]1CC[C@@H]1[C@@]3(C)CC[C@H](O)C(C)(C)[C@@H]3CC[C@@]21C)C(O)=O |r| Show InChI InChI=1S/C23H38O3/c1-20(2)16-9-13-23(5)17(21(16,3)11-10-18(20)24)7-6-15-14(19(25)26)8-12-22(15,23)4/h14-18,24H,6-13H2,1-5H3,(H,25,26)/t14-,15+,16-,17+,18-,21-,22+,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Sialidase
(Vibrio cholerae) | BDBM50466474
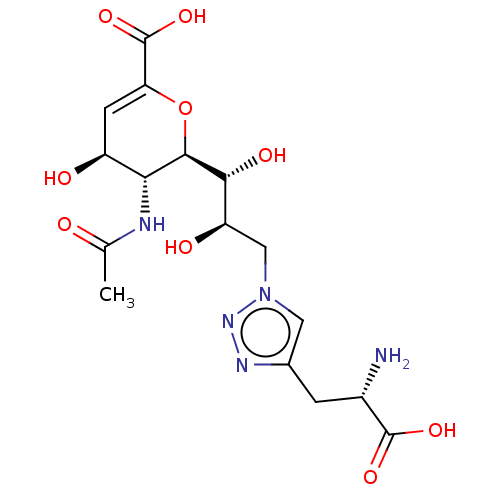
(CHEMBL4282280)Show SMILES [H][C@]1(OC(=C[C@H](O)[C@H]1NC(C)=O)C(O)=O)[C@H](O)[C@H](O)Cn1cc(C[C@H](N)C(O)=O)nn1 |r,c:3| Show InChI InChI=1S/C16H23N5O9/c1-6(22)18-12-9(23)3-11(16(28)29)30-14(12)13(25)10(24)5-21-4-7(19-20-21)2-8(17)15(26)27/h3-4,8-10,12-14,23-25H,2,5,17H2,1H3,(H,18,22)(H,26,27)(H,28,29)/t8-,9-,10+,12+,13+,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of Vibrio cholerae sialidase using Neu5Acalpha2-3GalbetapNP as substrate after 30 mins by colorimetric assay |
Bioorg Med Chem 26: 5751-5757 (2018)
Article DOI: 10.1016/j.bmc.2018.10.028
BindingDB Entry DOI: 10.7270/Q2BK1G2N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423991
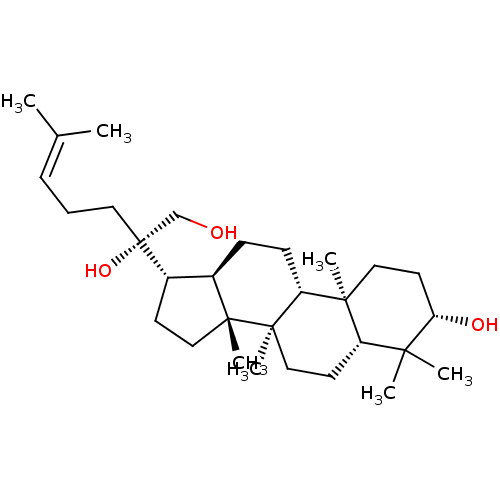
(CHEMBL2313414)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6][C@@]([#8])([#6]-[#8])[#6@H]-1-[#6]-[#6][C@]2([#6])[#6@@H]-1-[#6]-[#6]-[#6@@H]1[C@@]3([#6])[#6]-[#6]-[#6@H](-[#8])C([#6])([#6])[#6@@H]3-[#6]-[#6][C@@]21[#6] |r| Show InChI InChI=1S/C30H52O3/c1-20(2)9-8-15-30(33,19-31)22-12-17-28(6)21(22)10-11-24-27(5)16-14-25(32)26(3,4)23(27)13-18-29(24,28)7/h9,21-25,31-33H,8,10-19H2,1-7H3/t21-,22+,23+,24-,25+,27+,28-,29-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Sialidase-2
(Homo sapiens (Human)) | BDBM4706

((2R,3R,4S)-3-acetamido-4-hydroxy-2-[(1R,2R)-1,2,3-...)Show SMILES CC(=O)N[C@@H]1[C@@H](O)C=C(O[C@H]1[C@H](O)[C@H](O)CO)C(O)=O |r,c:7| Show InChI InChI=1S/C11H17NO8/c1-4(14)12-8-5(15)2-7(11(18)19)20-10(8)9(17)6(16)3-13/h2,5-6,8-10,13,15-17H,3H2,1H3,(H,12,14)(H,18,19)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cytosolic sialidase NEU2 using Neu5Acalpha2-3GalbetapNP as substrate after 30 mins by colorimetric assay |
Bioorg Med Chem 26: 5751-5757 (2018)
Article DOI: 10.1016/j.bmc.2018.10.028
BindingDB Entry DOI: 10.7270/Q2BK1G2N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423982
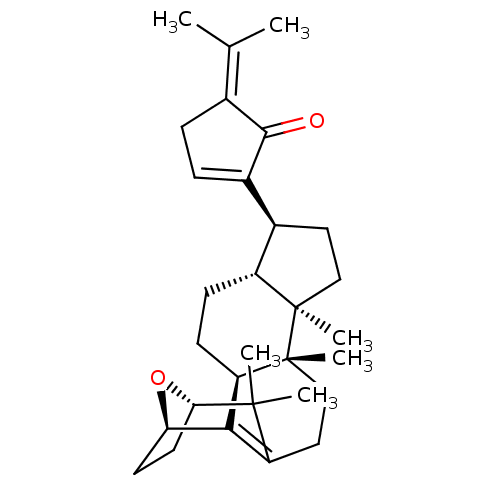
(GYPENSAPOGENIN A)Show SMILES [#6]\[#6](-[#6])=[#6]-1\[#6]-[#6]=[#6](-[#6@H]-2-[#6]-[#6][C@]3([#6])[#6@@H]-2-[#6]-[#6]-[#6@@H]2-[#6]-4=[#6](-[#6]-[#6][C@@]32[#6])C([#6])([#6])[#6@@H]-2-[#6]-[#6]-[#6@H]-4-[#8]-2)-[#6]-1=O |r,c:17,t:5| Show InChI InChI=1S/C30H42O2/c1-17(2)18-7-8-20(27(18)31)19-13-15-29(5)21(19)9-10-23-26-22(14-16-30(23,29)6)28(3,4)25-12-11-24(26)32-25/h8,19,21,23-25H,7,9-16H2,1-6H3/t19-,21-,23-,24-,25+,29-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Sialidase
(Vibrio cholerae) | BDBM50331692
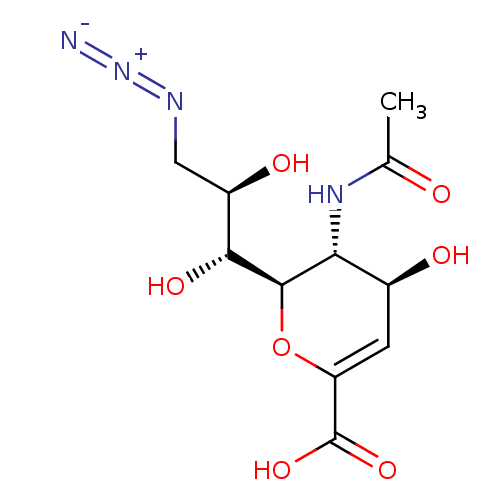
((2R,3R,4S)-3-acetamido-2-((1R,2R)-3-azido-1,2-dihy...)Show SMILES CC(=O)N[C@@H]1[C@@H](O)C=C(O[C@H]1[C@H](O)[C@H](O)CN=[N+]=[N-])C(O)=O |r,c:7| Show InChI InChI=1S/C11H16N4O7/c1-4(16)14-8-5(17)2-7(11(20)21)22-10(8)9(19)6(18)3-13-15-12/h2,5-6,8-10,17-19H,3H2,1H3,(H,14,16)(H,20,21)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of Vibrio cholerae sialidase using Neu5Acalpha2-3GalbetapNP as substrate after 30 mins by colorimetric assay |
Bioorg Med Chem 26: 5751-5757 (2018)
Article DOI: 10.1016/j.bmc.2018.10.028
BindingDB Entry DOI: 10.7270/Q2BK1G2N |
More data for this
Ligand-Target Pair | |
Sialidase
(Clostridium perfringens) | BDBM4706

((2R,3R,4S)-3-acetamido-4-hydroxy-2-[(1R,2R)-1,2,3-...)Show SMILES CC(=O)N[C@@H]1[C@@H](O)C=C(O[C@H]1[C@H](O)[C@H](O)CO)C(O)=O |r,c:7| Show InChI InChI=1S/C11H17NO8/c1-4(14)12-8-5(15)2-7(11(18)19)20-10(8)9(17)6(16)3-13/h2,5-6,8-10,13,15-17H,3H2,1H3,(H,12,14)(H,18,19)/t5-,6+,8+,9+,10+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium perfringens sialidase using Neu5Acalpha2-3GalbetapNP as substrate after 30 mins by colorimetric assay |
Bioorg Med Chem 26: 5751-5757 (2018)
Article DOI: 10.1016/j.bmc.2018.10.028
BindingDB Entry DOI: 10.7270/Q2BK1G2N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50115732
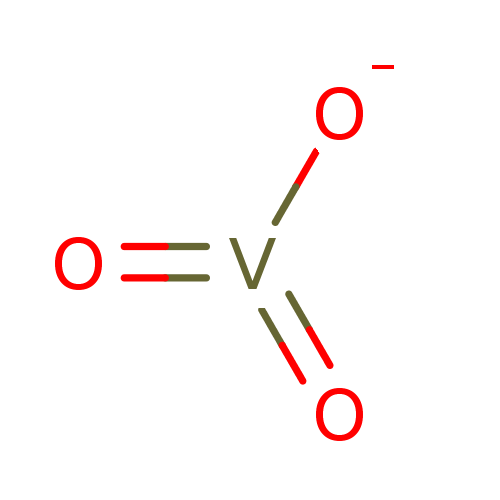
(CHEMBL294467 | Sodium vanadate) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423994
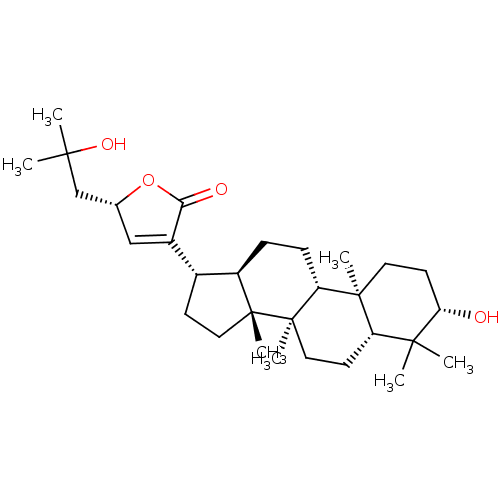
(CHEMBL2313421 | Gypensapogenin E)Show SMILES CC(C)(O)C[C@@H]1OC(=O)C(=C1)[C@H]1CC[C@]2(C)[C@@H]1CC[C@@H]1[C@@]3(C)CC[C@H](O)C(C)(C)[C@@H]3CC[C@@]21C |r,c:9| Show InChI InChI=1S/C30H48O4/c1-26(2,33)17-18-16-20(25(32)34-18)19-10-14-29(6)21(19)8-9-23-28(5)13-12-24(31)27(3,4)22(28)11-15-30(23,29)7/h16,18-19,21-24,31,33H,8-15,17H2,1-7H3/t18-,19-,21-,22+,23-,24+,28+,29-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423990
(20(S)-Protopanaxadiol | CHEMBL375563)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6][C@]([#6])([#8])[#6@H]-1-[#6]-[#6][C@]2([#6])[#6@@H]-1-[#6@H](-[#8])-[#6]-[#6@@H]1[C@@]3([#6])[#6]-[#6]-[#6@H](-[#8])C([#6])([#6])[#6@@H]3-[#6]-[#6][C@@]21[#6] Show InChI InChI=1S/C30H52O3/c1-19(2)10-9-14-30(8,33)20-11-16-29(7)25(20)21(31)18-23-27(5)15-13-24(32)26(3,4)22(27)12-17-28(23,29)6/h10,20-25,31-33H,9,11-18H2,1-8H3/t20-,21+,22-,23+,24-,25-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423981

(GYPENSAPOGENIN B)Show SMILES [#6]\[#6](-[#6])=[#6]-1\[#6]-[#6](=[#6]-[#6]-1=O)-[#6@H]-1-[#6]-[#6][C@]2([#6])[#6@@H]-1-[#6]-[#6]-[#6@@H]1-[#6]-3=[#6](-[#6]-[#6][C@@]21[#6])C([#6])([#6])[#6@@H]-1-[#6]-[#6]-[#6@H]-3-[#8]-1 |r,c:5,20| Show InChI InChI=1S/C30H42O2/c1-17(2)20-15-18(16-24(20)31)19-11-13-29(5)21(19)7-8-23-27-22(12-14-30(23,29)6)28(3,4)26-10-9-25(27)32-26/h16,19,21,23,25-26H,7-15H2,1-6H3/t19-,21-,23-,25-,26+,29-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423992

(CHEMBL2313423 | Gypensapogenin G)Show SMILES CC(=O)O[C@H]1CC[C@@]2(C)[C@@H](CC[C@]3(C)[C@@H]2CC[C@@H]2[C@H](CC[C@@]32C)C2=C[C@H](CC(C)(C)O)OC2=O)C1(C)C |r,t:26| Show InChI InChI=1S/C32H50O5/c1-19(33)36-26-13-14-30(6)24(29(26,4)5)12-16-32(8)25(30)10-9-23-21(11-15-31(23,32)7)22-17-20(37-27(22)34)18-28(2,3)35/h17,20-21,23-26,35H,9-16,18H2,1-8H3/t20-,21-,23-,24+,25-,26+,30+,31-,32-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423985

(CHEMBL2313418)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6@@H]-1-[#6][C@]([#8])([#6@H]-2-[#6]-[#6][C@]3([#6])[#6@@H]-2-[#6]-[#6]-[#6@@H]2[C@@]4([#6])[#6]-[#6]-[#6@H](-[#8])C([#6])([#6])[#6@@H]4-[#6]-[#6][C@@]32[#6])[#6](=O)-[#8]1 |r| Show InChI InChI=1S/C30H48O4/c1-18(2)16-19-17-30(33,25(32)34-19)21-10-14-28(6)20(21)8-9-23-27(5)13-12-24(31)26(3,4)22(27)11-15-29(23,28)7/h16,19-24,31,33H,8-15,17H2,1-7H3/t19-,20-,21+,22+,23-,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Sialidase
(Vibrio cholerae) | BDBM50466473

(CHEMBL4278803)Show SMILES [H][C@]1(OC(=C[C@H](O)[C@H]1NC(C)=O)C(O)=O)[C@H](O)[C@H](O)Cn1cc(C[C@H](NC(=O)[C@@H](N)CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(N)=O)nn1 |r,c:3| Show InChI InChI=1S/C32H50N10O15/c1-14(43)36-25-20(44)11-22(32(55)56)57-27(25)26(50)21(45)13-42-12-15(40-41-42)10-19(39-29(52)16(34)5-7-23(46)47)31(54)38-18(4-2-3-9-33)30(53)37-17(28(35)51)6-8-24(48)49/h11-12,16-21,25-27,44-45,50H,2-10,13,33-34H2,1H3,(H2,35,51)(H,36,43)(H,37,53)(H,38,54)(H,39,52)(H,46,47)(H,48,49)(H,55,56)/t16-,17-,18-,19-,20-,21+,25+,26+,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of Vibrio cholerae sialidase using Neu5Acalpha2-3GalbetapNP as substrate after 30 mins by colorimetric assay |
Bioorg Med Chem 26: 5751-5757 (2018)
Article DOI: 10.1016/j.bmc.2018.10.028
BindingDB Entry DOI: 10.7270/Q2BK1G2N |
More data for this
Ligand-Target Pair | |
Sialidase A
(Streptococcus pneumoniae) | BDBM50331692

((2R,3R,4S)-3-acetamido-2-((1R,2R)-3-azido-1,2-dihy...)Show SMILES CC(=O)N[C@@H]1[C@@H](O)C=C(O[C@H]1[C@H](O)[C@H](O)CN=[N+]=[N-])C(O)=O |r,c:7| Show InChI InChI=1S/C11H16N4O7/c1-4(16)14-8-5(17)2-7(11(20)21)22-10(8)9(19)6(18)3-13-15-12/h2,5-6,8-10,17-19H,3H2,1H3,(H,14,16)(H,20,21)/t5-,6+,8+,9+,10+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae NanA using Neu5Acalpha2-3GalbetapNP as substrate after 30 mins by colorimetric assay |
Bioorg Med Chem 26: 5751-5757 (2018)
Article DOI: 10.1016/j.bmc.2018.10.028
BindingDB Entry DOI: 10.7270/Q2BK1G2N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423993
(CHEMBL2313422 | Gypensapogenin F)Show SMILES CC(C)(O)C[C@H]1OC(=O)C(=C1)[C@H]1CC[C@]2(C)[C@@H]1CC[C@@H]1[C@@]3(C)CC[C@H](O)C(C)(C)[C@@H]3CC[C@@]21C |r,c:9| Show InChI InChI=1S/C30H48O4/c1-26(2,33)17-18-16-20(25(32)34-18)19-10-14-29(6)21(19)8-9-23-28(5)13-12-24(31)27(3,4)22(28)11-15-30(23,29)7/h16,18-19,21-24,31,33H,8-15,17H2,1-7H3/t18-,19+,21+,22-,23+,24-,28-,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423980
(GYPENSAPOGENIN C)Show SMILES [#6]\[#6](-[#6])=[#6]-1\[#6]-[#6]=[#6](-[#6@H]-2-[#6]-[#6][C@]3([#6])[#6@@H]-2-[#6]-[#6]-[#6@@H]2[C@@]4([#6])[#6]-[#6]-[#6@H](-[#8])C([#6])([#6])[#6@@H]4-[#6]-[#6][C@@]32[#6])-[#6]-1=O |r,t:5| Show InChI InChI=1S/C30H46O2/c1-18(2)19-8-9-21(26(19)32)20-12-16-29(6)22(20)10-11-24-28(5)15-14-25(31)27(3,4)23(28)13-17-30(24,29)7/h9,20,22-25,31H,8,10-17H2,1-7H3/t20-,22-,23+,24-,25+,28+,29-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423989

(CHEMBL2313415)Show SMILES C\C(=C\CCC(C)(C)O)[C@H]1CC[C@]2(C)[C@@H]1[C@H](O)C[C@@H]1[C@@]3(C)CC[C@H](O)C(C)(C)[C@@H]3CC[C@@]21C |r| Show InChI InChI=1S/C30H52O3/c1-19(10-9-14-26(2,3)33)20-11-16-30(8)25(20)21(31)18-23-28(6)15-13-24(32)27(4,5)22(28)12-17-29(23,30)7/h10,20-25,31-33H,9,11-18H2,1-8H3/b19-10-/t20-,21-,22+,23-,24+,25+,28+,29-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423988
(CHEMBL2313416)Show SMILES CC(=C)CCC[C@](C)(O)[C@H]1CC[C@]2(C)[C@@H]1[C@H](O)C[C@@H]1[C@@]3(C)CC[C@H](O)C(C)(C)[C@@H]3CC[C@@]21C |r| Show InChI InChI=1S/C30H52O3/c1-19(2)10-9-14-30(8,33)20-11-16-29(7)25(20)21(31)18-23-27(5)15-13-24(32)26(3,4)22(27)12-17-28(23,29)6/h20-25,31-33H,1,9-18H2,2-8H3/t20-,21+,22-,23+,24-,25-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423986
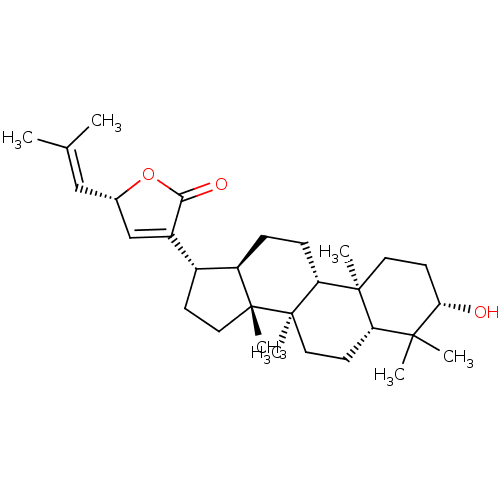
(GYPENSAPOGENIN D)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6@@H]-1-[#8]-[#6](=O)-[#6](=[#6]1)-[#6@H]-1-[#6]-[#6][C@]2([#6])[#6@@H]-1-[#6]-[#6]-[#6@@H]1[C@@]3([#6])[#6]-[#6]-[#6@H](-[#8])C([#6])([#6])[#6@@H]3-[#6]-[#6][C@@]21[#6] |r,c:8| Show InChI InChI=1S/C30H46O3/c1-18(2)16-19-17-21(26(32)33-19)20-10-14-29(6)22(20)8-9-24-28(5)13-12-25(31)27(3,4)23(28)11-15-30(24,29)7/h16-17,19-20,22-25,31H,8-15H2,1-7H3/t19-,20+,22+,23-,24+,25-,28-,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50423987

(CHEMBL2313417)Show SMILES CC(=C)[C@@H](O)CC[C@](C)(O)[C@H]1CC[C@]2(C)[C@@H]1[C@H](O)C[C@@H]1[C@@]3(C)CC[C@H](O)C(C)(C)[C@@H]3CC[C@@]21C |r| Show InChI InChI=1S/C30H52O4/c1-18(2)20(31)10-16-30(8,34)19-9-14-29(7)25(19)21(32)17-23-27(5)13-12-24(33)26(3,4)22(27)11-15-28(23,29)6/h19-25,31-34H,1,9-17H2,2-8H3/t19-,20-,21+,22-,23+,24-,25-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Bioorg Med Chem Lett 23: 297-300 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.097
BindingDB Entry DOI: 10.7270/Q2CJ8FS8 |
More data for this
Ligand-Target Pair | |
Sialidase
(Clostridium perfringens) | BDBM50331692

((2R,3R,4S)-3-acetamido-2-((1R,2R)-3-azido-1,2-dihy...)Show SMILES CC(=O)N[C@@H]1[C@@H](O)C=C(O[C@H]1[C@H](O)[C@H](O)CN=[N+]=[N-])C(O)=O |r,c:7| Show InChI InChI=1S/C11H16N4O7/c1-4(16)14-8-5(17)2-7(11(20)21)22-10(8)9(19)6(18)3-13-15-12/h2,5-6,8-10,17-19H,3H2,1H3,(H,14,16)(H,20,21)/t5-,6+,8+,9+,10+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium perfringens sialidase using Neu5Acalpha2-3GalbetapNP as substrate after 30 mins by colorimetric assay |
Bioorg Med Chem 26: 5751-5757 (2018)
Article DOI: 10.1016/j.bmc.2018.10.028
BindingDB Entry DOI: 10.7270/Q2BK1G2N |
More data for this
Ligand-Target Pair | |
Sialidase A
(Streptococcus pneumoniae) | BDBM50466472

(CHEMBL4289476)Show SMILES [H][C@]1(OC(=C[C@H](O)[C@H]1NC(C)=O)C(O)=O)[C@H](O)[C@H](O)Cn1cc(C[C@H](N)C(=O)N[C@H](CCC(O)=O)C(N)=O)nn1 |r,c:3| Show InChI InChI=1S/C21H31N7O11/c1-8(29)24-16-12(30)5-14(21(37)38)39-18(16)17(34)13(31)7-28-6-9(26-27-28)4-10(22)20(36)25-11(19(23)35)2-3-15(32)33/h5-6,10-13,16-18,30-31,34H,2-4,7,22H2,1H3,(H2,23,35)(H,24,29)(H,25,36)(H,32,33)(H,37,38)/t10-,11+,12-,13+,16+,17+,18+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae NanA using Neu5Acalpha2-3GalbetapNP as substrate after 30 mins by colorimetric assay |
Bioorg Med Chem 26: 5751-5757 (2018)
Article DOI: 10.1016/j.bmc.2018.10.028
BindingDB Entry DOI: 10.7270/Q2BK1G2N |
More data for this
Ligand-Target Pair | |
Sialidase
(Clostridium perfringens) | BDBM50466474

(CHEMBL4282280)Show SMILES [H][C@]1(OC(=C[C@H](O)[C@H]1NC(C)=O)C(O)=O)[C@H](O)[C@H](O)Cn1cc(C[C@H](N)C(O)=O)nn1 |r,c:3| Show InChI InChI=1S/C16H23N5O9/c1-6(22)18-12-9(23)3-11(16(28)29)30-14(12)13(25)10(24)5-21-4-7(19-20-21)2-8(17)15(26)27/h3-4,8-10,12-14,23-25H,2,5,17H2,1H3,(H,18,22)(H,26,27)(H,28,29)/t8-,9-,10+,12+,13+,14+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium perfringens sialidase using Neu5Acalpha2-3GalbetapNP as substrate after 30 mins by colorimetric assay |
Bioorg Med Chem 26: 5751-5757 (2018)
Article DOI: 10.1016/j.bmc.2018.10.028
BindingDB Entry DOI: 10.7270/Q2BK1G2N |
More data for this
Ligand-Target Pair | |
Sialidase
(Clostridium perfringens) | BDBM50466472

(CHEMBL4289476)Show SMILES [H][C@]1(OC(=C[C@H](O)[C@H]1NC(C)=O)C(O)=O)[C@H](O)[C@H](O)Cn1cc(C[C@H](N)C(=O)N[C@H](CCC(O)=O)C(N)=O)nn1 |r,c:3| Show InChI InChI=1S/C21H31N7O11/c1-8(29)24-16-12(30)5-14(21(37)38)39-18(16)17(34)13(31)7-28-6-9(26-27-28)4-10(22)20(36)25-11(19(23)35)2-3-15(32)33/h5-6,10-13,16-18,30-31,34H,2-4,7,22H2,1H3,(H2,23,35)(H,24,29)(H,25,36)(H,32,33)(H,37,38)/t10-,11+,12-,13+,16+,17+,18+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium perfringens sialidase using Neu5Acalpha2-3GalbetapNP as substrate after 30 mins by colorimetric assay |
Bioorg Med Chem 26: 5751-5757 (2018)
Article DOI: 10.1016/j.bmc.2018.10.028
BindingDB Entry DOI: 10.7270/Q2BK1G2N |
More data for this
Ligand-Target Pair | |
Sialidase
(Clostridium perfringens) | BDBM50466473

(CHEMBL4278803)Show SMILES [H][C@]1(OC(=C[C@H](O)[C@H]1NC(C)=O)C(O)=O)[C@H](O)[C@H](O)Cn1cc(C[C@H](NC(=O)[C@@H](N)CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(N)=O)nn1 |r,c:3| Show InChI InChI=1S/C32H50N10O15/c1-14(43)36-25-20(44)11-22(32(55)56)57-27(25)26(50)21(45)13-42-12-15(40-41-42)10-19(39-29(52)16(34)5-7-23(46)47)31(54)38-18(4-2-3-9-33)30(53)37-17(28(35)51)6-8-24(48)49/h11-12,16-21,25-27,44-45,50H,2-10,13,33-34H2,1H3,(H2,35,51)(H,36,43)(H,37,53)(H,38,54)(H,39,52)(H,46,47)(H,48,49)(H,55,56)/t16-,17-,18-,19-,20-,21+,25+,26+,27+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium perfringens sialidase using Neu5Acalpha2-3GalbetapNP as substrate after 30 mins by colorimetric assay |
Bioorg Med Chem 26: 5751-5757 (2018)
Article DOI: 10.1016/j.bmc.2018.10.028
BindingDB Entry DOI: 10.7270/Q2BK1G2N |
More data for this
Ligand-Target Pair | |
Sialidase A
(Streptococcus pneumoniae) | BDBM50466474

(CHEMBL4282280)Show SMILES [H][C@]1(OC(=C[C@H](O)[C@H]1NC(C)=O)C(O)=O)[C@H](O)[C@H](O)Cn1cc(C[C@H](N)C(O)=O)nn1 |r,c:3| Show InChI InChI=1S/C16H23N5O9/c1-6(22)18-12-9(23)3-11(16(28)29)30-14(12)13(25)10(24)5-21-4-7(19-20-21)2-8(17)15(26)27/h3-4,8-10,12-14,23-25H,2,5,17H2,1H3,(H,18,22)(H,26,27)(H,28,29)/t8-,9-,10+,12+,13+,14+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae NanA using Neu5Acalpha2-3GalbetapNP as substrate after 30 mins by colorimetric assay |
Bioorg Med Chem 26: 5751-5757 (2018)
Article DOI: 10.1016/j.bmc.2018.10.028
BindingDB Entry DOI: 10.7270/Q2BK1G2N |
More data for this
Ligand-Target Pair | |
Sialidase-2
(Homo sapiens (Human)) | BDBM50331692

((2R,3R,4S)-3-acetamido-2-((1R,2R)-3-azido-1,2-dihy...)Show SMILES CC(=O)N[C@@H]1[C@@H](O)C=C(O[C@H]1[C@H](O)[C@H](O)CN=[N+]=[N-])C(O)=O |r,c:7| Show InChI InChI=1S/C11H16N4O7/c1-4(16)14-8-5(17)2-7(11(20)21)22-10(8)9(19)6(18)3-13-15-12/h2,5-6,8-10,17-19H,3H2,1H3,(H,14,16)(H,20,21)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cytosolic sialidase NEU2 using Neu5Acalpha2-3GalbetapNP as substrate after 30 mins by colorimetric assay |
Bioorg Med Chem 26: 5751-5757 (2018)
Article DOI: 10.1016/j.bmc.2018.10.028
BindingDB Entry DOI: 10.7270/Q2BK1G2N |
More data for this
Ligand-Target Pair | |
Sialidase A
(Streptococcus pneumoniae) | BDBM50466473

(CHEMBL4278803)Show SMILES [H][C@]1(OC(=C[C@H](O)[C@H]1NC(C)=O)C(O)=O)[C@H](O)[C@H](O)Cn1cc(C[C@H](NC(=O)[C@@H](N)CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(N)=O)nn1 |r,c:3| Show InChI InChI=1S/C32H50N10O15/c1-14(43)36-25-20(44)11-22(32(55)56)57-27(25)26(50)21(45)13-42-12-15(40-41-42)10-19(39-29(52)16(34)5-7-23(46)47)31(54)38-18(4-2-3-9-33)30(53)37-17(28(35)51)6-8-24(48)49/h11-12,16-21,25-27,44-45,50H,2-10,13,33-34H2,1H3,(H2,35,51)(H,36,43)(H,37,53)(H,38,54)(H,39,52)(H,46,47)(H,48,49)(H,55,56)/t16-,17-,18-,19-,20-,21+,25+,26+,27+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-Davis
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae NanA using Neu5Acalpha2-3GalbetapNP as substrate after 30 mins by colorimetric assay |
Bioorg Med Chem 26: 5751-5757 (2018)
Article DOI: 10.1016/j.bmc.2018.10.028
BindingDB Entry DOI: 10.7270/Q2BK1G2N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data