 Found 214 hits with Last Name = 'yap' and Initial = 'k'
Found 214 hits with Last Name = 'yap' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin G
(Homo sapiens (Human)) | BDBM50520048

(CHEMBL4450993)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N2 Show InChI InChI=1S/C71H92N14O20S2/c1-4-38(2)58-68(102)81-51-37-107-106-36-50(80-61(95)45(30-40-14-7-5-8-15-40)74-55(88)34-72-60(94)47(33-57(91)92)77-66(100)52-18-11-27-83(52)70(104)48(78-65(51)99)32-41-16-9-6-10-17-41)64(98)73-39(3)59(93)76-46(31-42-21-23-43(87)24-22-42)62(96)79-49(35-86)63(97)75-44(25-26-56(89)90)69(103)85-29-13-20-54(85)71(105)84-28-12-19-53(84)67(101)82-58/h5-10,14-17,21-24,38-39,44-54,58,86-87H,4,11-13,18-20,25-37H2,1-3H3,(H,72,94)(H,73,98)(H,74,88)(H,75,97)(H,76,93)(H,77,100)(H,78,99)(H,79,96)(H,80,95)(H,81,102)(H,82,101)(H,89,90)(H,91,92)/t38-,39-,44-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,58-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G using Suc-AAPF-MCA as substrate at pH 7.2 and 298 K measured every 60 secs for 600 secs |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50520068

(CHEMBL4447828)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)C2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)[C@@H](C)O)[C@@H](C)CC |r| Show InChI InChI=1S/C67H104N18O18S2/c1-6-35(3)51-62(99)79-44-33-104-105-34-45(78-55(92)39(20-13-25-71-67(69)70)73-49(88)31-72-54(91)41(30-50(89)90)75-60(97)46-21-14-26-83(46)64(101)42(76-58(44)95)29-38-17-9-8-10-18-38)59(96)82-53(37(5)87)63(100)74-40(19-11-12-24-68)56(93)77-43(32-86)57(94)81-52(36(4)7-2)66(103)85-28-16-23-48(85)65(102)84-27-15-22-47(84)61(98)80-51/h8-10,17-18,35-37,39-48,51-53,86-87H,6-7,11-16,19-34,68H2,1-5H3,(H,72,91)(H,73,88)(H,74,100)(H,75,97)(H,76,95)(H,77,93)(H,78,92)(H,79,99)(H,80,98)(H,81,94)(H,82,96)(H,89,90)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45?,46-,47-,48-,51-,52-,53-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of bovine beta-trypsin using L-BAPNA as substrate preincubated for 5 mins followed by substrate addition measured after 60 mins |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50520051

(CHEMBL4592765)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 Show InChI InChI=1S/C70H98N18O21S2/c1-3-36(2)56-66(106)84-48-35-111-110-34-47(83-58(98)40(13-7-25-74-70(72)73)76-53(92)32-75-57(97)44(31-55(95)96)80-64(104)49-14-8-26-86(49)68(108)45(81-63(48)103)30-37-11-5-4-6-12-37)62(102)77-41(21-23-52(71)91)59(99)79-43(29-38-17-19-39(90)20-18-38)60(100)82-46(33-89)61(101)78-42(22-24-54(93)94)67(107)88-28-10-16-51(88)69(109)87-27-9-15-50(87)65(105)85-56/h4-6,11-12,17-20,36,40-51,56,89-90H,3,7-10,13-16,21-35H2,1-2H3,(H2,71,91)(H,75,97)(H,76,92)(H,77,102)(H,78,101)(H,79,99)(H,80,104)(H,81,103)(H,82,100)(H,83,98)(H,84,106)(H,85,105)(H,93,94)(H,95,96)(H4,72,73,74)/t36-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,56-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50520051

(CHEMBL4592765)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 Show InChI InChI=1S/C70H98N18O21S2/c1-3-36(2)56-66(106)84-48-35-111-110-34-47(83-58(98)40(13-7-25-74-70(72)73)76-53(92)32-75-57(97)44(31-55(95)96)80-64(104)49-14-8-26-86(49)68(108)45(81-63(48)103)30-37-11-5-4-6-12-37)62(102)77-41(21-23-52(71)91)59(99)79-43(29-38-17-19-39(90)20-18-38)60(100)82-46(33-89)61(101)78-42(22-24-54(93)94)67(107)88-28-10-16-51(88)69(109)87-27-9-15-50(87)65(105)85-56/h4-6,11-12,17-20,36,40-51,56,89-90H,3,7-10,13-16,21-35H2,1-2H3,(H2,71,91)(H,75,97)(H,76,92)(H,77,102)(H,78,101)(H,79,99)(H,80,104)(H,81,103)(H,82,100)(H,83,98)(H,84,106)(H,85,105)(H,93,94)(H,95,96)(H4,72,73,74)/t36-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,56-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G using Suc-AAPF-MCA as substrate at pH 7.2 and 298 K measured every 60 secs for 600 secs |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B2
(Homo sapiens) | BDBM50520048

(CHEMBL4450993)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N2 Show InChI InChI=1S/C71H92N14O20S2/c1-4-38(2)58-68(102)81-51-37-107-106-36-50(80-61(95)45(30-40-14-7-5-8-15-40)74-55(88)34-72-60(94)47(33-57(91)92)77-66(100)52-18-11-27-83(52)70(104)48(78-65(51)99)32-41-16-9-6-10-17-41)64(98)73-39(3)59(93)76-46(31-42-21-23-43(87)24-22-42)62(96)79-49(35-86)63(97)75-44(25-26-56(89)90)69(103)85-29-13-20-54(85)71(105)84-28-12-19-53(84)67(101)82-58/h5-10,14-17,21-24,38-39,44-54,58,86-87H,4,11-13,18-20,25-37H2,1-3H3,(H,72,94)(H,73,98)(H,74,88)(H,75,97)(H,76,93)(H,77,100)(H,78,99)(H,79,96)(H,80,95)(H,81,102)(H,82,101)(H,89,90)(H,91,92)/t38-,39-,44-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,58-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human chymotrypsin using Suc-AAPF-MCA as substrate at pH 8 and 298 K measured every 60 secs for 600 secs |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B2
(Homo sapiens) | BDBM50520063

(CHEMBL4586444)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)[C@@H](C)O Show InChI InChI=1S/C69H97N17O21S2/c1-4-35(2)54-64(103)81-46-33-108-109-34-47(80-57(96)40(14-8-24-72-69(70)71)74-51(90)31-73-56(95)43(30-53(93)94)76-62(101)48-15-9-25-84(48)67(106)44(78-60(46)99)29-37-12-6-5-7-13-37)61(100)83-55(36(3)88)65(104)77-42(28-38-18-20-39(89)21-19-38)58(97)79-45(32-87)59(98)75-41(22-23-52(91)92)66(105)86-27-11-17-50(86)68(107)85-26-10-16-49(85)63(102)82-54/h5-7,12-13,18-21,35-36,40-50,54-55,87-89H,4,8-11,14-17,22-34H2,1-3H3,(H,73,95)(H,74,90)(H,75,98)(H,76,101)(H,77,104)(H,78,99)(H,79,97)(H,80,96)(H,81,103)(H,82,102)(H,83,100)(H,91,92)(H,93,94)(H4,70,71,72)/t35-,36+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,54-,55-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human chymotrypsin using Suc-AAPF-MCA as substrate at pH 8 and 298 K measured every 60 secs for 600 secs |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B2
(Homo sapiens) | BDBM50520058
(CHEMBL4469390)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(cc2)-c2ccccc2)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N2 |r| Show InChI InChI=1S/C81H98N14O18S2/c1-4-46(2)68-78(110)91-62-45-115-114-44-61(90-71(103)55(37-48-17-8-5-9-18-48)84-66(98)42-82-70(102)57(41-67(99)100)86-76(108)63-23-14-34-93(63)79(111)58(88-75(62)107)39-49-19-10-6-11-20-49)74(106)83-47(3)69(101)85-56(38-51-28-32-54(97)33-29-51)72(104)89-60(43-96)73(105)87-59(40-50-26-30-53(31-27-50)52-21-12-7-13-22-52)80(112)95-36-16-25-65(95)81(113)94-35-15-24-64(94)77(109)92-68/h5-13,17-22,26-33,46-47,55-65,68,96-97H,4,14-16,23-25,34-45H2,1-3H3,(H,82,102)(H,83,106)(H,84,98)(H,85,101)(H,86,108)(H,87,105)(H,88,107)(H,89,104)(H,90,103)(H,91,110)(H,92,109)(H,99,100)/t46-,47-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,68-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human chymotrypsin using Suc-AAPF-MCA as substrate at pH 8 and 298 K measured every 60 secs for 600 secs |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50520048

(CHEMBL4450993)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N2 Show InChI InChI=1S/C71H92N14O20S2/c1-4-38(2)58-68(102)81-51-37-107-106-36-50(80-61(95)45(30-40-14-7-5-8-15-40)74-55(88)34-72-60(94)47(33-57(91)92)77-66(100)52-18-11-27-83(52)70(104)48(78-65(51)99)32-41-16-9-6-10-17-41)64(98)73-39(3)59(93)76-46(31-42-21-23-43(87)24-22-42)62(96)79-49(35-86)63(97)75-44(25-26-56(89)90)69(103)85-29-13-20-54(85)71(105)84-28-12-19-53(84)67(101)82-58/h5-10,14-17,21-24,38-39,44-54,58,86-87H,4,11-13,18-20,25-37H2,1-3H3,(H,72,94)(H,73,98)(H,74,88)(H,75,97)(H,76,93)(H,77,100)(H,78,99)(H,79,96)(H,80,95)(H,81,102)(H,82,101)(H,89,90)(H,91,92)/t38-,39-,44-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,58-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50520049

(CHEMBL4560112)Show SMILES NC(=O)CC[C@@H]1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccc(Cl)cc3)NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 |r| Show InChI InChI=1S/C69H93ClN18O22S2/c70-37-16-14-36(15-17-37)28-42-60(102)83-45(32-89)61(103)79-41(20-23-54(94)95)66(108)88-27-7-13-50(88)68(110)87-26-6-12-49(87)64(106)78-40(19-22-53(92)93)59(101)85-47-34-112-111-33-46(62(104)77-39(58(100)80-42)18-21-51(71)90)84-57(99)38(10-4-24-74-69(72)73)76-52(91)31-75-56(98)43(30-55(96)97)81-65(107)48-11-5-25-86(48)67(109)44(82-63(47)105)29-35-8-2-1-3-9-35/h1-3,8-9,14-17,38-50,89H,4-7,10-13,18-34H2,(H2,71,90)(H,75,98)(H,76,91)(H,77,104)(H,78,106)(H,79,103)(H,80,100)(H,81,107)(H,82,105)(H,83,102)(H,84,99)(H,85,101)(H,92,93)(H,94,95)(H,96,97)(H4,72,73,74)/t38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50520060
(CHEMBL4465306)Show SMILES Cc1ccc(C[C@@H]2NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]3CSSC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]4CCCN4C(=O)[C@@H]4CCCN4C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC2=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N3)cc1 |r| Show InChI InChI=1S/C70H96N18O22S2/c1-36-15-17-38(18-16-36)29-43-61(102)83-46(33-89)62(103)79-42(21-24-55(94)95)67(108)88-28-8-14-51(88)69(110)87-27-7-13-50(87)65(106)78-41(20-23-54(92)93)60(101)85-48-35-112-111-34-47(63(104)77-40(59(100)80-43)19-22-52(71)90)84-58(99)39(11-5-25-74-70(72)73)76-53(91)32-75-57(98)44(31-56(96)97)81-66(107)49-12-6-26-86(49)68(109)45(82-64(48)105)30-37-9-3-2-4-10-37/h2-4,9-10,15-18,39-51,89H,5-8,11-14,19-35H2,1H3,(H2,71,90)(H,75,98)(H,76,91)(H,77,104)(H,78,106)(H,79,103)(H,80,100)(H,81,107)(H,82,105)(H,83,102)(H,84,99)(H,85,101)(H,92,93)(H,94,95)(H,96,97)(H4,72,73,74)/t39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50520063

(CHEMBL4586444)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)[C@@H](C)O Show InChI InChI=1S/C69H97N17O21S2/c1-4-35(2)54-64(103)81-46-33-108-109-34-47(80-57(96)40(14-8-24-72-69(70)71)74-51(90)31-73-56(95)43(30-53(93)94)76-62(101)48-15-9-25-84(48)67(106)44(78-60(46)99)29-37-12-6-5-7-13-37)61(100)83-55(36(3)88)65(104)77-42(28-38-18-20-39(89)21-19-38)58(97)79-45(32-87)59(98)75-41(22-23-52(91)92)66(105)86-27-11-17-50(86)68(107)85-26-10-16-49(85)63(102)82-54/h5-7,12-13,18-21,35-36,40-50,54-55,87-89H,4,8-11,14-17,22-34H2,1-3H3,(H,73,95)(H,74,90)(H,75,98)(H,76,101)(H,77,104)(H,78,99)(H,79,97)(H,80,96)(H,81,103)(H,82,102)(H,83,100)(H,91,92)(H,93,94)(H4,70,71,72)/t35-,36+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,54-,55-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50520050

(CHEMBL4457132)Show SMILES NC(=O)CC[C@@H]1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccc(O)cc3)NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 Show InChI InChI=1S/C69H94N18O23S2/c70-51(90)21-18-39-58(100)79-42(28-36-14-16-37(89)17-15-36)60(102)82-45(32-88)61(103)78-41(20-23-54(94)95)66(108)87-27-7-13-50(87)68(110)86-26-6-12-49(86)64(106)77-40(19-22-53(92)93)59(101)84-47-34-112-111-33-46(62(104)76-39)83-57(99)38(10-4-24-73-69(71)72)75-52(91)31-74-56(98)43(30-55(96)97)80-65(107)48-11-5-25-85(48)67(109)44(81-63(47)105)29-35-8-2-1-3-9-35/h1-3,8-9,14-17,38-50,88-89H,4-7,10-13,18-34H2,(H2,70,90)(H,74,98)(H,75,91)(H,76,104)(H,77,106)(H,78,103)(H,79,100)(H,80,107)(H,81,105)(H,82,102)(H,83,99)(H,84,101)(H,92,93)(H,94,95)(H,96,97)(H4,71,72,73)/t38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50520058

(CHEMBL4469390)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(cc2)-c2ccccc2)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N2 |r| Show InChI InChI=1S/C81H98N14O18S2/c1-4-46(2)68-78(110)91-62-45-115-114-44-61(90-71(103)55(37-48-17-8-5-9-18-48)84-66(98)42-82-70(102)57(41-67(99)100)86-76(108)63-23-14-34-93(63)79(111)58(88-75(62)107)39-49-19-10-6-11-20-49)74(106)83-47(3)69(101)85-56(38-51-28-32-54(97)33-29-51)72(104)89-60(43-96)73(105)87-59(40-50-26-30-53(31-27-50)52-21-12-7-13-22-52)80(112)95-36-16-25-65(95)81(113)94-35-15-24-64(94)77(109)92-68/h5-13,17-22,26-33,46-47,55-65,68,96-97H,4,14-16,23-25,34-45H2,1-3H3,(H,82,102)(H,83,106)(H,84,98)(H,85,101)(H,86,108)(H,87,105)(H,88,107)(H,89,104)(H,90,103)(H,91,110)(H,92,109)(H,99,100)/t46-,47-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,68-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G using Suc-AAPF-MCA as substrate at pH 7.2 and 298 K measured every 60 secs for 600 secs |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50520064

(CHEMBL4590739)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccc(O)cc3)NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 Show InChI InChI=1S/C68H93N17O23S2/c1-34(87)54-64(105)77-41(27-36-15-17-37(88)18-16-36)58(99)79-44(31-86)59(100)75-40(20-22-52(92)93)65(106)85-26-8-14-49(85)67(108)84-25-7-13-48(84)62(103)74-39(19-21-51(90)91)57(98)80-45-32-109-110-33-46(61(102)82-54)81-56(97)38(11-5-23-71-68(69)70)73-50(89)30-72-55(96)42(29-53(94)95)76-63(104)47-12-6-24-83(47)66(107)43(78-60(45)101)28-35-9-3-2-4-10-35/h2-4,9-10,15-18,34,38-49,54,86-88H,5-8,11-14,19-33H2,1H3,(H,72,96)(H,73,89)(H,74,103)(H,75,100)(H,76,104)(H,77,105)(H,78,101)(H,79,99)(H,80,98)(H,81,97)(H,82,102)(H,90,91)(H,92,93)(H,94,95)(H4,69,70,71)/t34-,38+,39+,40+,41+,42+,43+,44+,45+,46+,47+,48+,49+,54+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50520063

(CHEMBL4586444)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)[C@@H](C)O Show InChI InChI=1S/C69H97N17O21S2/c1-4-35(2)54-64(103)81-46-33-108-109-34-47(80-57(96)40(14-8-24-72-69(70)71)74-51(90)31-73-56(95)43(30-53(93)94)76-62(101)48-15-9-25-84(48)67(106)44(78-60(46)99)29-37-12-6-5-7-13-37)61(100)83-55(36(3)88)65(104)77-42(28-38-18-20-39(89)21-19-38)58(97)79-45(32-87)59(98)75-41(22-23-52(91)92)66(105)86-27-11-17-50(86)68(107)85-26-10-16-49(85)63(102)82-54/h5-7,12-13,18-21,35-36,40-50,54-55,87-89H,4,8-11,14-17,22-34H2,1-3H3,(H,73,95)(H,74,90)(H,75,98)(H,76,101)(H,77,104)(H,78,99)(H,79,97)(H,80,96)(H,81,103)(H,82,102)(H,83,100)(H,91,92)(H,93,94)(H4,70,71,72)/t35-,36+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,54-,55-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G using Suc-AAPF-MCA as substrate at pH 7.2 and 298 K measured every 60 secs for 600 secs |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50520066

(CHEMBL4442550)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N2 Show InChI InChI=1S/C70H90N14O20S2/c1-4-37(2)57-67(101)80-50-36-106-105-35-49(79-60(94)43(28-39-14-7-5-8-15-39)73-54(87)33-71-59(93)45(31-55(88)89)75-65(99)51-18-11-25-82(51)68(102)46(76-64(50)98)30-40-16-9-6-10-17-40)63(97)72-38(3)58(92)74-44(29-41-21-23-42(86)24-22-41)61(95)78-48(34-85)62(96)77-47(32-56(90)91)69(103)84-27-13-20-53(84)70(104)83-26-12-19-52(83)66(100)81-57/h5-10,14-17,21-24,37-38,43-53,57,85-86H,4,11-13,18-20,25-36H2,1-3H3,(H,71,93)(H,72,97)(H,73,87)(H,74,92)(H,75,99)(H,76,98)(H,77,96)(H,78,95)(H,79,94)(H,80,101)(H,81,100)(H,88,89)(H,90,91)/t37-,38-,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,53-,57-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50520059

(CHEMBL4561218)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)[C@@H](C)CC Show InChI InChI=1S/C71H102N18O19S2/c1-5-37(3)56-67(105)84-49-36-110-109-35-48(83-59(97)42(16-10-26-75-71(73)74)77-54(93)33-76-58(96)45(32-55(94)95)80-65(103)50-17-11-27-87(50)68(106)46(81-64(49)102)31-39-14-8-7-9-15-39)63(101)78-43(24-25-53(72)92)60(98)79-44(30-40-20-22-41(91)23-21-40)61(99)82-47(34-90)62(100)86-57(38(4)6-2)70(108)89-29-13-19-52(89)69(107)88-28-12-18-51(88)66(104)85-56/h7-9,14-15,20-23,37-38,42-52,56-57,90-91H,5-6,10-13,16-19,24-36H2,1-4H3,(H2,72,92)(H,76,96)(H,77,93)(H,78,101)(H,79,98)(H,80,103)(H,81,102)(H,82,99)(H,83,97)(H,84,105)(H,85,104)(H,86,100)(H,94,95)(H4,73,74,75)/t37-,38-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,56-,57-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B2
(Homo sapiens) | BDBM50520064

(CHEMBL4590739)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccc(O)cc3)NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 Show InChI InChI=1S/C68H93N17O23S2/c1-34(87)54-64(105)77-41(27-36-15-17-37(88)18-16-36)58(99)79-44(31-86)59(100)75-40(20-22-52(92)93)65(106)85-26-8-14-49(85)67(108)84-25-7-13-48(84)62(103)74-39(19-21-51(90)91)57(98)80-45-32-109-110-33-46(61(102)82-54)81-56(97)38(11-5-23-71-68(69)70)73-50(89)30-72-55(96)42(29-53(94)95)76-63(104)47-12-6-24-83(47)66(107)43(78-60(45)101)28-35-9-3-2-4-10-35/h2-4,9-10,15-18,34,38-49,54,86-88H,5-8,11-14,19-33H2,1H3,(H,72,96)(H,73,89)(H,74,103)(H,75,100)(H,76,104)(H,77,105)(H,78,101)(H,79,99)(H,80,98)(H,81,97)(H,82,102)(H,90,91)(H,92,93)(H,94,95)(H4,69,70,71)/t34-,38+,39+,40+,41+,42+,43+,44+,45+,46+,47+,48+,49+,54+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human chymotrypsin using Suc-AAPF-MCA as substrate at pH 8 and 298 K measured every 60 secs for 600 secs |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50520064

(CHEMBL4590739)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccc(O)cc3)NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 Show InChI InChI=1S/C68H93N17O23S2/c1-34(87)54-64(105)77-41(27-36-15-17-37(88)18-16-36)58(99)79-44(31-86)59(100)75-40(20-22-52(92)93)65(106)85-26-8-14-49(85)67(108)84-25-7-13-48(84)62(103)74-39(19-21-51(90)91)57(98)80-45-32-109-110-33-46(61(102)82-54)81-56(97)38(11-5-23-71-68(69)70)73-50(89)30-72-55(96)42(29-53(94)95)76-63(104)47-12-6-24-83(47)66(107)43(78-60(45)101)28-35-9-3-2-4-10-35/h2-4,9-10,15-18,34,38-49,54,86-88H,5-8,11-14,19-33H2,1H3,(H,72,96)(H,73,89)(H,74,103)(H,75,100)(H,76,104)(H,77,105)(H,78,101)(H,79,99)(H,80,98)(H,81,97)(H,82,102)(H,90,91)(H,92,93)(H,94,95)(H4,69,70,71)/t34-,38+,39+,40+,41+,42+,43+,44+,45+,46+,47+,48+,49+,54+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G using Suc-AAPF-MCA as substrate at pH 7.2 and 298 K measured every 60 secs for 600 secs |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50520052
(CHEMBL4554739)Show SMILES NC(=O)CC[C@@H]1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccc(I)cc3)NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 |r| Show InChI InChI=1S/C69H93IN18O22S2/c70-37-16-14-36(15-17-37)28-42-60(102)83-45(32-89)61(103)79-41(20-23-54(94)95)66(108)88-27-7-13-50(88)68(110)87-26-6-12-49(87)64(106)78-40(19-22-53(92)93)59(101)85-47-34-112-111-33-46(62(104)77-39(58(100)80-42)18-21-51(71)90)84-57(99)38(10-4-24-74-69(72)73)76-52(91)31-75-56(98)43(30-55(96)97)81-65(107)48-11-5-25-86(48)67(109)44(82-63(47)105)29-35-8-2-1-3-9-35/h1-3,8-9,14-17,38-50,89H,4-7,10-13,18-34H2,(H2,71,90)(H,75,98)(H,76,91)(H,77,104)(H,78,106)(H,79,103)(H,80,100)(H,81,107)(H,82,105)(H,83,102)(H,84,99)(H,85,101)(H,92,93)(H,94,95)(H,96,97)(H4,72,73,74)/t38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50520058

(CHEMBL4469390)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccc(cc2)-c2ccccc2)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N2 |r| Show InChI InChI=1S/C81H98N14O18S2/c1-4-46(2)68-78(110)91-62-45-115-114-44-61(90-71(103)55(37-48-17-8-5-9-18-48)84-66(98)42-82-70(102)57(41-67(99)100)86-76(108)63-23-14-34-93(63)79(111)58(88-75(62)107)39-49-19-10-6-11-20-49)74(106)83-47(3)69(101)85-56(38-51-28-32-54(97)33-29-51)72(104)89-60(43-96)73(105)87-59(40-50-26-30-53(31-27-50)52-21-12-7-13-22-52)80(112)95-36-16-25-65(95)81(113)94-35-15-24-64(94)77(109)92-68/h5-13,17-22,26-33,46-47,55-65,68,96-97H,4,14-16,23-25,34-45H2,1-3H3,(H,82,102)(H,83,106)(H,84,98)(H,85,101)(H,86,108)(H,87,105)(H,88,107)(H,89,104)(H,90,103)(H,91,110)(H,92,109)(H,99,100)/t46-,47-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,68-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50520057

(CHEMBL4441622)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N2 Show InChI InChI=1S/C75H94N14O18S2/c1-4-42(2)62-72(104)85-56-41-109-108-40-55(84-65(97)49(33-44-17-8-5-9-18-44)78-60(92)38-76-64(96)51(37-61(93)94)80-70(102)57-23-14-30-87(57)73(105)52(82-69(56)101)35-45-19-10-6-11-20-45)68(100)77-43(3)63(95)79-50(34-47-26-28-48(91)29-27-47)66(98)83-54(39-90)67(99)81-53(36-46-21-12-7-13-22-46)74(106)89-32-16-25-59(89)75(107)88-31-15-24-58(88)71(103)86-62/h5-13,17-22,26-29,42-43,49-59,62,90-91H,4,14-16,23-25,30-41H2,1-3H3,(H,76,96)(H,77,100)(H,78,92)(H,79,95)(H,80,102)(H,81,99)(H,82,101)(H,83,98)(H,84,97)(H,85,104)(H,86,103)(H,93,94)/t42-,43-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50520061
(CHEMBL4556207)Show SMILES NC(=O)CC[C@@H]1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccc(cc3)[N+]([O-])=O)NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 |r| Show InChI InChI=1S/C69H93N19O24S2/c70-51(90)21-18-39-58(100)79-42(28-36-14-16-37(17-15-36)88(111)112)60(102)82-45(32-89)61(103)78-41(20-23-54(94)95)66(108)87-27-7-13-50(87)68(110)86-26-6-12-49(86)64(106)77-40(19-22-53(92)93)59(101)84-47-34-114-113-33-46(62(104)76-39)83-57(99)38(10-4-24-73-69(71)72)75-52(91)31-74-56(98)43(30-55(96)97)80-65(107)48-11-5-25-85(48)67(109)44(81-63(47)105)29-35-8-2-1-3-9-35/h1-3,8-9,14-17,38-50,89H,4-7,10-13,18-34H2,(H2,70,90)(H,74,98)(H,75,91)(H,76,104)(H,77,106)(H,78,103)(H,79,100)(H,80,107)(H,81,105)(H,82,102)(H,83,99)(H,84,101)(H,92,93)(H,94,95)(H,96,97)(H4,71,72,73)/t38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50520055
(CHEMBL4464900)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N2)[C@@H](C)CC Show InChI InChI=1S/C72H96N14O18S2/c1-6-39(3)58-69(101)81-52-38-106-105-37-51(80-62(94)46(31-42-17-10-8-11-18-42)75-56(89)35-73-61(93)48(34-57(90)91)77-67(99)53-21-14-28-84(53)70(102)49(78-66(52)98)33-43-19-12-9-13-20-43)65(97)74-41(5)60(92)76-47(32-44-24-26-45(88)27-25-44)63(95)79-50(36-87)64(96)83-59(40(4)7-2)72(104)86-30-16-23-55(86)71(103)85-29-15-22-54(85)68(100)82-58/h8-13,17-20,24-27,39-41,46-55,58-59,87-88H,6-7,14-16,21-23,28-38H2,1-5H3,(H,73,93)(H,74,97)(H,75,89)(H,76,92)(H,77,99)(H,78,98)(H,79,95)(H,80,94)(H,81,101)(H,82,100)(H,83,96)(H,90,91)/t39-,40-,41-,46-,47-,48-,49-,50-,51-,52-,53-,54-,55-,58-,59-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50520050

(CHEMBL4457132)Show SMILES NC(=O)CC[C@@H]1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccc(O)cc3)NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 Show InChI InChI=1S/C69H94N18O23S2/c70-51(90)21-18-39-58(100)79-42(28-36-14-16-37(89)17-15-36)60(102)82-45(32-88)61(103)78-41(20-23-54(94)95)66(108)87-27-7-13-50(87)68(110)86-26-6-12-49(86)64(106)77-40(19-22-53(92)93)59(101)84-47-34-112-111-33-46(62(104)76-39)83-57(99)38(10-4-24-73-69(71)72)75-52(91)31-74-56(98)43(30-55(96)97)80-65(107)48-11-5-25-85(48)67(109)44(81-63(47)105)29-35-8-2-1-3-9-35/h1-3,8-9,14-17,38-50,88-89H,4-7,10-13,18-34H2,(H2,70,90)(H,74,98)(H,75,91)(H,76,104)(H,77,106)(H,78,103)(H,79,100)(H,80,107)(H,81,105)(H,82,102)(H,83,99)(H,84,101)(H,92,93)(H,94,95)(H,96,97)(H4,71,72,73)/t38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G using Suc-AAPF-MCA as substrate at pH 7.2 and 298 K measured every 60 secs for 600 secs |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50520053

(CHEMBL4449838)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)[C@@H](C)O)[C@@H](C)CC Show InChI InChI=1S/C70H101N17O19S2/c1-6-36(3)54-65(102)81-47-34-107-108-35-48(80-58(95)42(17-11-25-73-70(71)72)75-52(91)32-74-57(94)44(31-53(92)93)76-63(100)49-18-12-26-85(49)67(104)45(78-61(47)98)30-39-15-9-8-10-16-39)62(99)84-56(38(5)89)66(103)77-43(29-40-21-23-41(90)24-22-40)59(96)79-46(33-88)60(97)83-55(37(4)7-2)69(106)87-28-14-20-51(87)68(105)86-27-13-19-50(86)64(101)82-54/h8-10,15-16,21-24,36-38,42-51,54-56,88-90H,6-7,11-14,17-20,25-35H2,1-5H3,(H,74,94)(H,75,91)(H,76,100)(H,77,103)(H,78,98)(H,79,96)(H,80,95)(H,81,102)(H,82,101)(H,83,97)(H,84,99)(H,92,93)(H4,71,72,73)/t36-,37-,38+,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,54-,55-,56-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50520049

(CHEMBL4560112)Show SMILES NC(=O)CC[C@@H]1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccc(Cl)cc3)NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 |r| Show InChI InChI=1S/C69H93ClN18O22S2/c70-37-16-14-36(15-17-37)28-42-60(102)83-45(32-89)61(103)79-41(20-23-54(94)95)66(108)88-27-7-13-50(88)68(110)87-26-6-12-49(87)64(106)78-40(19-22-53(92)93)59(101)85-47-34-112-111-33-46(62(104)77-39(58(100)80-42)18-21-51(71)90)84-57(99)38(10-4-24-74-69(72)73)76-52(91)31-75-56(98)43(30-55(96)97)81-65(107)48-11-5-25-86(48)67(109)44(82-63(47)105)29-35-8-2-1-3-9-35/h1-3,8-9,14-17,38-50,89H,4-7,10-13,18-34H2,(H2,71,90)(H,75,98)(H,76,91)(H,77,104)(H,78,106)(H,79,103)(H,80,100)(H,81,107)(H,82,105)(H,83,102)(H,84,99)(H,85,101)(H,92,93)(H,94,95)(H,96,97)(H4,72,73,74)/t38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G using Suc-AAPF-MCA as substrate at pH 7.2 and 298 K measured every 60 secs for 600 secs |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B2
(Homo sapiens) | BDBM50520051

(CHEMBL4592765)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 Show InChI InChI=1S/C70H98N18O21S2/c1-3-36(2)56-66(106)84-48-35-111-110-34-47(83-58(98)40(13-7-25-74-70(72)73)76-53(92)32-75-57(97)44(31-55(95)96)80-64(104)49-14-8-26-86(49)68(108)45(81-63(48)103)30-37-11-5-4-6-12-37)62(102)77-41(21-23-52(71)91)59(99)79-43(29-38-17-19-39(90)20-18-38)60(100)82-46(33-89)61(101)78-42(22-24-54(93)94)67(107)88-28-10-16-51(88)69(109)87-27-9-15-50(87)65(105)85-56/h4-6,11-12,17-20,36,40-51,56,89-90H,3,7-10,13-16,21-35H2,1-2H3,(H2,71,91)(H,75,97)(H,76,92)(H,77,102)(H,78,101)(H,79,99)(H,80,104)(H,81,103)(H,82,100)(H,83,98)(H,84,106)(H,85,105)(H,93,94)(H,95,96)(H4,72,73,74)/t36-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,56-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human chymotrypsin using Suc-AAPF-MCA as substrate at pH 8 and 298 K measured every 60 secs for 600 secs |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50520060

(CHEMBL4465306)Show SMILES Cc1ccc(C[C@@H]2NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]3CSSC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]4CCCN4C(=O)[C@@H]4CCCN4C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC2=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N3)cc1 |r| Show InChI InChI=1S/C70H96N18O22S2/c1-36-15-17-38(18-16-36)29-43-61(102)83-46(33-89)62(103)79-42(21-24-55(94)95)67(108)88-28-8-14-51(88)69(110)87-27-7-13-50(87)65(106)78-41(20-23-54(92)93)60(101)85-48-35-112-111-34-47(63(104)77-40(59(100)80-43)19-22-52(71)90)84-58(99)39(11-5-25-74-70(72)73)76-53(91)32-75-57(98)44(31-56(96)97)81-66(107)49-12-6-26-86(49)68(109)45(82-64(48)105)30-37-9-3-2-4-10-37/h2-4,9-10,15-18,39-51,89H,5-8,11-14,19-35H2,1H3,(H2,71,90)(H,75,98)(H,76,91)(H,77,104)(H,78,106)(H,79,103)(H,80,100)(H,81,107)(H,82,105)(H,83,102)(H,84,99)(H,85,101)(H,92,93)(H,94,95)(H,96,97)(H4,72,73,74)/t39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G using Suc-AAPF-MCA as substrate at pH 7.2 and 298 K measured every 60 secs for 600 secs |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B2
(Homo sapiens) | BDBM50520049

(CHEMBL4560112)Show SMILES NC(=O)CC[C@@H]1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccc(Cl)cc3)NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 |r| Show InChI InChI=1S/C69H93ClN18O22S2/c70-37-16-14-36(15-17-37)28-42-60(102)83-45(32-89)61(103)79-41(20-23-54(94)95)66(108)88-27-7-13-50(88)68(110)87-26-6-12-49(87)64(106)78-40(19-22-53(92)93)59(101)85-47-34-112-111-33-46(62(104)77-39(58(100)80-42)18-21-51(71)90)84-57(99)38(10-4-24-74-69(72)73)76-52(91)31-75-56(98)43(30-55(96)97)81-65(107)48-11-5-25-86(48)67(109)44(82-63(47)105)29-35-8-2-1-3-9-35/h1-3,8-9,14-17,38-50,89H,4-7,10-13,18-34H2,(H2,71,90)(H,75,98)(H,76,91)(H,77,104)(H,78,106)(H,79,103)(H,80,100)(H,81,107)(H,82,105)(H,83,102)(H,84,99)(H,85,101)(H,92,93)(H,94,95)(H,96,97)(H4,72,73,74)/t38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human chymotrypsin using Suc-AAPF-MCA as substrate at pH 8 and 298 K measured every 60 secs for 600 secs |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50267001

(CHEMBL4074776)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N2)[C@@H](C)O)[C@@H](C)CC |r| Show InChI InChI=1S/C63H94N16O20S2/c1-7-29(3)47-58(94)71-39-27-100-101-28-40(72-59(95)49(31(5)81)73-46(86)25-66-51(87)36(23-44(64)84)67-56(92)41-12-9-19-77(41)61(97)37(24-45(65)85)69-54(39)90)55(91)76-50(32(6)82)60(96)68-35(22-33-15-17-34(83)18-16-33)52(88)70-38(26-80)53(89)75-48(30(4)8-2)63(99)79-21-11-14-43(79)62(98)78-20-10-13-42(78)57(93)74-47/h15-18,29-32,35-43,47-50,80-83H,7-14,19-28H2,1-6H3,(H2,64,84)(H2,65,85)(H,66,87)(H,67,92)(H,68,96)(H,69,90)(H,70,88)(H,71,94)(H,72,95)(H,73,86)(H,74,93)(H,75,89)(H,76,91)/t29-,30-,31+,32+,35-,36-,37-,38-,39-,40-,41-,42-,43-,47-,48-,49-,50-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50267000
(CHEMBL4083086)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N2)[C@@H](C)O)[C@@H](C)CC |r| Show InChI InChI=1S/C63H94N16O19S2/c1-7-30(3)47-58(93)71-39-28-99-100-29-40(72-59(94)49(32(5)81)73-46(85)26-66-51(86)36(24-44(64)83)67-56(91)41-17-12-20-77(41)61(96)37(25-45(65)84)69-54(39)89)55(90)76-50(33(6)82)60(95)68-35(23-34-15-10-9-11-16-34)52(87)70-38(27-80)53(88)75-48(31(4)8-2)63(98)79-22-14-19-43(79)62(97)78-21-13-18-42(78)57(92)74-47/h9-11,15-16,30-33,35-43,47-50,80-82H,7-8,12-14,17-29H2,1-6H3,(H2,64,83)(H2,65,84)(H,66,86)(H,67,91)(H,68,95)(H,69,89)(H,70,87)(H,71,93)(H,72,94)(H,73,85)(H,74,92)(H,75,88)(H,76,90)/t30-,31-,32+,33+,35-,36-,37-,38-,39-,40-,41-,42-,43-,47-,48-,49-,50-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50520056
(CHEMBL4462421)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)[C@@H](C)CC Show InChI InChI=1S/C70H100N18O19S2/c1-5-36(3)55-66(104)83-48-35-109-108-34-47(82-58(96)41(16-10-24-74-70(72)73)76-53(92)32-75-57(95)44(31-54(93)94)79-64(102)49-17-11-25-86(49)67(105)45(80-63(48)101)29-38-14-8-7-9-15-38)62(100)78-43(30-52(71)91)60(98)77-42(28-39-20-22-40(90)23-21-39)59(97)81-46(33-89)61(99)85-56(37(4)6-2)69(107)88-27-13-19-51(88)68(106)87-26-12-18-50(87)65(103)84-55/h7-9,14-15,20-23,36-37,41-51,55-56,89-90H,5-6,10-13,16-19,24-35H2,1-4H3,(H2,71,91)(H,75,95)(H,76,92)(H,77,98)(H,78,100)(H,79,102)(H,80,101)(H,81,97)(H,82,96)(H,83,104)(H,84,103)(H,85,99)(H,93,94)(H4,72,73,74)/t36-,37-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,55-,56-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B2
(Homo sapiens) | BDBM50520061

(CHEMBL4556207)Show SMILES NC(=O)CC[C@@H]1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccc(cc3)[N+]([O-])=O)NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 |r| Show InChI InChI=1S/C69H93N19O24S2/c70-51(90)21-18-39-58(100)79-42(28-36-14-16-37(17-15-36)88(111)112)60(102)82-45(32-89)61(103)78-41(20-23-54(94)95)66(108)87-27-7-13-50(87)68(110)86-26-6-12-49(86)64(106)77-40(19-22-53(92)93)59(101)84-47-34-114-113-33-46(62(104)76-39)83-57(99)38(10-4-24-73-69(71)72)75-52(91)31-74-56(98)43(30-55(96)97)80-65(107)48-11-5-25-85(48)67(109)44(81-63(47)105)29-35-8-2-1-3-9-35/h1-3,8-9,14-17,38-50,89H,4-7,10-13,18-34H2,(H2,70,90)(H,74,98)(H,75,91)(H,76,104)(H,77,106)(H,78,103)(H,79,100)(H,80,107)(H,81,105)(H,82,102)(H,83,99)(H,84,101)(H,92,93)(H,94,95)(H,96,97)(H4,71,72,73)/t38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human chymotrypsin using Suc-AAPF-MCA as substrate at pH 8 and 298 K measured every 60 secs for 600 secs |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50520052

(CHEMBL4554739)Show SMILES NC(=O)CC[C@@H]1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccc(I)cc3)NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 |r| Show InChI InChI=1S/C69H93IN18O22S2/c70-37-16-14-36(15-17-37)28-42-60(102)83-45(32-89)61(103)79-41(20-23-54(94)95)66(108)88-27-7-13-50(88)68(110)87-26-6-12-49(87)64(106)78-40(19-22-53(92)93)59(101)85-47-34-112-111-33-46(62(104)77-39(58(100)80-42)18-21-51(71)90)84-57(99)38(10-4-24-74-69(72)73)76-52(91)31-75-56(98)43(30-55(96)97)81-65(107)48-11-5-25-86(48)67(109)44(82-63(47)105)29-35-8-2-1-3-9-35/h1-3,8-9,14-17,38-50,89H,4-7,10-13,18-34H2,(H2,71,90)(H,75,98)(H,76,91)(H,77,104)(H,78,106)(H,79,103)(H,80,100)(H,81,107)(H,82,105)(H,83,102)(H,84,99)(H,85,101)(H,92,93)(H,94,95)(H,96,97)(H4,72,73,74)/t38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G using Suc-AAPF-MCA as substrate at pH 7.2 and 298 K measured every 60 secs for 600 secs |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B2
(Homo sapiens) | BDBM50520060

(CHEMBL4465306)Show SMILES Cc1ccc(C[C@@H]2NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]3CSSC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]4CCCN4C(=O)[C@@H]4CCCN4C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC2=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N3)cc1 |r| Show InChI InChI=1S/C70H96N18O22S2/c1-36-15-17-38(18-16-36)29-43-61(102)83-46(33-89)62(103)79-42(21-24-55(94)95)67(108)88-28-8-14-51(88)69(110)87-27-7-13-50(87)65(106)78-41(20-23-54(92)93)60(101)85-48-35-112-111-34-47(63(104)77-40(59(100)80-43)19-22-52(71)90)84-58(99)39(11-5-25-74-70(72)73)76-53(91)32-75-57(98)44(31-56(96)97)81-66(107)49-12-6-26-86(49)68(109)45(82-64(48)105)30-37-9-3-2-4-10-37/h2-4,9-10,15-18,39-51,89H,5-8,11-14,19-35H2,1H3,(H2,71,90)(H,75,98)(H,76,91)(H,77,104)(H,78,106)(H,79,103)(H,80,100)(H,81,107)(H,82,105)(H,83,102)(H,84,99)(H,85,101)(H,92,93)(H,94,95)(H,96,97)(H4,72,73,74)/t39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human chymotrypsin using Suc-AAPF-MCA as substrate at pH 8 and 298 K measured every 60 secs for 600 secs |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50520065

(CHEMBL4593335)Show SMILES CCCC[C@@H]1NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(=O)N2 Show InChI InChI=1S/C70H99N15O19S2/c1-6-9-18-42-59(93)79-48-35-105-106-36-49(64(98)77-46(30-39-16-11-10-12-17-39)68(102)83-26-13-19-50(83)65(99)76-45(32-55(90)91)58(92)72-33-54(89)73-42)80-67(101)56(37(4)7-2)81-66(100)51-20-14-27-84(51)69(103)52-21-15-28-85(52)70(104)57(38(5)8-3)82-62(96)47(34-86)78-60(94)43(29-40-22-24-41(87)25-23-40)74-61(95)44(31-53(71)88)75-63(48)97/h10-12,16-17,22-25,37-38,42-52,56-57,86-87H,6-9,13-15,18-21,26-36H2,1-5H3,(H2,71,88)(H,72,92)(H,73,89)(H,74,95)(H,75,97)(H,76,99)(H,77,98)(H,78,94)(H,79,93)(H,80,101)(H,81,100)(H,82,96)(H,90,91)/t37-,38-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,56-,57-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells using NleTDY-pNA as substrate assessed as cleavage of pNA at pH 7.2 a... |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B2
(Homo sapiens) | BDBM50520050

(CHEMBL4457132)Show SMILES NC(=O)CC[C@@H]1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccc(O)cc3)NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 Show InChI InChI=1S/C69H94N18O23S2/c70-51(90)21-18-39-58(100)79-42(28-36-14-16-37(89)17-15-36)60(102)82-45(32-88)61(103)78-41(20-23-54(94)95)66(108)87-27-7-13-50(87)68(110)86-26-6-12-49(86)64(106)77-40(19-22-53(92)93)59(101)84-47-34-112-111-33-46(62(104)76-39)83-57(99)38(10-4-24-73-69(71)72)75-52(91)31-74-56(98)43(30-55(96)97)80-65(107)48-11-5-25-85(48)67(109)44(81-63(47)105)29-35-8-2-1-3-9-35/h1-3,8-9,14-17,38-50,88-89H,4-7,10-13,18-34H2,(H2,70,90)(H,74,98)(H,75,91)(H,76,104)(H,77,106)(H,78,103)(H,79,100)(H,80,107)(H,81,105)(H,82,102)(H,83,99)(H,84,101)(H,92,93)(H,94,95)(H,96,97)(H4,71,72,73)/t38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human chymotrypsin using Suc-AAPF-MCA as substrate at pH 8 and 298 K measured every 60 secs for 600 secs |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chromobox protein homolog 7
(Homo sapiens (Human)) | BDBM50189696
(CHEMBL3827924)Show SMILES COc1cccc(C(=O)N2CCN(CC2)C(=O)COc2cc(C)c(NC(N)=O)c(C)c2)c1OC Show InChI InChI=1S/C24H30N4O6/c1-15-12-17(13-16(2)21(15)26-24(25)31)34-14-20(29)27-8-10-28(11-9-27)23(30)18-6-5-7-19(32-3)22(18)33-4/h5-7,12-13H,8-11,14H2,1-4H3,(H3,25,26,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
| Assay Description
Binding affinity to CBX7ChD (7 to 66 residues) (unknown origin) expressed in RIPL-BL21 (DE3)-CodonPlus competent cells in presence of FITC-labeled SE... |
ACS Med Chem Lett 7: 601-5 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00042
BindingDB Entry DOI: 10.7270/Q28054JT |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50520061

(CHEMBL4556207)Show SMILES NC(=O)CC[C@@H]1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]3CCCN3C(=O)[C@@H]3CCCN3C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc3ccc(cc3)[N+]([O-])=O)NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 |r| Show InChI InChI=1S/C69H93N19O24S2/c70-51(90)21-18-39-58(100)79-42(28-36-14-16-37(17-15-36)88(111)112)60(102)82-45(32-89)61(103)78-41(20-23-54(94)95)66(108)87-27-7-13-50(87)68(110)86-26-6-12-49(86)64(106)77-40(19-22-53(92)93)59(101)84-47-34-114-113-33-46(62(104)76-39)83-57(99)38(10-4-24-73-69(71)72)75-52(91)31-74-56(98)43(30-55(96)97)80-65(107)48-11-5-25-85(48)67(109)44(81-63(47)105)29-35-8-2-1-3-9-35/h1-3,8-9,14-17,38-50,89H,4-7,10-13,18-34H2,(H2,70,90)(H,74,98)(H,75,91)(H,76,104)(H,77,106)(H,78,103)(H,79,100)(H,80,107)(H,81,105)(H,82,102)(H,83,99)(H,84,101)(H,92,93)(H,94,95)(H,96,97)(H4,71,72,73)/t38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin G using Suc-AAPF-MCA as substrate at pH 7.2 and 298 K measured every 60 secs for 600 secs |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |
Chromobox protein homolog 7
(Homo sapiens (Human)) | BDBM50189699

(CHEMBL3827834)Show SMILES COc1cccc(C(=O)N2CCN(CC2)C(=O)COc2ccc(NC(N)=O)c(C)c2)c1OC Show InChI InChI=1S/C23H28N4O6/c1-15-13-16(7-8-18(15)25-23(24)30)33-14-20(28)26-9-11-27(12-10-26)22(29)17-5-4-6-19(31-2)21(17)32-3/h4-8,13H,9-12,14H2,1-3H3,(H3,24,25,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
| Assay Description
Binding affinity to CBX7ChD (7 to 66 residues) (unknown origin) expressed in RIPL-BL21 (DE3)-CodonPlus competent cells in presence of FITC-labeled SE... |
ACS Med Chem Lett 7: 601-5 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00042
BindingDB Entry DOI: 10.7270/Q28054JT |
More data for this
Ligand-Target Pair | |
Chromobox protein homolog 7
(Homo sapiens (Human)) | BDBM50189697
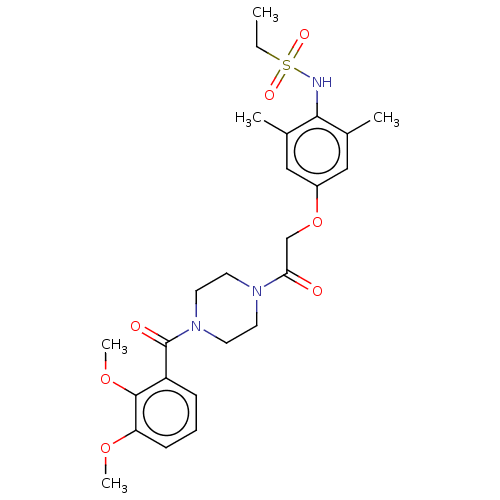
(CHEMBL3827733)Show SMILES CCS(=O)(=O)Nc1c(C)cc(OCC(=O)N2CCN(CC2)C(=O)c2cccc(OC)c2OC)cc1C Show InChI InChI=1S/C25H33N3O7S/c1-6-36(31,32)26-23-17(2)14-19(15-18(23)3)35-16-22(29)27-10-12-28(13-11-27)25(30)20-8-7-9-21(33-4)24(20)34-5/h7-9,14-15,26H,6,10-13,16H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
| Assay Description
Binding affinity to CBX7ChD (7 to 66 residues) (unknown origin) expressed in RIPL-BL21 (DE3)-CodonPlus competent cells in presence of FITC-labeled SE... |
ACS Med Chem Lett 7: 601-5 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00042
BindingDB Entry DOI: 10.7270/Q28054JT |
More data for this
Ligand-Target Pair | |
Chromobox protein homolog 7
(Homo sapiens (Human)) | BDBM50189698
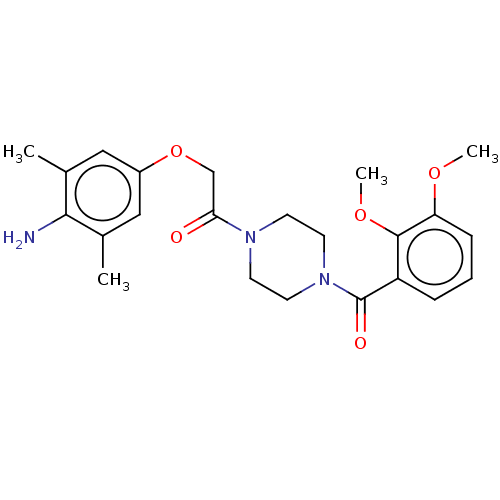
(CHEMBL3827336)Show SMILES COc1cccc(C(=O)N2CCN(CC2)C(=O)COc2cc(C)c(N)c(C)c2)c1OC Show InChI InChI=1S/C23H29N3O5/c1-15-12-17(13-16(2)21(15)24)31-14-20(27)25-8-10-26(11-9-25)23(28)18-6-5-7-19(29-3)22(18)30-4/h5-7,12-13H,8-11,14,24H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
| Assay Description
Binding affinity to CBX7ChD (7 to 66 residues) (unknown origin) expressed in RIPL-BL21 (DE3)-CodonPlus competent cells in presence of FITC-labeled SE... |
ACS Med Chem Lett 7: 601-5 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00042
BindingDB Entry DOI: 10.7270/Q28054JT |
More data for this
Ligand-Target Pair | |
Chromobox protein homolog 7
(Homo sapiens (Human)) | BDBM50189711

(CHEMBL3827805)Show SMILES CCS(=O)(=O)Nc1ccc(OCC(=O)N2CCN(CC2)C(=O)c2cccc(OC)c2OC)cc1C Show InChI InChI=1S/C24H31N3O7S/c1-5-35(30,31)25-20-10-9-18(15-17(20)2)34-16-22(28)26-11-13-27(14-12-26)24(29)19-7-6-8-21(32-3)23(19)33-4/h6-10,15,25H,5,11-14,16H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
| Assay Description
Binding affinity to CBX7ChD (7 to 66 residues) (unknown origin) expressed in RIPL-BL21 (DE3)-CodonPlus competent cells in presence of FITC-labeled SE... |
ACS Med Chem Lett 7: 601-5 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00042
BindingDB Entry DOI: 10.7270/Q28054JT |
More data for this
Ligand-Target Pair | |
Chromobox protein homolog 7
(Homo sapiens (Human)) | BDBM50189780
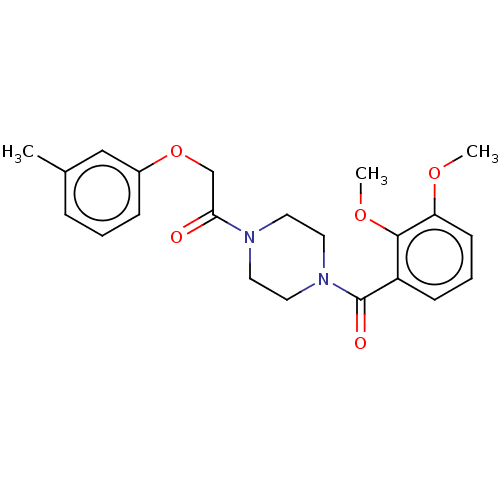
(CHEMBL3827954)Show SMILES COc1cccc(C(=O)N2CCN(CC2)C(=O)COc2cccc(C)c2)c1OC Show InChI InChI=1S/C22H26N2O5/c1-16-6-4-7-17(14-16)29-15-20(25)23-10-12-24(13-11-23)22(26)18-8-5-9-19(27-2)21(18)28-3/h4-9,14H,10-13,15H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
| Assay Description
Binding affinity to CBX7ChD (7 to 66 residues) (unknown origin) expressed in RIPL-BL21 (DE3)-CodonPlus competent cells in presence of FITC-labeled SE... |
ACS Med Chem Lett 7: 601-5 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00042
BindingDB Entry DOI: 10.7270/Q28054JT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Chromobox protein homolog 7
(Homo sapiens (Human)) | BDBM50189762
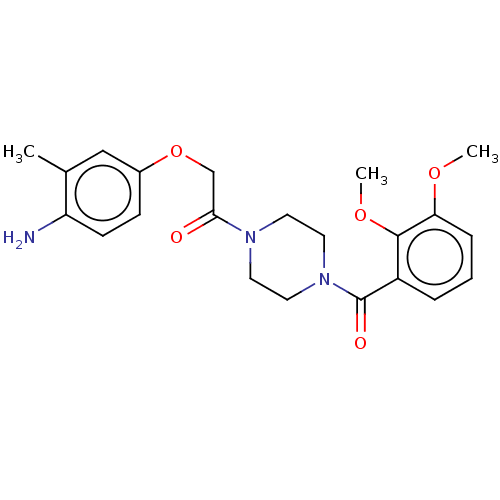
(CHEMBL3827451)Show SMILES COc1cccc(C(=O)N2CCN(CC2)C(=O)COc2ccc(N)c(C)c2)c1OC Show InChI InChI=1S/C22H27N3O5/c1-15-13-16(7-8-18(15)23)30-14-20(26)24-9-11-25(12-10-24)22(27)17-5-4-6-19(28-2)21(17)29-3/h4-8,13H,9-12,14,23H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
| Assay Description
Binding affinity to CBX7ChD (7 to 66 residues) (unknown origin) expressed in RIPL-BL21 (DE3)-CodonPlus competent cells in presence of FITC-labeled SE... |
ACS Med Chem Lett 7: 601-5 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00042
BindingDB Entry DOI: 10.7270/Q28054JT |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM60828
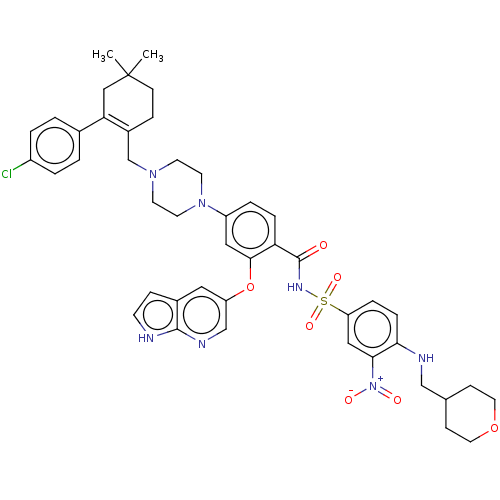
(ABT-199 | BDBM189459 | US10213433, Compound 5 | US...)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)[N+]([O-])=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California, Riverside
| Assay Description
One hundred microliters of a 600 ng/mL solution of biotin-BID (Biotin-lc-EDIIRNIARHLAQVGDSMDR-NH2, where lc indicates a hydrocarbon chain of six meth... |
ACS Chem Biol 12: 444-455 (2017)
Article DOI: 10.1021/acschembio.6b00962
BindingDB Entry DOI: 10.7270/Q2P26WZ3 |
More data for this
Ligand-Target Pair | |
Bcl-2-related protein A1 [1-149]
(Homo sapiens (Human)) | BDBM218788

(Ac-CATQLRRFGDKLNFRQKLLN-NH2 | hNOXA)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CS)NC(C)=O)[C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C108H180N36O28S/c1-54(2)43-71(139-96(163)69(35-37-81(112)148)135-105(172)86(59(10)145)144-88(155)58(9)126-104(171)79(53-173)127-60(11)146)97(164)132-65(31-22-40-122-106(116)117)90(157)130-67(33-24-42-124-108(120)121)94(161)141-75(47-61-25-14-12-15-26-61)89(156)125-52-84(151)128-78(51-85(152)153)103(170)131-64(30-19-21-39-110)93(160)138-74(46-57(7)8)100(167)143-77(50-83(114)150)102(169)142-76(48-62-27-16-13-17-28-62)101(168)133-66(32-23-41-123-107(118)119)91(158)134-68(34-36-80(111)147)95(162)129-63(29-18-20-38-109)92(159)137-73(45-56(5)6)99(166)140-72(44-55(3)4)98(165)136-70(87(115)154)49-82(113)149/h12-17,25-28,54-59,63-79,86,145,173H,18-24,29-53,109-110H2,1-11H3,(H2,111,147)(H2,112,148)(H2,113,149)(H2,114,150)(H2,115,154)(H,125,156)(H,126,171)(H,127,146)(H,128,151)(H,129,162)(H,130,157)(H,131,170)(H,132,164)(H,133,168)(H,134,158)(H,135,172)(H,136,165)(H,137,159)(H,138,160)(H,139,163)(H,140,166)(H,141,161)(H,142,169)(H,143,167)(H,144,155)(H,152,153)(H4,116,117,122)(H4,118,119,123)(H4,120,121,124)/t58-,59+,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,86-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California, Riverside
| Assay Description
One hundred microliters of a 600 ng/mL solution of biotin-BID (Biotin-lc-EDIIRNIARHLAQVGDSMDR-NH2, where lc indicates a hydrocarbon chain of six meth... |
ACS Chem Biol 12: 444-455 (2017)
Article DOI: 10.1021/acschembio.6b00962
BindingDB Entry DOI: 10.7270/Q2P26WZ3 |
More data for this
Ligand-Target Pair | |
Bcl-2-related protein A1 [1-149]
(Homo sapiens (Human)) | BDBM218795
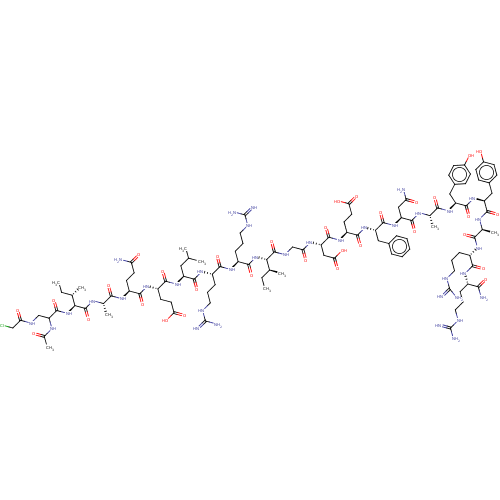
(130E7 | Ac-λIAQELRRIGDEFNAYYARR-NH2)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)C(CNC(=O)CCl)NC(C)=O)[C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C110H171ClN36O32/c1-11-54(5)86(146-97(170)68(25-19-43-126-110(121)122)137-93(166)67(24-18-42-125-109(119)120)138-100(173)72(44-53(3)4)142-95(168)70(35-38-83(155)156)139-94(167)69(34-37-79(112)151)136-90(163)58(9)131-106(179)87(55(6)12-2)147-104(177)78(132-59(10)148)51-127-81(153)50-111)105(178)128-52-82(154)133-77(49-85(159)160)103(176)140-71(36-39-84(157)158)96(169)143-75(45-60-20-14-13-15-21-60)102(175)145-76(48-80(113)152)99(172)130-57(8)91(164)141-74(47-62-28-32-64(150)33-29-62)101(174)144-73(46-61-26-30-63(149)31-27-61)98(171)129-56(7)89(162)135-66(23-17-41-124-108(117)118)92(165)134-65(88(114)161)22-16-40-123-107(115)116/h13-15,20-21,26-33,53-58,65-78,86-87,149-150H,11-12,16-19,22-25,34-52H2,1-10H3,(H2,112,151)(H2,113,152)(H2,114,161)(H,127,153)(H,128,178)(H,129,171)(H,130,172)(H,131,179)(H,132,148)(H,133,154)(H,134,165)(H,135,162)(H,136,163)(H,137,166)(H,138,173)(H,139,167)(H,140,176)(H,141,164)(H,142,168)(H,143,169)(H,144,174)(H,145,175)(H,146,170)(H,147,177)(H,155,156)(H,157,158)(H,159,160)(H4,115,116,123)(H4,117,118,124)(H4,119,120,125)(H4,121,122,126)/t54-,55-,56-,57-,58-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78?,86-,87-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California, Riverside
| Assay Description
One hundred microliters of a 600 ng/mL solution of biotin-BID (Biotin-lc-EDIIRNIARHLAQVGDSMDR-NH2, where lc indicates a hydrocarbon chain of six meth... |
ACS Chem Biol 12: 444-455 (2017)
Article DOI: 10.1021/acschembio.6b00962
BindingDB Entry DOI: 10.7270/Q2P26WZ3 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50520051

(CHEMBL4592765)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2CCCN2C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]2CSSC[C@H](NC1=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 Show InChI InChI=1S/C70H98N18O21S2/c1-3-36(2)56-66(106)84-48-35-111-110-34-47(83-58(98)40(13-7-25-74-70(72)73)76-53(92)32-75-57(97)44(31-55(95)96)80-64(104)49-14-8-26-86(49)68(108)45(81-63(48)103)30-37-11-5-4-6-12-37)62(102)77-41(21-23-52(71)91)59(99)79-43(29-38-17-19-39(90)20-18-38)60(100)82-46(33-89)61(101)78-42(22-24-54(93)94)67(107)88-28-10-16-51(88)69(109)87-27-9-15-50(87)65(105)85-56/h4-6,11-12,17-20,36,40-51,56,89-90H,3,7-10,13-16,21-35H2,1-2H3,(H2,71,91)(H,75,97)(H,76,92)(H,77,102)(H,78,101)(H,79,99)(H,80,104)(H,81,103)(H,82,100)(H,83,98)(H,84,106)(H,85,105)(H,93,94)(H,95,96)(H4,72,73,74)/t36-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,56-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human chymase expressed in Pichia pastoris X-33 cells assessed as decrease in angiotensin 1 cleavage to angiotensin 2 incub... |
J Med Chem 63: 816-826 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01811
BindingDB Entry DOI: 10.7270/Q2RX9GG7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data


















































