 Found 155 hits with Last Name = 'yi' and Initial = 'sy'
Found 155 hits with Last Name = 'yi' and Initial = 'sy' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50160612

(CHEMBL362823 | [2-(2,3-Dihydro-benzo[1,4]dioxin-5-...)Show SMILES Fc1ccc2[nH]cc([C@H]3CC[C@@H](CC3)NCCOc3cccc4OCCOc34)c2c1 |wU:11.14,wD:8.7,(7.58,-3.4,;7.58,-1.87,;8.92,-1.08,;8.92,.47,;7.58,1.23,;7.26,2.74,;5.71,2.89,;5.11,1.49,;3.59,1.17,;2.56,2.31,;1.06,2,;.57,.54,;1.62,-.62,;3.12,-.29,;-.93,.21,;-1.42,-1.25,;-2.91,-1.57,;-3.94,-.41,;-5.46,-.75,;-5.46,-2.29,;-6.8,-3.06,;-8.13,-2.29,;-8.13,-.75,;-9.46,,;-9.46,1.54,;-8.13,2.31,;-6.8,1.54,;-6.82,.02,;6.25,.46,;6.25,-1.08,)| Show InChI InChI=1S/C24H27FN2O3/c25-17-6-9-21-19(14-17)20(15-27-21)16-4-7-18(8-5-16)26-10-11-28-22-2-1-3-23-24(22)30-13-12-29-23/h1-3,6,9,14-16,18,26-27H,4-5,7-8,10-13H2/t16-,18- | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.05 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against Serotonin transporter determined bydisplacement of [3H]-paroxetine from rat cortical membranes |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50160611
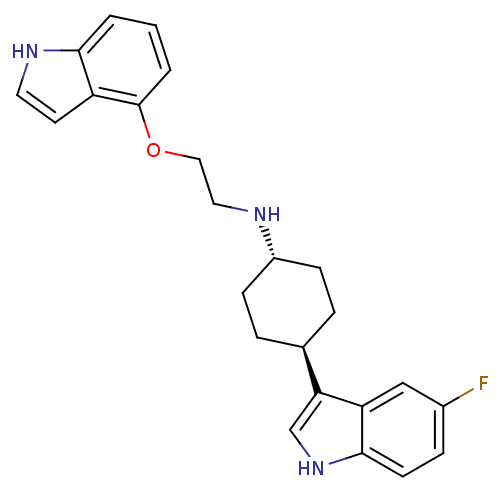
(CHEMBL359549 | [4-(5-Fluoro-1H-indol-3-yl)-cyclohe...)Show SMILES Fc1ccc2[nH]cc([C@H]3CC[C@@H](CC3)NCCOc3cccc4[nH]ccc34)c2c1 |wU:11.14,wD:8.7,(7.29,-3.35,;7.29,-1.79,;8.62,-1.03,;8.62,.53,;7.29,1.29,;6.96,2.81,;5.43,2.97,;4.81,1.55,;3.29,1.24,;2.26,2.38,;.76,2.06,;.27,.6,;1.32,-.56,;2.82,-.24,;-1.23,.27,;-1.72,-1.19,;-3.22,-1.5,;-4.25,-.36,;-5.77,-.69,;-5.77,-2.23,;-7.11,-3,;-8.44,-2.23,;-8.47,-.69,;-9.6,.35,;-8.98,1.77,;-7.42,1.58,;-7.12,.09,;5.95,.53,;5.95,-1.03,)| Show InChI InChI=1S/C24H26FN3O/c25-17-6-9-23-20(14-17)21(15-28-23)16-4-7-18(8-5-16)26-12-13-29-24-3-1-2-22-19(24)10-11-27-22/h1-3,6,9-11,14-16,18,26-28H,4-5,7-8,12-13H2/t16-,18- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against human 5-hydroxytryptamine 1A receptors transfected into CHO cells. |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50160611

(CHEMBL359549 | [4-(5-Fluoro-1H-indol-3-yl)-cyclohe...)Show SMILES Fc1ccc2[nH]cc([C@H]3CC[C@@H](CC3)NCCOc3cccc4[nH]ccc34)c2c1 |wU:11.14,wD:8.7,(7.29,-3.35,;7.29,-1.79,;8.62,-1.03,;8.62,.53,;7.29,1.29,;6.96,2.81,;5.43,2.97,;4.81,1.55,;3.29,1.24,;2.26,2.38,;.76,2.06,;.27,.6,;1.32,-.56,;2.82,-.24,;-1.23,.27,;-1.72,-1.19,;-3.22,-1.5,;-4.25,-.36,;-5.77,-.69,;-5.77,-2.23,;-7.11,-3,;-8.44,-2.23,;-8.47,-.69,;-9.6,.35,;-8.98,1.77,;-7.42,1.58,;-7.12,.09,;5.95,.53,;5.95,-1.03,)| Show InChI InChI=1S/C24H26FN3O/c25-17-6-9-23-20(14-17)21(15-28-23)16-4-7-18(8-5-16)26-12-13-29-24-3-1-2-22-19(24)10-11-27-22/h1-3,6,9-11,14-16,18,26-28H,4-5,7-8,12-13H2/t16-,18- | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against Serotonin transporter determined bydisplacement of [3H]-paroxetine from rat cortical membranes |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50160602

(5-Fluoro-3-{4-[4-(2-methoxy-phenyl)-piperazin-1-yl...)Show SMILES COc1ccccc1N1CCN(CC1)[C@H]1CC[C@H](CC1)c1c[nH]c2ccc(F)cc12 |wU:14.15,17.22,(-5.05,2.08,;-6.57,1.76,;-7.06,.3,;-8.58,-.03,;-9.05,-1.48,;-8.02,-2.65,;-6.52,-2.32,;-6.03,-.87,;-4.53,-.54,;-3.5,-1.72,;-1.98,-1.38,;-1.51,.07,;-2.52,1.24,;-4.04,.91,;.01,.4,;.5,1.87,;2,2.18,;3.03,1.02,;2.56,-.44,;1.06,-.75,;4.55,1.35,;5.16,2.76,;6.7,2.59,;7.03,1.09,;8.37,.33,;8.37,-1.23,;7.03,-1.99,;7.03,-3.54,;5.7,-1.23,;5.7,.33,)| Show InChI InChI=1S/C25H30FN3O/c1-30-25-5-3-2-4-24(25)29-14-12-28(13-15-29)20-9-6-18(7-10-20)22-17-27-23-11-8-19(26)16-21(22)23/h2-5,8,11,16-18,20,27H,6-7,9-10,12-15H2,1H3/t18-,20+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against Serotonin transporter determined bydisplacement of [3H]-paroxetine from rat cortical membranes |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50160601

(CHEMBL180817 | [4-(5-Fluoro-1H-indol-3-yl)-cyclohe...)Show SMILES COc1ccccc1OCCN[C@H]1CC[C@H](CC1)c1c[nH]c2ccc(F)cc12 |wU:15.19,12.12,(-6.23,2.4,;-7.55,1.63,;-7.55,.09,;-8.91,-.7,;-8.91,-2.24,;-7.55,-3.01,;-6.22,-2.24,;-6.22,-.68,;-4.71,-.37,;-3.69,-1.52,;-2.17,-1.2,;-1.7,.27,;-.19,.6,;.29,2.05,;1.8,2.37,;2.83,1.21,;2.36,-.24,;.85,-.57,;4.32,1.53,;4.95,2.93,;6.46,2.78,;6.79,1.28,;8.13,.53,;8.13,-1.03,;6.79,-1.8,;6.79,-3.34,;5.46,-1.03,;5.46,.53,)| Show InChI InChI=1S/C23H27FN2O2/c1-27-22-4-2-3-5-23(22)28-13-12-25-18-9-6-16(7-10-18)20-15-26-21-11-8-17(24)14-19(20)21/h2-5,8,11,14-16,18,25-26H,6-7,9-10,12-13H2,1H3/t16-,18+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against Serotonin transporter determined bydisplacement of [3H]-paroxetine from rat cortical membranes |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50160613
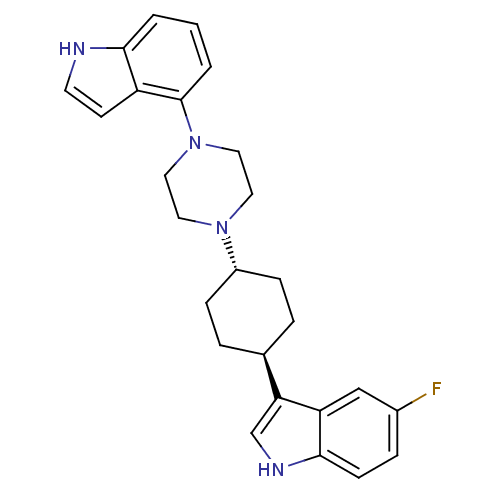
(5-Fluoro-3-[trans-4-[4-(1H-indol-4-yl)-1-piperazin...)Show SMILES Fc1ccc2[nH]cc([C@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4[nH]ccc34)c2c1 |r,wU:8.7,wD:11.14,(-4.03,-2.02,;-3.69,-.51,;-2.22,-.05,;-1.89,1.44,;-3.01,2.47,;-2.99,4.01,;-4.45,4.5,;-5.37,3.27,;-6.9,3.28,;-7.69,1.96,;-9.22,1.97,;-9.98,3.31,;-9.21,4.64,;-7.66,4.62,;-11.52,3.32,;-12.3,1.99,;-13.84,2.01,;-14.59,3.34,;-13.81,4.67,;-12.29,4.67,;-16.14,3.34,;-16.9,4.68,;-18.43,4.69,;-19.22,3.36,;-18.46,2.01,;-18.93,.54,;-17.68,-.36,;-16.42,.55,;-16.91,2.02,;-4.47,2.02,;-4.81,.53,)| Show InChI InChI=1S/C26H29FN4/c27-19-6-9-25-22(16-19)23(17-29-25)18-4-7-20(8-5-18)30-12-14-31(15-13-30)26-3-1-2-24-21(26)10-11-28-24/h1-3,6,9-11,16-18,20,28-29H,4-5,7-8,12-15H2/t18-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against human 5-hydroxytryptamine 1A receptors transfected into CHO cells. |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50160609
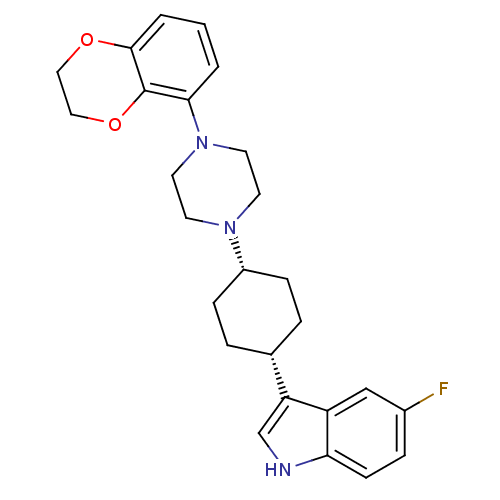
(3-{4-[4-(2,3-Dihydro-benzo[1,4]dioxin-5-yl)-pipera...)Show SMILES Fc1ccc2[nH]cc([C@@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4OCCOc34)c2c1 |wU:11.14,8.7,(7.68,-3.68,;7.68,-2.14,;9.01,-1.36,;9.01,.19,;7.68,.95,;7.35,2.47,;5.83,2.61,;5.2,1.21,;3.7,.9,;2.67,2.03,;1.15,1.72,;.69,.27,;1.71,-.9,;3.23,-.58,;-.83,-.05,;-1.3,-1.53,;-2.82,-1.83,;-3.85,-.69,;-3.36,.79,;-1.86,1.09,;-5.37,-1.01,;-5.84,-2.46,;-7.36,-2.77,;-8.39,-1.62,;-7.9,-.15,;-8.92,.98,;-8.43,2.47,;-6.91,2.77,;-5.91,1.61,;-6.4,.16,;6.34,.18,;6.34,-1.36,)| Show InChI InChI=1S/C26H30FN3O2/c27-19-6-9-23-21(16-19)22(17-28-23)18-4-7-20(8-5-18)29-10-12-30(13-11-29)24-2-1-3-25-26(24)32-15-14-31-25/h1-3,6,9,16-18,20,28H,4-5,7-8,10-15H2/t18-,20+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against Serotonin transporter determined bydisplacement of [3H]-paroxetine from rat cortical membranes |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50160603
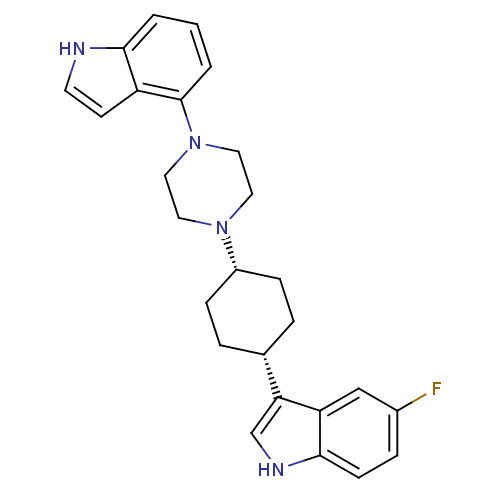
(5-Fluoro-3-[cis-4-[4-(1H-indol-4-yl)-1-piperazinyl...)Show SMILES Fc1ccc2[nH]cc([C@@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4[nH]ccc34)c2c1 |r,wU:11.14,8.7,(-4.03,-2.02,;-3.69,-.51,;-2.22,-.05,;-1.89,1.44,;-3.01,2.47,;-2.99,4.01,;-4.45,4.5,;-5.37,3.27,;-6.9,3.28,;-7.66,4.62,;-9.21,4.64,;-9.98,3.31,;-9.22,1.97,;-7.69,1.96,;-11.52,3.32,;-12.3,1.99,;-13.84,2.01,;-14.59,3.34,;-13.81,4.67,;-12.29,4.67,;-16.14,3.34,;-16.9,4.68,;-18.43,4.69,;-19.22,3.36,;-18.46,2.01,;-18.93,.54,;-17.68,-.36,;-16.42,.55,;-16.91,2.02,;-4.47,2.02,;-4.81,.53,)| Show InChI InChI=1S/C26H29FN4/c27-19-6-9-25-22(16-19)23(17-29-25)18-4-7-20(8-5-18)30-12-14-31(15-13-30)26-3-1-2-24-21(26)10-11-28-24/h1-3,6,9-11,16-18,20,28-29H,4-5,7-8,12-15H2/t18-,20+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against Serotonin transporter determined bydisplacement of [3H]-paroxetine from rat cortical membranes |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50160605
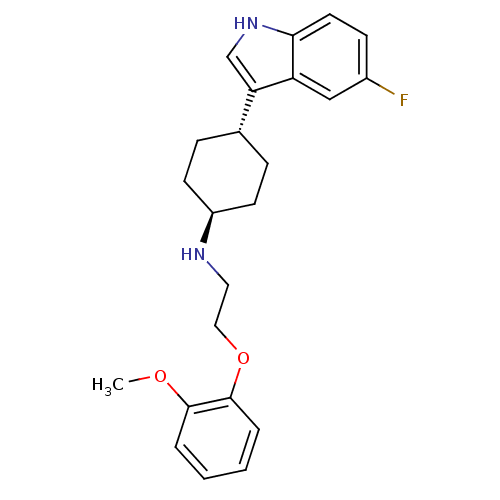
(CHEMBL359505 | [4-(5-Fluoro-1H-indol-3-yl)-cyclohe...)Show SMILES COc1ccccc1OCCN[C@H]1CC[C@@H](CC1)c1c[nH]c2ccc(F)cc12 |wU:12.12,wD:15.19,(-6.22,2.4,;-7.55,1.61,;-7.55,.09,;-8.91,-.68,;-8.91,-2.24,;-7.55,-2.99,;-6.22,-2.24,;-6.22,-.68,;-4.71,-.37,;-3.68,-1.5,;-2.17,-1.2,;-1.7,.27,;-.19,.6,;.29,2.05,;1.8,2.36,;2.83,1.23,;2.36,-.26,;.85,-.57,;4.32,1.53,;4.95,2.93,;6.47,2.79,;6.79,1.28,;8.13,.53,;8.13,-1.03,;6.79,-1.8,;6.79,-3.34,;5.47,-1.03,;5.47,.51,)| Show InChI InChI=1S/C23H27FN2O2/c1-27-22-4-2-3-5-23(22)28-13-12-25-18-9-6-16(7-10-18)20-15-26-21-11-8-17(24)14-19(20)21/h2-5,8,11,14-16,18,25-26H,6-7,9-10,12-13H2,1H3/t16-,18- | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against Serotonin transporter determined bydisplacement of [3H]-paroxetine from rat cortical membranes |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50160606

(3-{4-[4-(2,3-Dihydro-benzo[1,4]dioxin-5-yl)-pipera...)Show SMILES Fc1ccc2[nH]cc([C@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4OCCOc34)c2c1 |wU:11.14,wD:8.7,(7.68,-3.68,;7.68,-2.14,;9.01,-1.36,;9.01,.19,;7.68,.95,;7.35,2.47,;5.83,2.61,;5.2,1.21,;3.7,.9,;2.67,2.03,;1.15,1.72,;.69,.27,;1.71,-.9,;3.23,-.58,;-.83,-.05,;-1.3,-1.53,;-2.82,-1.83,;-3.85,-.69,;-3.36,.79,;-1.86,1.09,;-5.37,-1.01,;-5.84,-2.46,;-7.36,-2.77,;-8.39,-1.62,;-7.9,-.15,;-8.92,.98,;-8.43,2.47,;-6.91,2.77,;-5.91,1.61,;-6.4,.16,;6.34,.18,;6.34,-1.36,)| Show InChI InChI=1S/C26H30FN3O2/c27-19-6-9-23-21(16-19)22(17-28-23)18-4-7-20(8-5-18)29-10-12-30(13-11-29)24-2-1-3-25-26(24)32-15-14-31-25/h1-3,6,9,16-18,20,28H,4-5,7-8,10-15H2/t18-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against human 5-hydroxytryptamine 1A receptors transfected into CHO cells. |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50160610

(CHEMBL178304 | [2-(2,3-Dihydro-benzo[1,4]dioxin-5-...)Show SMILES Fc1ccc2[nH]cc([C@@H]3CC[C@@H](CC3)NCCOc3cccc4OCCOc34)c2c1 |wU:8.7,11.14,(7.58,-3.4,;7.58,-1.87,;8.92,-1.08,;8.92,.47,;7.58,1.23,;7.26,2.74,;5.71,2.89,;5.11,1.49,;3.59,1.17,;2.56,2.31,;1.06,2,;.57,.54,;1.62,-.62,;3.12,-.29,;-.93,.21,;-1.42,-1.25,;-2.91,-1.57,;-3.94,-.41,;-5.46,-.75,;-5.46,-2.29,;-6.8,-3.06,;-8.13,-2.29,;-8.13,-.75,;-9.46,,;-9.46,1.54,;-8.13,2.31,;-6.8,1.54,;-6.82,.02,;6.25,.46,;6.25,-1.08,)| Show InChI InChI=1S/C24H27FN2O3/c25-17-6-9-21-19(14-17)20(15-27-21)16-4-7-18(8-5-16)26-10-11-28-22-2-1-3-23-24(22)30-13-12-29-23/h1-3,6,9,14-16,18,26-27H,4-5,7-8,10-13H2/t16-,18+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against Serotonin transporter determined bydisplacement of [3H]-paroxetine from rat cortical membranes |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50160607
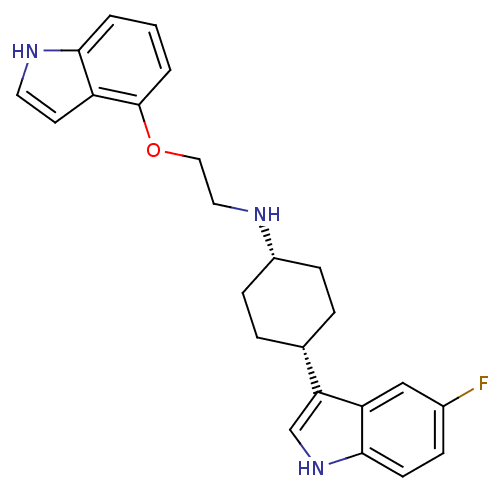
(CHEMBL178467 | [4-(5-Fluoro-1H-indol-3-yl)-cyclohe...)Show SMILES Fc1ccc2[nH]cc([C@@H]3CC[C@@H](CC3)NCCOc3cccc4[nH]ccc34)c2c1 |wU:8.7,11.14,(7.29,-3.35,;7.29,-1.79,;8.62,-1.03,;8.62,.53,;7.29,1.29,;6.96,2.81,;5.43,2.97,;4.81,1.55,;3.29,1.24,;2.26,2.38,;.76,2.06,;.27,.6,;1.32,-.56,;2.82,-.24,;-1.23,.27,;-1.72,-1.19,;-3.22,-1.5,;-4.25,-.36,;-5.77,-.69,;-5.77,-2.23,;-7.11,-3,;-8.44,-2.23,;-8.47,-.69,;-9.59,.35,;-8.98,1.77,;-7.42,1.58,;-7.12,.09,;5.95,.53,;5.95,-1.03,)| Show InChI InChI=1S/C24H26FN3O/c25-17-6-9-23-20(14-17)21(15-28-23)16-4-7-18(8-5-16)26-12-13-29-24-3-1-2-22-19(24)10-11-27-22/h1-3,6,9-11,14-16,18,26-28H,4-5,7-8,12-13H2/t16-,18+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against Serotonin transporter determined bydisplacement of [3H]-paroxetine from rat cortical membranes |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50160608
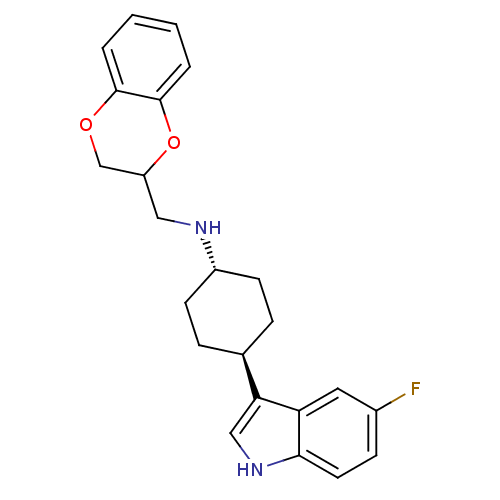
((2,3-Dihydro-benzo[1,4]dioxin-2-ylmethyl)-[4-(5-fl...)Show SMILES Fc1ccc2[nH]cc([C@H]3CC[C@@H](CC3)NCC3COc4ccccc4O3)c2c1 |wU:11.14,wD:8.7,(5.88,4.43,;6.21,2.92,;7.68,2.47,;8,.97,;6.87,-.05,;6.88,-1.62,;5.42,-2.08,;4.51,-.86,;2.99,-.87,;2.2,.48,;.65,.46,;-.12,-.87,;.68,-2.22,;2.22,-2.2,;-1.63,-.89,;-2.42,.44,;-3.96,.44,;-4.75,1.77,;-6.32,1.75,;-7.06,.4,;-8.61,.37,;-9.37,-.96,;-8.58,-2.29,;-7.02,-2.27,;-6.28,-.94,;-4.74,-.92,;5.41,.41,;5.09,1.89,)| Show InChI InChI=1S/C23H25FN2O2/c24-16-7-10-21-19(11-16)20(13-26-21)15-5-8-17(9-6-15)25-12-18-14-27-22-3-1-2-4-23(22)28-18/h1-4,7,10-11,13,15,17-18,25-26H,5-6,8-9,12,14H2/t15-,17-,18? | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against Serotonin transporter determined bydisplacement of [3H]-paroxetine from rat cortical membranes |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50160604

(5-Fluoro-3-{4-[4-(2-methoxy-phenyl)-piperazin-1-yl...)Show SMILES COc1ccccc1N1CCN(CC1)[C@H]1CC[C@@H](CC1)c1c[nH]c2ccc(F)cc12 |wU:14.15,wD:17.22,(-5.05,2.08,;-6.57,1.76,;-7.06,.3,;-8.58,-.03,;-9.05,-1.48,;-8.02,-2.65,;-6.52,-2.32,;-6.03,-.87,;-4.53,-.54,;-3.5,-1.72,;-1.98,-1.38,;-1.49,.07,;-2.52,1.24,;-4.04,.91,;.01,.4,;.5,1.87,;2,2.18,;3.03,1.02,;2.56,-.44,;1.06,-.75,;4.55,1.35,;5.16,2.76,;6.7,2.59,;7.03,1.09,;8.37,.33,;8.37,-1.23,;7.03,-1.99,;7.03,-3.54,;5.7,-1.23,;5.7,.33,)| Show InChI InChI=1S/C25H30FN3O/c1-30-25-5-3-2-4-24(25)29-14-12-28(13-15-29)20-9-6-18(7-10-20)22-17-27-23-11-8-19(26)16-21(22)23/h2-5,8,11,16-18,20,27H,6-7,9-10,12-15H2,1H3/t18-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against human 5-hydroxytryptamine 1A receptors transfected into CHO cells. |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50160612

(CHEMBL362823 | [2-(2,3-Dihydro-benzo[1,4]dioxin-5-...)Show SMILES Fc1ccc2[nH]cc([C@H]3CC[C@@H](CC3)NCCOc3cccc4OCCOc34)c2c1 |wU:11.14,wD:8.7,(7.58,-3.4,;7.58,-1.87,;8.92,-1.08,;8.92,.47,;7.58,1.23,;7.26,2.74,;5.71,2.89,;5.11,1.49,;3.59,1.17,;2.56,2.31,;1.06,2,;.57,.54,;1.62,-.62,;3.12,-.29,;-.93,.21,;-1.42,-1.25,;-2.91,-1.57,;-3.94,-.41,;-5.46,-.75,;-5.46,-2.29,;-6.8,-3.06,;-8.13,-2.29,;-8.13,-.75,;-9.46,,;-9.46,1.54,;-8.13,2.31,;-6.8,1.54,;-6.82,.02,;6.25,.46,;6.25,-1.08,)| Show InChI InChI=1S/C24H27FN2O3/c25-17-6-9-21-19(14-17)20(15-27-21)16-4-7-18(8-5-16)26-10-11-28-22-2-1-3-23-24(22)30-13-12-29-23/h1-3,6,9,14-16,18,26-27H,4-5,7-8,10-13H2/t16-,18- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against human 5-hydroxytryptamine 1A receptors transfected into CHO cells. |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50160605

(CHEMBL359505 | [4-(5-Fluoro-1H-indol-3-yl)-cyclohe...)Show SMILES COc1ccccc1OCCN[C@H]1CC[C@@H](CC1)c1c[nH]c2ccc(F)cc12 |wU:12.12,wD:15.19,(-6.22,2.4,;-7.55,1.61,;-7.55,.09,;-8.91,-.68,;-8.91,-2.24,;-7.55,-2.99,;-6.22,-2.24,;-6.22,-.68,;-4.71,-.37,;-3.68,-1.5,;-2.17,-1.2,;-1.7,.27,;-.19,.6,;.29,2.05,;1.8,2.36,;2.83,1.23,;2.36,-.26,;.85,-.57,;4.32,1.53,;4.95,2.93,;6.47,2.79,;6.79,1.28,;8.13,.53,;8.13,-1.03,;6.79,-1.8,;6.79,-3.34,;5.47,-1.03,;5.47,.51,)| Show InChI InChI=1S/C23H27FN2O2/c1-27-22-4-2-3-5-23(22)28-13-12-25-18-9-6-16(7-10-18)20-15-26-21-11-8-17(24)14-19(20)21/h2-5,8,11,14-16,18,25-26H,6-7,9-10,12-13H2,1H3/t16-,18- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against human 5-hydroxytryptamine 1A receptors transfected into CHO cells. |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50160614
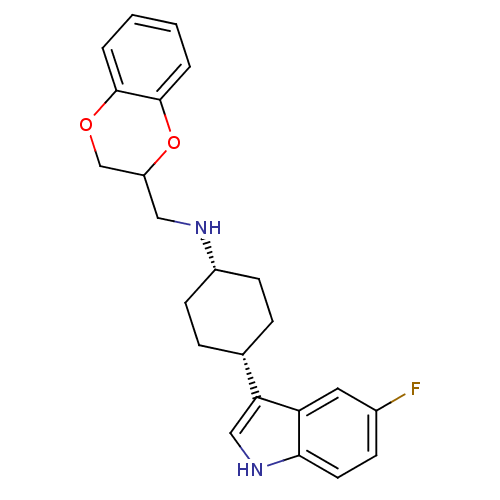
((2,3-Dihydro-benzo[1,4]dioxin-2-ylmethyl)-[4-(5-fl...)Show SMILES Fc1ccc2[nH]cc([C@@H]3CC[C@@H](CC3)NCC3COc4ccccc4O3)c2c1 |wU:8.7,11.14,(6.09,4.09,;6.35,2.58,;7.79,2.04,;8.03,.53,;6.84,-.43,;6.79,-1.97,;5.3,-2.36,;4.46,-1.1,;2.92,-1.01,;2.2,.37,;.66,.44,;-.16,-.87,;.54,-2.25,;2.08,-2.32,;-1.69,-.8,;-2.39,.57,;-3.93,.64,;-4.65,2.02,;-6.22,2.09,;-7.03,.78,;-8.57,.85,;-9.39,-.45,;-8.66,-1.83,;-7.15,-1.9,;-6.31,-.59,;-4.77,-.66,;5.42,.11,;5.18,1.62,)| Show InChI InChI=1S/C23H25FN2O2/c24-16-7-10-21-19(11-16)20(13-26-21)15-5-8-17(9-6-15)25-12-18-14-27-22-3-1-2-4-23(22)28-18/h1-4,7,10-11,13,15,17-18,25-26H,5-6,8-9,12,14H2/t15-,17+,18? | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against Serotonin transporter determined bydisplacement of [3H]-paroxetine from rat cortical membranes |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50160603

(5-Fluoro-3-[cis-4-[4-(1H-indol-4-yl)-1-piperazinyl...)Show SMILES Fc1ccc2[nH]cc([C@@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4[nH]ccc34)c2c1 |r,wU:11.14,8.7,(-4.03,-2.02,;-3.69,-.51,;-2.22,-.05,;-1.89,1.44,;-3.01,2.47,;-2.99,4.01,;-4.45,4.5,;-5.37,3.27,;-6.9,3.28,;-7.66,4.62,;-9.21,4.64,;-9.98,3.31,;-9.22,1.97,;-7.69,1.96,;-11.52,3.32,;-12.3,1.99,;-13.84,2.01,;-14.59,3.34,;-13.81,4.67,;-12.29,4.67,;-16.14,3.34,;-16.9,4.68,;-18.43,4.69,;-19.22,3.36,;-18.46,2.01,;-18.93,.54,;-17.68,-.36,;-16.42,.55,;-16.91,2.02,;-4.47,2.02,;-4.81,.53,)| Show InChI InChI=1S/C26H29FN4/c27-19-6-9-25-22(16-19)23(17-29-25)18-4-7-20(8-5-18)30-12-14-31(15-13-30)26-3-1-2-24-21(26)10-11-28-24/h1-3,6,9-11,16-18,20,28-29H,4-5,7-8,12-15H2/t18-,20+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 36.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against human 5-hydroxytryptamine 1A receptors transfected into CHO cells. |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50160604

(5-Fluoro-3-{4-[4-(2-methoxy-phenyl)-piperazin-1-yl...)Show SMILES COc1ccccc1N1CCN(CC1)[C@H]1CC[C@@H](CC1)c1c[nH]c2ccc(F)cc12 |wU:14.15,wD:17.22,(-5.05,2.08,;-6.57,1.76,;-7.06,.3,;-8.58,-.03,;-9.05,-1.48,;-8.02,-2.65,;-6.52,-2.32,;-6.03,-.87,;-4.53,-.54,;-3.5,-1.72,;-1.98,-1.38,;-1.49,.07,;-2.52,1.24,;-4.04,.91,;.01,.4,;.5,1.87,;2,2.18,;3.03,1.02,;2.56,-.44,;1.06,-.75,;4.55,1.35,;5.16,2.76,;6.7,2.59,;7.03,1.09,;8.37,.33,;8.37,-1.23,;7.03,-1.99,;7.03,-3.54,;5.7,-1.23,;5.7,.33,)| Show InChI InChI=1S/C25H30FN3O/c1-30-25-5-3-2-4-24(25)29-14-12-28(13-15-29)20-9-6-18(7-10-20)22-17-27-23-11-8-19(26)16-21(22)23/h2-5,8,11,16-18,20,27H,6-7,9-10,12-15H2,1H3/t18-,20- | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 46.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against Serotonin transporter determined bydisplacement of [3H]-paroxetine from rat cortical membranes |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50160613

(5-Fluoro-3-[trans-4-[4-(1H-indol-4-yl)-1-piperazin...)Show SMILES Fc1ccc2[nH]cc([C@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4[nH]ccc34)c2c1 |r,wU:8.7,wD:11.14,(-4.03,-2.02,;-3.69,-.51,;-2.22,-.05,;-1.89,1.44,;-3.01,2.47,;-2.99,4.01,;-4.45,4.5,;-5.37,3.27,;-6.9,3.28,;-7.69,1.96,;-9.22,1.97,;-9.98,3.31,;-9.21,4.64,;-7.66,4.62,;-11.52,3.32,;-12.3,1.99,;-13.84,2.01,;-14.59,3.34,;-13.81,4.67,;-12.29,4.67,;-16.14,3.34,;-16.9,4.68,;-18.43,4.69,;-19.22,3.36,;-18.46,2.01,;-18.93,.54,;-17.68,-.36,;-16.42,.55,;-16.91,2.02,;-4.47,2.02,;-4.81,.53,)| Show InChI InChI=1S/C26H29FN4/c27-19-6-9-25-22(16-19)23(17-29-25)18-4-7-20(8-5-18)30-12-14-31(15-13-30)26-3-1-2-24-21(26)10-11-28-24/h1-3,6,9-11,16-18,20,28-29H,4-5,7-8,12-15H2/t18-,20- | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 48.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against Serotonin transporter determined bydisplacement of [3H]-paroxetine from rat cortical membranes |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50160609

(3-{4-[4-(2,3-Dihydro-benzo[1,4]dioxin-5-yl)-pipera...)Show SMILES Fc1ccc2[nH]cc([C@@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4OCCOc34)c2c1 |wU:11.14,8.7,(7.68,-3.68,;7.68,-2.14,;9.01,-1.36,;9.01,.19,;7.68,.95,;7.35,2.47,;5.83,2.61,;5.2,1.21,;3.7,.9,;2.67,2.03,;1.15,1.72,;.69,.27,;1.71,-.9,;3.23,-.58,;-.83,-.05,;-1.3,-1.53,;-2.82,-1.83,;-3.85,-.69,;-3.36,.79,;-1.86,1.09,;-5.37,-1.01,;-5.84,-2.46,;-7.36,-2.77,;-8.39,-1.62,;-7.9,-.15,;-8.92,.98,;-8.43,2.47,;-6.91,2.77,;-5.91,1.61,;-6.4,.16,;6.34,.18,;6.34,-1.36,)| Show InChI InChI=1S/C26H30FN3O2/c27-19-6-9-23-21(16-19)22(17-28-23)18-4-7-20(8-5-18)29-10-12-30(13-11-29)24-2-1-3-25-26(24)32-15-14-31-25/h1-3,6,9,16-18,20,28H,4-5,7-8,10-15H2/t18-,20+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against human 5-hydroxytryptamine 1A receptors transfected into CHO cells. |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50160606

(3-{4-[4-(2,3-Dihydro-benzo[1,4]dioxin-5-yl)-pipera...)Show SMILES Fc1ccc2[nH]cc([C@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4OCCOc34)c2c1 |wU:11.14,wD:8.7,(7.68,-3.68,;7.68,-2.14,;9.01,-1.36,;9.01,.19,;7.68,.95,;7.35,2.47,;5.83,2.61,;5.2,1.21,;3.7,.9,;2.67,2.03,;1.15,1.72,;.69,.27,;1.71,-.9,;3.23,-.58,;-.83,-.05,;-1.3,-1.53,;-2.82,-1.83,;-3.85,-.69,;-3.36,.79,;-1.86,1.09,;-5.37,-1.01,;-5.84,-2.46,;-7.36,-2.77,;-8.39,-1.62,;-7.9,-.15,;-8.92,.98,;-8.43,2.47,;-6.91,2.77,;-5.91,1.61,;-6.4,.16,;6.34,.18,;6.34,-1.36,)| Show InChI InChI=1S/C26H30FN3O2/c27-19-6-9-23-21(16-19)22(17-28-23)18-4-7-20(8-5-18)29-10-12-30(13-11-29)24-2-1-3-25-26(24)32-15-14-31-25/h1-3,6,9,16-18,20,28H,4-5,7-8,10-15H2/t18-,20- | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 67.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against Serotonin transporter determined bydisplacement of [3H]-paroxetine from rat cortical membranes |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50160602

(5-Fluoro-3-{4-[4-(2-methoxy-phenyl)-piperazin-1-yl...)Show SMILES COc1ccccc1N1CCN(CC1)[C@H]1CC[C@H](CC1)c1c[nH]c2ccc(F)cc12 |wU:14.15,17.22,(-5.05,2.08,;-6.57,1.76,;-7.06,.3,;-8.58,-.03,;-9.05,-1.48,;-8.02,-2.65,;-6.52,-2.32,;-6.03,-.87,;-4.53,-.54,;-3.5,-1.72,;-1.98,-1.38,;-1.51,.07,;-2.52,1.24,;-4.04,.91,;.01,.4,;.5,1.87,;2,2.18,;3.03,1.02,;2.56,-.44,;1.06,-.75,;4.55,1.35,;5.16,2.76,;6.7,2.59,;7.03,1.09,;8.37,.33,;8.37,-1.23,;7.03,-1.99,;7.03,-3.54,;5.7,-1.23,;5.7,.33,)| Show InChI InChI=1S/C25H30FN3O/c1-30-25-5-3-2-4-24(25)29-14-12-28(13-15-29)20-9-6-18(7-10-20)22-17-27-23-11-8-19(26)16-21(22)23/h2-5,8,11,16-18,20,27H,6-7,9-10,12-15H2,1H3/t18-,20+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against human 5-hydroxytryptamine 1A receptors transfected into CHO cells. |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50160607

(CHEMBL178467 | [4-(5-Fluoro-1H-indol-3-yl)-cyclohe...)Show SMILES Fc1ccc2[nH]cc([C@@H]3CC[C@@H](CC3)NCCOc3cccc4[nH]ccc34)c2c1 |wU:8.7,11.14,(7.29,-3.35,;7.29,-1.79,;8.62,-1.03,;8.62,.53,;7.29,1.29,;6.96,2.81,;5.43,2.97,;4.81,1.55,;3.29,1.24,;2.26,2.38,;.76,2.06,;.27,.6,;1.32,-.56,;2.82,-.24,;-1.23,.27,;-1.72,-1.19,;-3.22,-1.5,;-4.25,-.36,;-5.77,-.69,;-5.77,-2.23,;-7.11,-3,;-8.44,-2.23,;-8.47,-.69,;-9.59,.35,;-8.98,1.77,;-7.42,1.58,;-7.12,.09,;5.95,.53,;5.95,-1.03,)| Show InChI InChI=1S/C24H26FN3O/c25-17-6-9-23-20(14-17)21(15-28-23)16-4-7-18(8-5-16)26-12-13-29-24-3-1-2-22-19(24)10-11-27-22/h1-3,6,9-11,14-16,18,26-28H,4-5,7-8,12-13H2/t16-,18+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against human 5-hydroxytryptamine 1A receptors transfected into CHO cells. |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50160608

((2,3-Dihydro-benzo[1,4]dioxin-2-ylmethyl)-[4-(5-fl...)Show SMILES Fc1ccc2[nH]cc([C@H]3CC[C@@H](CC3)NCC3COc4ccccc4O3)c2c1 |wU:11.14,wD:8.7,(5.88,4.43,;6.21,2.92,;7.68,2.47,;8,.97,;6.87,-.05,;6.88,-1.62,;5.42,-2.08,;4.51,-.86,;2.99,-.87,;2.2,.48,;.65,.46,;-.12,-.87,;.68,-2.22,;2.22,-2.2,;-1.63,-.89,;-2.42,.44,;-3.96,.44,;-4.75,1.77,;-6.32,1.75,;-7.06,.4,;-8.61,.37,;-9.37,-.96,;-8.58,-2.29,;-7.02,-2.27,;-6.28,-.94,;-4.74,-.92,;5.41,.41,;5.09,1.89,)| Show InChI InChI=1S/C23H25FN2O2/c24-16-7-10-21-19(11-16)20(13-26-21)15-5-8-17(9-6-15)25-12-18-14-27-22-3-1-2-4-23(22)28-18/h1-4,7,10-11,13,15,17-18,25-26H,5-6,8-9,12,14H2/t15-,17-,18? | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 194 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against human 5-hydroxytryptamine 1A receptors transfected into CHO cells. |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50160601

(CHEMBL180817 | [4-(5-Fluoro-1H-indol-3-yl)-cyclohe...)Show SMILES COc1ccccc1OCCN[C@H]1CC[C@H](CC1)c1c[nH]c2ccc(F)cc12 |wU:15.19,12.12,(-6.23,2.4,;-7.55,1.63,;-7.55,.09,;-8.91,-.7,;-8.91,-2.24,;-7.55,-3.01,;-6.22,-2.24,;-6.22,-.68,;-4.71,-.37,;-3.69,-1.52,;-2.17,-1.2,;-1.7,.27,;-.19,.6,;.29,2.05,;1.8,2.37,;2.83,1.21,;2.36,-.24,;.85,-.57,;4.32,1.53,;4.95,2.93,;6.46,2.78,;6.79,1.28,;8.13,.53,;8.13,-1.03,;6.79,-1.8,;6.79,-3.34,;5.46,-1.03,;5.46,.53,)| Show InChI InChI=1S/C23H27FN2O2/c1-27-22-4-2-3-5-23(22)28-13-12-25-18-9-6-16(7-10-18)20-15-26-21-11-8-17(24)14-19(20)21/h2-5,8,11,14-16,18,25-26H,6-7,9-10,12-13H2,1H3/t16-,18+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 307 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against human 5-hydroxytryptamine 1A receptors transfected into CHO cells. |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50160614

((2,3-Dihydro-benzo[1,4]dioxin-2-ylmethyl)-[4-(5-fl...)Show SMILES Fc1ccc2[nH]cc([C@@H]3CC[C@@H](CC3)NCC3COc4ccccc4O3)c2c1 |wU:8.7,11.14,(6.09,4.09,;6.35,2.58,;7.79,2.04,;8.03,.53,;6.84,-.43,;6.79,-1.97,;5.3,-2.36,;4.46,-1.1,;2.92,-1.01,;2.2,.37,;.66,.44,;-.16,-.87,;.54,-2.25,;2.08,-2.32,;-1.69,-.8,;-2.39,.57,;-3.93,.64,;-4.65,2.02,;-6.22,2.09,;-7.03,.78,;-8.57,.85,;-9.39,-.45,;-8.66,-1.83,;-7.15,-1.9,;-6.31,-.59,;-4.77,-.66,;5.42,.11,;5.18,1.62,)| Show InChI InChI=1S/C23H25FN2O2/c24-16-7-10-21-19(11-16)20(13-26-21)15-5-8-17(9-6-15)25-12-18-14-27-22-3-1-2-4-23(22)28-18/h1-4,7,10-11,13,15,17-18,25-26H,5-6,8-9,12,14H2/t15-,17+,18? | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 332 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against human 5-hydroxytryptamine 1A receptors transfected into CHO cells. |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50160610

(CHEMBL178304 | [2-(2,3-Dihydro-benzo[1,4]dioxin-5-...)Show SMILES Fc1ccc2[nH]cc([C@@H]3CC[C@@H](CC3)NCCOc3cccc4OCCOc34)c2c1 |wU:8.7,11.14,(7.58,-3.4,;7.58,-1.87,;8.92,-1.08,;8.92,.47,;7.58,1.23,;7.26,2.74,;5.71,2.89,;5.11,1.49,;3.59,1.17,;2.56,2.31,;1.06,2,;.57,.54,;1.62,-.62,;3.12,-.29,;-.93,.21,;-1.42,-1.25,;-2.91,-1.57,;-3.94,-.41,;-5.46,-.75,;-5.46,-2.29,;-6.8,-3.06,;-8.13,-2.29,;-8.13,-.75,;-9.46,,;-9.46,1.54,;-8.13,2.31,;-6.8,1.54,;-6.82,.02,;6.25,.46,;6.25,-1.08,)| Show InChI InChI=1S/C24H27FN2O3/c25-17-6-9-21-19(14-17)20(15-27-21)16-4-7-18(8-5-16)26-10-11-28-22-2-1-3-23-24(22)30-13-12-29-23/h1-3,6,9,14-16,18,26-27H,4-5,7-8,10-13H2/t16-,18+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 484 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity determined against human 5-hydroxytryptamine 1A receptors transfected into CHO cells. |
Bioorg Med Chem Lett 15: 911-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.064
BindingDB Entry DOI: 10.7270/Q2VD6XZH |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598850
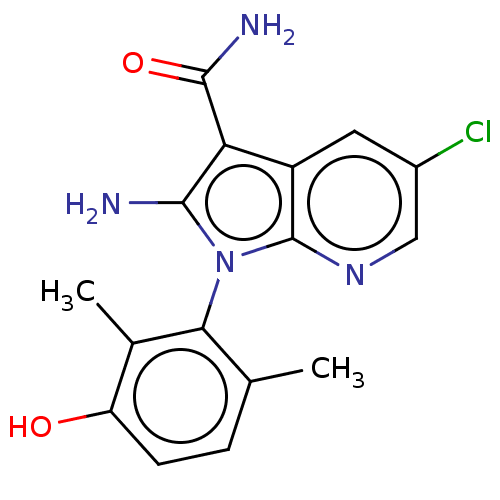
(CHEMBL5196713)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2cc(Cl)cnc12 |(.92,2.78,;1.81,1.93,;3.29,2.36,;4.41,1.3,;4.04,-.2,;4.93,-1.05,;2.56,-.63,;2.27,-1.83,;1.45,.43,;-.05,.11,;-.68,-1.3,;-.06,-2.36,;-2.21,-1.13,;-3.24,-2.28,;-4.44,-2.03,;-2.86,-3.45,;-2.53,.37,;-3.87,1.14,;-3.87,2.68,;-4.93,3.3,;-2.53,3.45,;-1.2,2.68,;-1.2,1.14,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598854
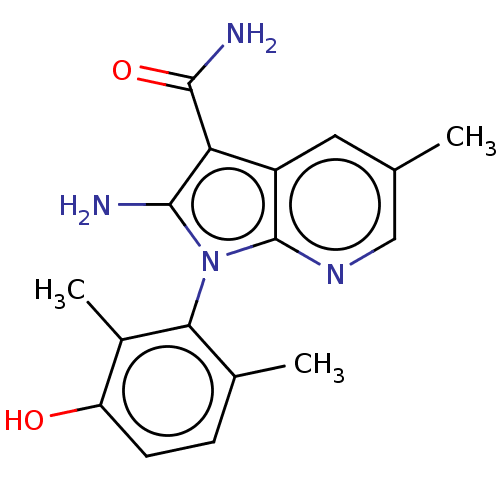
(CHEMBL5174684)Show SMILES Cc1cnc2n(c(N)c(C(N)=O)c2c1)-c1c(C)ccc(O)c1C |(-4.93,3.3,;-3.87,2.68,;-2.53,3.45,;-1.2,2.68,;-1.2,1.14,;-.05,.11,;-.68,-1.3,;-.06,-2.36,;-2.21,-1.13,;-3.24,-2.28,;-4.44,-2.03,;-2.86,-3.45,;-2.53,.37,;-3.87,1.14,;1.45,.43,;1.81,1.93,;.92,2.78,;3.29,2.36,;4.41,1.3,;4.04,-.2,;4.93,-1.05,;2.56,-.63,;2.27,-1.83,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598845

(CHEMBL5201803)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc(cnc12)C1CC1 |(3.4,-2.47,;3.69,-1.27,;5.17,-.84,;5.53,.66,;4.42,1.72,;4.71,2.92,;2.94,1.29,;2.05,2.14,;2.58,-.21,;1.07,-.53,;.45,-1.94,;1.06,-3.01,;-1.09,-1.78,;-2.11,-2.92,;-3.32,-2.67,;-1.73,-4.1,;-1.4,-.27,;-2.74,.5,;-2.74,2.04,;-1.4,2.81,;-.07,2.04,;-.07,.5,;-4.07,2.81,;-5.53,2.81,;-4.76,4.1,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598851

(CHEMBL5199076)Show SMILES Cc1cc2c(C(N)=O)c(N)n(-c3c(C)ccc(O)c3C)c2nc1C |(-4.93,2.68,;-3.87,2.07,;-3.87,.52,;-2.53,-.25,;-2.21,-1.75,;-3.24,-2.9,;-4.44,-2.64,;-2.86,-4.07,;-.68,-1.91,;-.06,-2.98,;-.05,-.5,;1.45,-.18,;1.81,1.31,;.92,2.16,;3.29,1.75,;4.41,.68,;4.04,-.81,;4.93,-1.66,;2.56,-1.25,;2.27,-2.44,;-1.2,.52,;-1.2,2.07,;-2.53,2.84,;-2.53,4.07,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598830
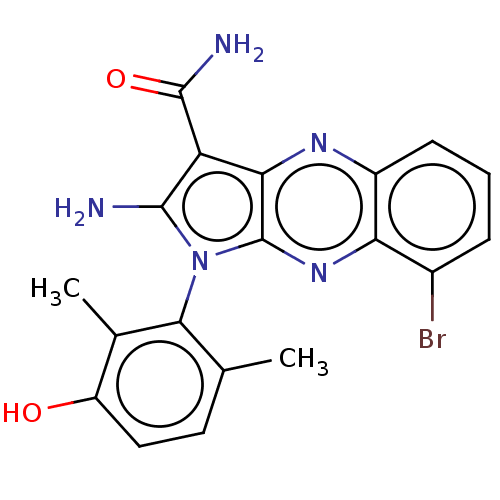
(CHEMBL5186220)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc3cccc(Br)c3nc12 |(2.67,-2.98,;2.96,-1.78,;4.44,-1.35,;4.8,.14,;3.69,1.21,;3.98,2.41,;2.21,.78,;1.32,1.63,;1.85,-.72,;.34,-1.04,;-.28,-2.45,;.33,-3.52,;-1.81,-2.29,;-2.84,-3.43,;-4.05,-3.18,;-2.46,-4.61,;-2.13,-.78,;-3.47,-.01,;-3.47,1.53,;-4.8,2.3,;-4.8,3.84,;-3.47,4.61,;-2.13,3.84,;-1.07,4.45,;-2.13,2.3,;-.8,1.53,;-.8,-.01,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598832
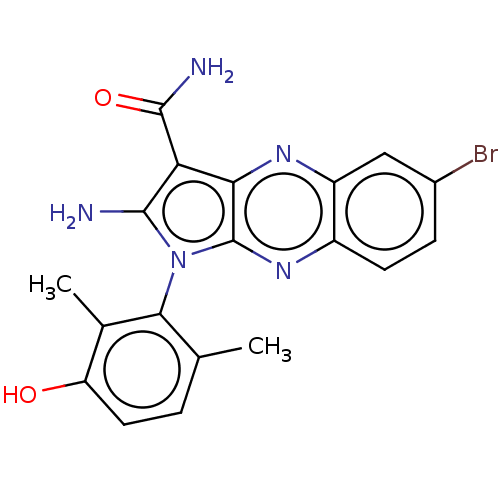
(CHEMBL5185146)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc3cc(Br)ccc3nc12 |(3.2,-2.98,;3.49,-1.78,;4.97,-1.35,;5.34,.14,;4.22,1.21,;4.51,2.41,;2.74,.78,;1.85,1.63,;2.38,-.72,;.88,-1.04,;.25,-2.45,;.87,-3.52,;-1.28,-2.29,;-2.31,-3.43,;-3.51,-3.18,;-1.93,-4.61,;-1.6,-.78,;-2.93,-.01,;-2.93,1.53,;-4.27,2.3,;-4.27,3.84,;-5.34,4.45,;-2.93,4.61,;-1.6,3.84,;-1.6,2.3,;-.27,1.53,;-.27,-.01,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598855
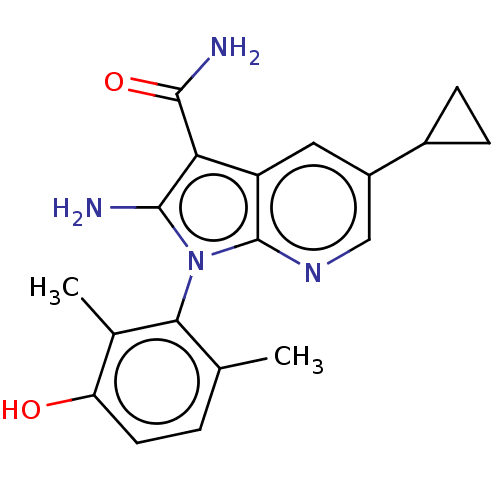
(CHEMBL5190904)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2cc(cnc12)C1CC1 |(1.78,2.14,;2.68,1.29,;4.15,1.72,;5.27,.66,;4.9,-.84,;5.8,-1.69,;3.43,-1.27,;3.13,-2.47,;2.32,-.21,;.81,-.53,;.18,-1.94,;.8,-3.01,;-1.35,-1.78,;-2.38,-2.92,;-3.58,-2.67,;-1.99,-4.09,;-1.67,-.27,;-3,.5,;-3,2.04,;-1.67,2.81,;-.33,2.04,;-.33,.5,;-4.34,2.81,;-5.8,2.81,;-5.03,4.09,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598833
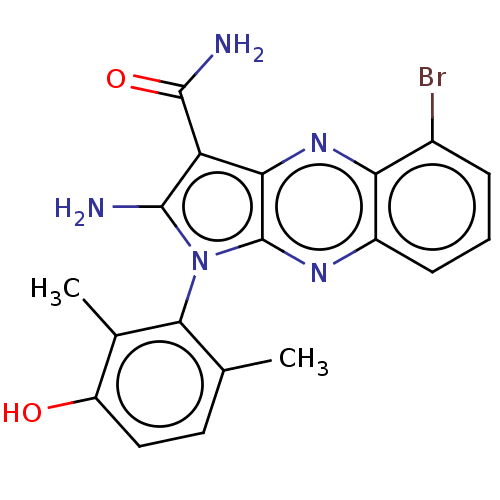
(CHEMBL5181222)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc3c(Br)cccc3nc12 |(3.2,-2.98,;3.49,-1.78,;4.97,-1.35,;5.34,.14,;4.22,1.21,;4.51,2.41,;2.74,.78,;1.85,1.63,;2.38,-.72,;.88,-1.04,;.25,-2.45,;.87,-3.52,;-1.28,-2.29,;-2.31,-3.43,;-3.51,-3.18,;-1.93,-4.61,;-1.6,-.78,;-2.93,-.01,;-2.93,1.53,;-4.27,2.3,;-5.34,1.68,;-4.27,3.84,;-2.93,4.61,;-1.6,3.84,;-1.6,2.3,;-.27,1.53,;-.27,-.01,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598835
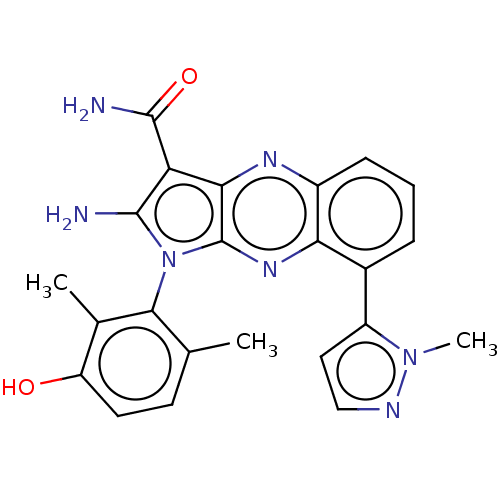
(CHEMBL5169345)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc3cccc(-c4ccnn4C)c3nc12 |(2.67,-4.15,;2.96,-2.95,;4.44,-2.52,;4.8,-1.03,;3.69,.04,;3.98,1.24,;2.21,-.39,;1.32,.45,;1.85,-1.89,;.34,-2.21,;-.28,-3.62,;.33,-4.69,;-1.81,-3.46,;-2.84,-4.61,;-4.05,-4.35,;-2.46,-5.78,;-2.13,-1.95,;-3.47,-1.18,;-3.47,.36,;-4.8,1.13,;-4.8,2.67,;-3.47,3.44,;-2.13,2.67,;-.8,3.44,;.59,2.8,;1.62,3.94,;.85,5.27,;-.66,4.96,;-1.58,5.78,;-2.13,1.13,;-.8,.36,;-.8,-1.18,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598836

(CHEMBL5190822)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc3cccc(C4=CCCC4)c3nc12 |t:24,(2.67,-3.9,;2.96,-2.7,;4.44,-2.27,;4.8,-.77,;3.69,.29,;3.98,1.49,;2.21,-.14,;1.32,.71,;1.85,-1.64,;.34,-1.96,;-.28,-3.37,;.33,-4.44,;-1.81,-3.21,;-2.84,-4.35,;-4.05,-4.1,;-2.46,-5.53,;-2.13,-1.7,;-3.47,-.93,;-3.47,.61,;-4.8,1.38,;-4.8,2.92,;-3.47,3.69,;-2.13,2.92,;-.8,3.69,;-.66,5.21,;.85,5.53,;1.62,4.19,;.59,3.05,;-2.13,1.38,;-.8,.61,;-.8,-.93,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598834
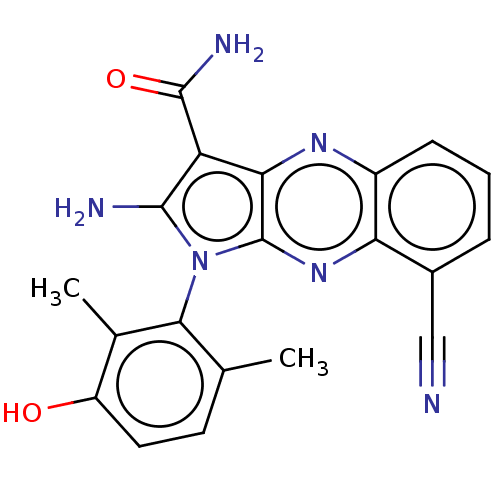
(CHEMBL5198360)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc3cccc(C#N)c3nc12 |(2.67,-3.29,;2.96,-2.09,;4.44,-1.66,;4.8,-.16,;3.69,.9,;3.98,2.1,;2.21,.47,;1.32,1.32,;1.85,-1.03,;.34,-1.35,;-.28,-2.76,;.33,-3.83,;-1.81,-2.6,;-2.84,-3.74,;-4.05,-3.49,;-2.46,-4.91,;-2.13,-1.09,;-3.47,-.32,;-3.47,1.22,;-4.8,1.99,;-4.8,3.53,;-3.47,4.3,;-2.13,3.53,;-.8,4.3,;.27,4.91,;-2.13,1.99,;-.8,1.22,;-.8,-.32,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598838
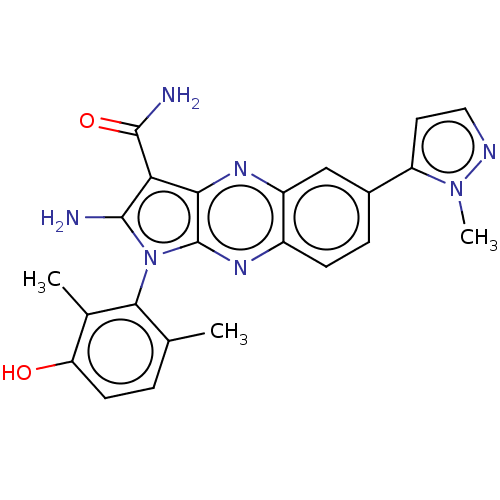
(CHEMBL5172086)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc3cc(ccc3nc12)-c1ccnn1C |(4.54,-3.9,;4.84,-2.71,;6.31,-2.27,;6.68,-.78,;5.56,.29,;5.86,1.48,;4.09,-.15,;3.19,.7,;3.73,-1.65,;2.22,-1.97,;1.59,-3.37,;2.21,-4.44,;.06,-3.21,;-.97,-4.36,;-2.17,-4.1,;-.58,-5.53,;-.26,-1.71,;-1.59,-.94,;-1.59,.6,;-2.93,1.37,;-2.93,2.91,;-1.59,3.68,;-.26,2.91,;-.26,1.37,;1.07,.6,;1.07,-.94,;-4.26,3.68,;-4.4,5.21,;-5.91,5.53,;-6.68,4.2,;-5.65,3.05,;-5.9,1.84,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
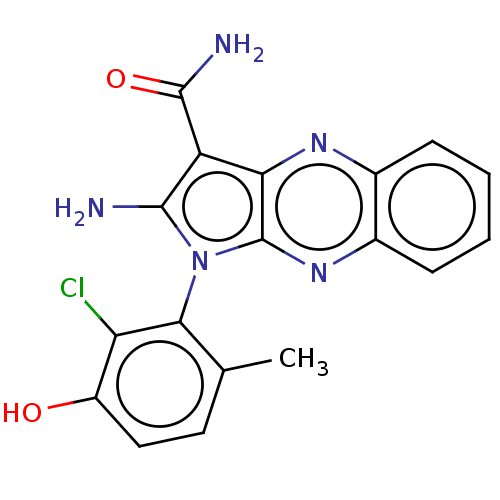
(Homo sapiens (Human)) | BDBM50598820
(CHEMBL5191665)Show SMILES Cc1ccc(O)c(Cl)c1-n1c(N)c(C(N)=O)c2nc3ccccc3nc12 |(2.67,-2.98,;2.96,-1.78,;4.44,-1.35,;4.8,.14,;3.69,1.21,;3.98,2.41,;2.21,.78,;1.32,1.63,;1.85,-.72,;.34,-1.04,;-.28,-2.45,;.33,-3.52,;-1.81,-2.29,;-2.84,-3.43,;-4.05,-3.18,;-2.46,-4.61,;-2.13,-.78,;-3.47,-.01,;-3.47,1.53,;-4.8,2.3,;-4.8,3.84,;-3.47,4.61,;-2.13,3.84,;-2.13,2.3,;-.8,1.53,;-.8,-.01,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
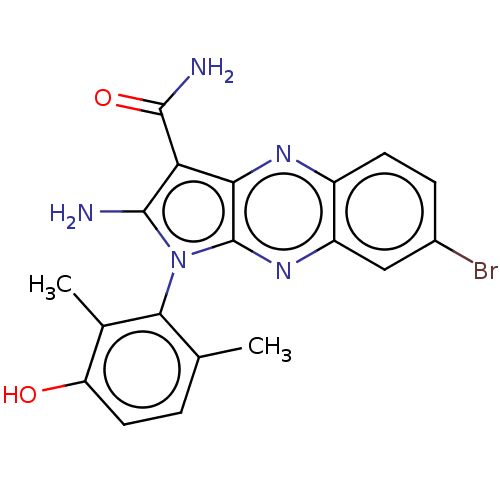
(Homo sapiens (Human)) | BDBM50598831
(CHEMBL5195417)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc3ccc(Br)cc3nc12 |(2.67,-3.6,;2.96,-2.4,;4.44,-1.97,;4.8,-.47,;3.69,.59,;3.98,1.79,;2.21,.16,;1.32,1.01,;1.85,-1.34,;.34,-1.66,;-.28,-3.07,;.33,-4.13,;-1.81,-2.91,;-2.84,-4.05,;-4.05,-3.8,;-2.46,-5.22,;-2.13,-1.4,;-3.47,-.63,;-3.47,.91,;-4.8,1.68,;-4.8,3.22,;-3.47,3.99,;-3.47,5.22,;-2.13,3.22,;-2.13,1.68,;-.8,.91,;-.8,-.63,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
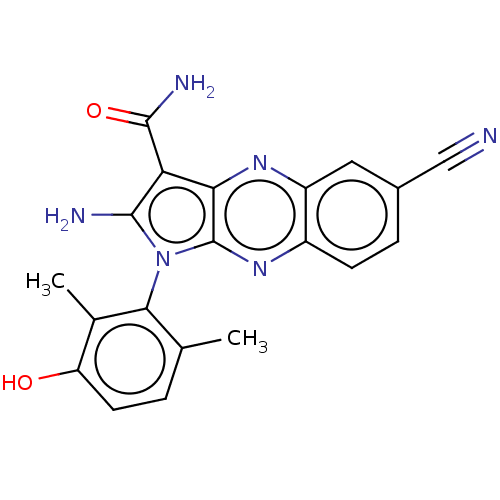
(Homo sapiens (Human)) | BDBM50598837
(CHEMBL5187022)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc3cc(ccc3nc12)C#N |(3.87,-3.29,;4.16,-2.09,;5.64,-1.66,;6,-.16,;4.89,.9,;5.18,2.1,;3.41,.47,;2.52,1.32,;3.05,-1.03,;1.54,-1.35,;.92,-2.76,;1.53,-3.83,;-.61,-2.6,;-1.64,-3.74,;-2.85,-3.49,;-1.26,-4.91,;-.93,-1.09,;-2.27,-.32,;-2.27,1.22,;-3.6,1.99,;-3.6,3.53,;-2.27,4.3,;-.93,3.53,;-.93,1.99,;.4,1.22,;.4,-.32,;-4.94,4.3,;-6,4.91,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598840
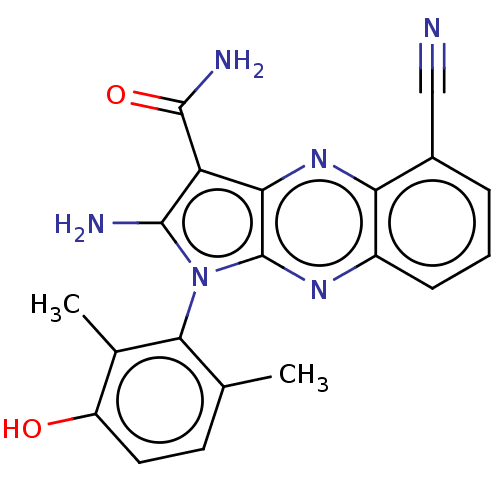
(CHEMBL5199806)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc3c(cccc3nc12)C#N |(3.87,-2.98,;4.16,-1.78,;5.64,-1.35,;6,.14,;4.89,1.21,;5.18,2.41,;3.41,.78,;2.52,1.63,;3.05,-.72,;1.54,-1.04,;.92,-2.45,;1.53,-3.52,;-.61,-2.29,;-1.64,-3.43,;-2.85,-3.18,;-1.26,-4.61,;-.93,-.78,;-2.27,-.01,;-2.27,1.53,;-3.6,2.3,;-3.6,3.84,;-2.27,4.61,;-.93,3.84,;-.93,2.3,;.4,1.53,;.4,-.01,;-4.94,1.53,;-6,.91,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598839
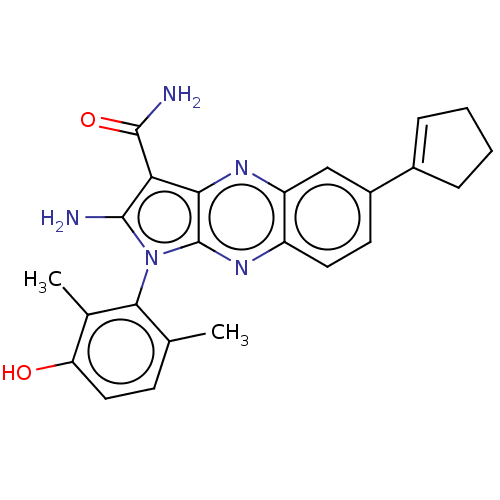
(CHEMBL5184178)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc3cc(ccc3nc12)C1=CCCC1 |t:30,(4.54,-3.9,;4.84,-2.71,;6.31,-2.27,;6.68,-.78,;5.56,.29,;5.86,1.48,;4.09,-.15,;3.19,.7,;3.73,-1.64,;2.22,-1.97,;1.59,-3.37,;2.21,-4.44,;.06,-3.21,;-.97,-4.36,;-2.17,-4.1,;-.58,-5.53,;-.26,-1.71,;-1.59,-.94,;-1.59,.6,;-2.93,1.37,;-2.93,2.91,;-1.59,3.68,;-.26,2.91,;-.26,1.37,;1.07,.6,;1.07,-.94,;-4.26,3.68,;-5.65,3.05,;-6.68,4.2,;-5.91,5.53,;-4.4,5.21,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598855

(CHEMBL5190904)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2cc(cnc12)C1CC1 |(1.78,2.14,;2.68,1.29,;4.15,1.72,;5.27,.66,;4.9,-.84,;5.8,-1.69,;3.43,-1.27,;3.13,-2.47,;2.32,-.21,;.81,-.53,;.18,-1.94,;.8,-3.01,;-1.35,-1.78,;-2.38,-2.92,;-3.58,-2.67,;-1.99,-4.09,;-1.67,-.27,;-3,.5,;-3,2.04,;-1.67,2.81,;-.33,2.04,;-.33,.5,;-4.34,2.81,;-5.8,2.81,;-5.03,4.09,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598844
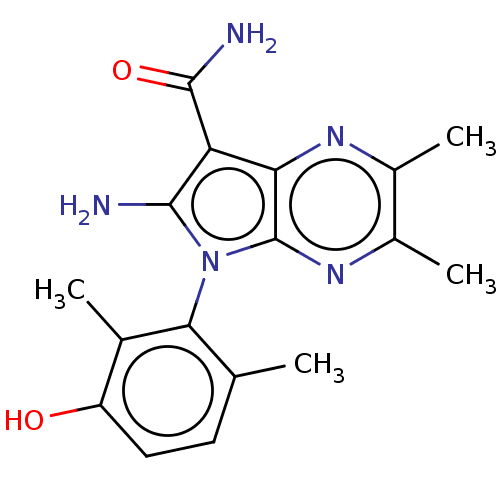
(CHEMBL5200005)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc(C)c(C)nc12 |(2.54,-2.44,;2.83,-1.24,;4.31,-.81,;4.67,.68,;3.56,1.75,;3.85,2.95,;2.08,1.31,;1.18,2.16,;1.72,-.18,;.21,-.5,;-.42,-1.91,;.2,-2.98,;-1.95,-1.75,;-2.98,-2.9,;-4.18,-2.64,;-2.59,-4.07,;-2.27,-.25,;-3.6,.52,;-3.6,2.07,;-4.67,2.68,;-2.27,2.84,;-2.27,4.07,;-.93,2.07,;-.93,.52,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
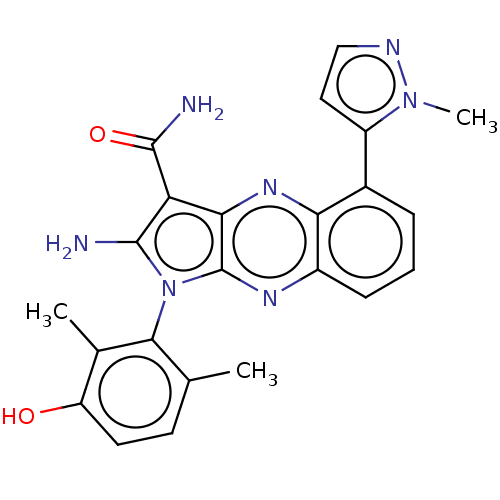
Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase
(Homo sapiens (Human)) | BDBM50598841
(CHEMBL5196095)Show SMILES Cc1ccc(O)c(C)c1-n1c(N)c(C(N)=O)c2nc3c(cccc3nc12)-c1ccnn1C |(4.54,-2.98,;4.84,-1.78,;6.31,-1.35,;6.68,.14,;5.56,1.21,;5.86,2.41,;4.09,.78,;3.19,1.63,;3.73,-.72,;2.22,-1.04,;1.59,-2.45,;2.21,-3.52,;.06,-2.29,;-.97,-3.43,;-2.17,-3.18,;-.58,-4.61,;-.26,-.78,;-1.59,-.01,;-1.59,1.53,;-2.93,2.3,;-2.93,3.84,;-1.59,4.61,;-.26,3.84,;-.26,2.3,;1.08,1.53,;1.08,-.01,;-4.26,1.53,;-5.65,2.17,;-6.68,1.02,;-5.91,-.31,;-4.4,.01,;-3.48,-.81,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 3
(Homo sapiens (Human)) | BDBM50598820

(CHEMBL5191665)Show SMILES Cc1ccc(O)c(Cl)c1-n1c(N)c(C(N)=O)c2nc3ccccc3nc12 |(2.67,-2.98,;2.96,-1.78,;4.44,-1.35,;4.8,.14,;3.69,1.21,;3.98,2.41,;2.21,.78,;1.32,1.63,;1.85,-.72,;.34,-1.04,;-.28,-2.45,;.33,-3.52,;-1.81,-2.29,;-2.84,-3.43,;-4.05,-3.18,;-2.46,-4.61,;-2.13,-.78,;-3.47,-.01,;-3.47,1.53,;-4.8,2.3,;-4.8,3.84,;-3.47,4.61,;-2.13,3.84,;-2.13,2.3,;-.8,1.53,;-.8,-.01,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 3
(Homo sapiens (Human)) | BDBM50100316
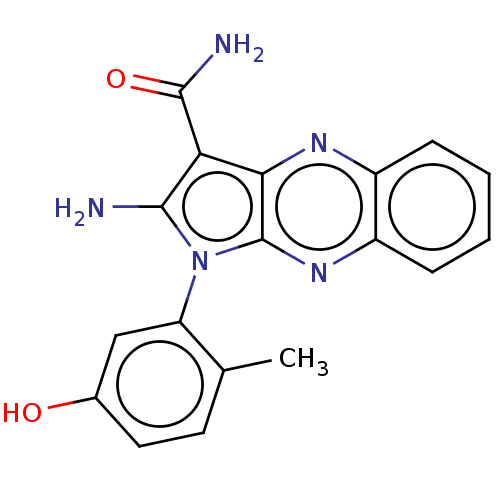
(CHEMBL3321809)Show SMILES Cc1ccc(O)cc1-n1c(N)c(C(N)=O)c2nc3ccccc3nc12 |(9.45,-34.75,;10.95,-35.07,;11.43,-36.54,;12.94,-36.86,;13.97,-35.71,;15.47,-36.03,;13.49,-34.25,;11.98,-33.93,;11.51,-32.46,;12.41,-31.22,;13.95,-31.22,;11.51,-29.97,;11.98,-28.51,;13.47,-28.11,;10.95,-27.36,;10.04,-30.45,;8.71,-29.68,;7.38,-30.45,;6.04,-29.68,;4.71,-30.45,;4.71,-31.99,;6.04,-32.76,;7.38,-31.99,;8.71,-32.76,;10.04,-31.99,)| Show InChI InChI=1S/C18H15N5O2/c1-9-6-7-10(24)8-13(9)23-16(19)14(17(20)25)15-18(23)22-12-5-3-2-4-11(12)21-15/h2-8,24H,19H2,1H3,(H2,20,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00552
BindingDB Entry DOI: 10.7270/Q2028WKQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data