

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581847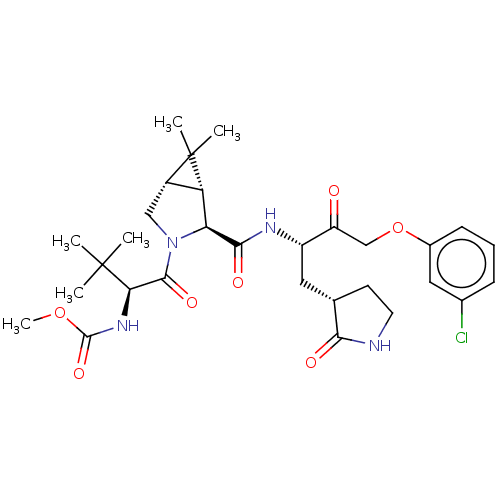 (WO2022208262, Example 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581848 (WO2022208262, Example 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | <0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50160612 (CHEMBL362823 | [2-(2,3-Dihydro-benzo[1,4]dioxin-5-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.05 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity determined against Serotonin transporter determined bydisplacement of [3H]-paroxetine from rat cortical membranes | Bioorg Med Chem Lett 15: 911-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.064 BindingDB Entry DOI: 10.7270/Q2VD6XZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50160611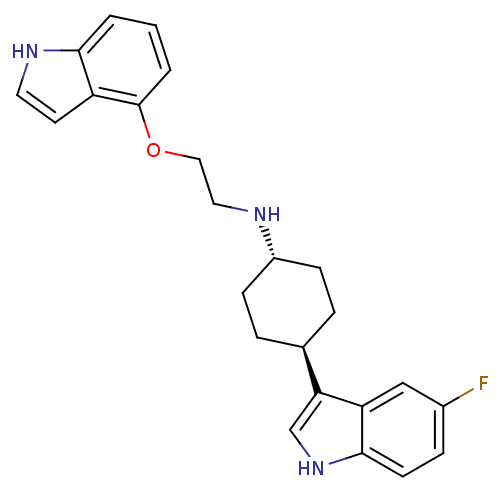 (CHEMBL359549 | [4-(5-Fluoro-1H-indol-3-yl)-cyclohe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity determined against human 5-hydroxytryptamine 1A receptors transfected into CHO cells. | Bioorg Med Chem Lett 15: 911-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.064 BindingDB Entry DOI: 10.7270/Q2VD6XZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581851 (WO2022208262, Example 24) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 1.41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535166 (WO2022013684, Example 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50137733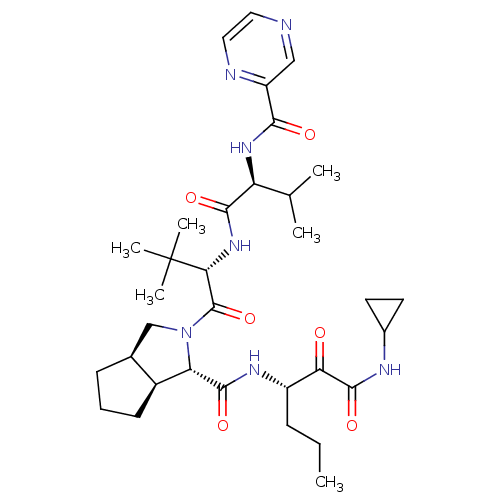 ((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity of the compound against cathepsin B | Bioorg Med Chem Lett 14: 257-61 (2003) BindingDB Entry DOI: 10.7270/Q20P0ZDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50160611 (CHEMBL359549 | [4-(5-Fluoro-1H-indol-3-yl)-cyclohe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity determined against Serotonin transporter determined bydisplacement of [3H]-paroxetine from rat cortical membranes | Bioorg Med Chem Lett 15: 911-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.064 BindingDB Entry DOI: 10.7270/Q2VD6XZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581852 (WO2022208262, Example 25) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 1.76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581850 (WO2022208262, Example 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 1.78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581846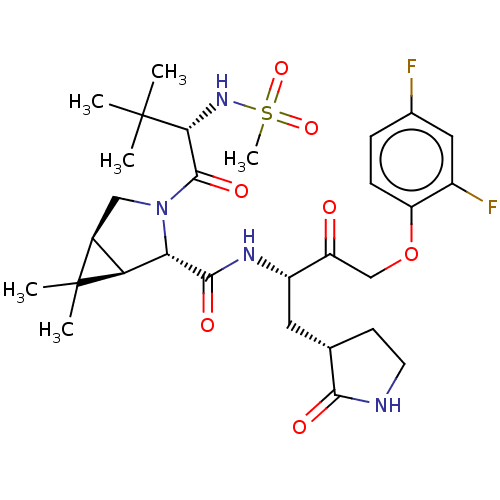 (WO2022208262, Example 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 1.78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581839 (WO2022208262, Example 1 | WO2022208262, Example 35) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 2.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581855 (WO2022208262, Example 28) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 2.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581857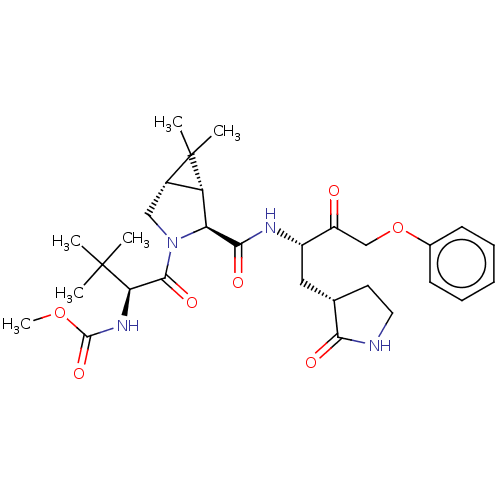 (WO2022208262, Example 30) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 2.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581858 (WO2022208262, Example 32) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 2.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581839 (WO2022208262, Example 1 | WO2022208262, Example 35) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 2.76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581860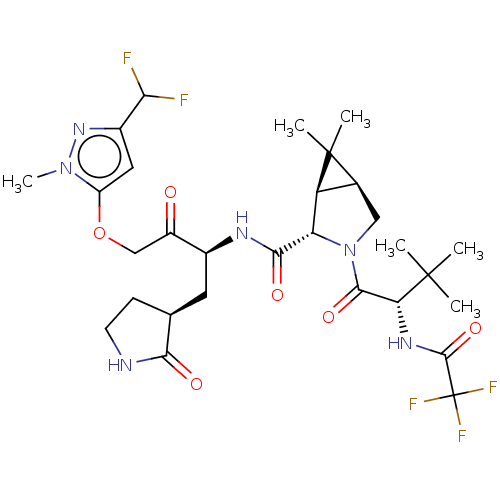 (WO2022208262, Example 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 2.87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581842 (WO2022208262, Example 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 2.88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581859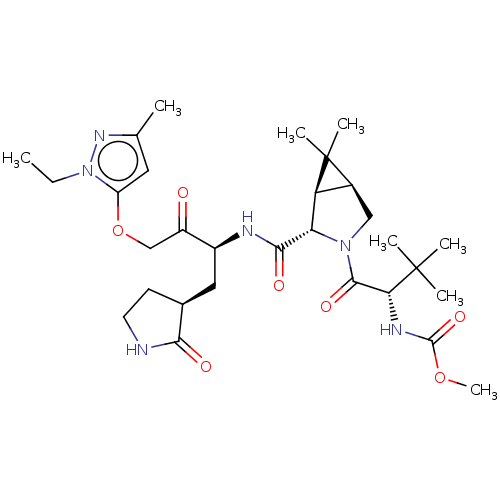 (WO2022208262, Example 33) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 3.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50160602 (5-Fluoro-3-{4-[4-(2-methoxy-phenyl)-piperazin-1-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity determined against Serotonin transporter determined bydisplacement of [3H]-paroxetine from rat cortical membranes | Bioorg Med Chem Lett 15: 911-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.064 BindingDB Entry DOI: 10.7270/Q2VD6XZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581840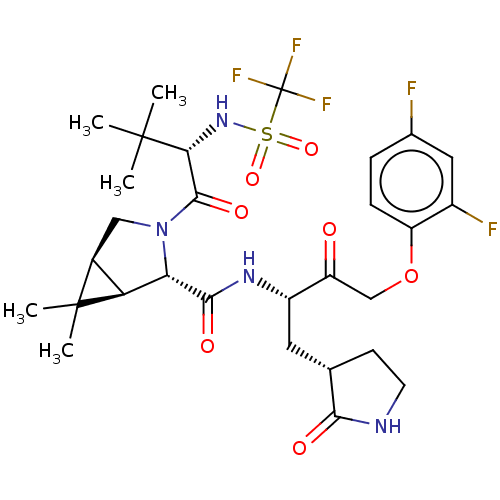 (WO2022208262, Example 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581863 (WO2022208262, Example 39) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50137736 ((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity of the compound against cathepsin B | Bioorg Med Chem Lett 14: 257-61 (2003) BindingDB Entry DOI: 10.7270/Q20P0ZDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50160601 (CHEMBL180817 | [4-(5-Fluoro-1H-indol-3-yl)-cyclohe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity determined against Serotonin transporter determined bydisplacement of [3H]-paroxetine from rat cortical membranes | Bioorg Med Chem Lett 15: 911-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.064 BindingDB Entry DOI: 10.7270/Q2VD6XZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50137720 ((1S,3aR,6aS)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity of the compound against cathepsin B | Bioorg Med Chem Lett 14: 257-61 (2003) BindingDB Entry DOI: 10.7270/Q20P0ZDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581862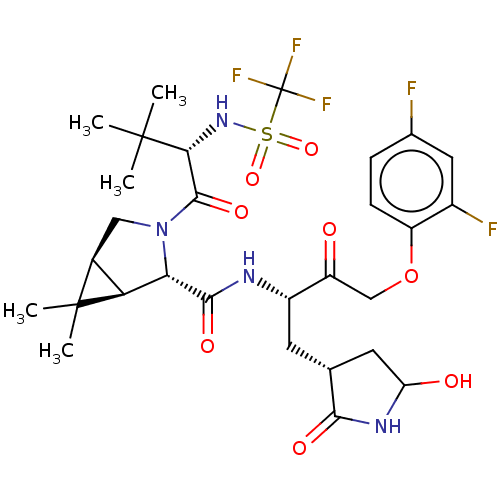 (WO2022208262, Example 38) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 4.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581864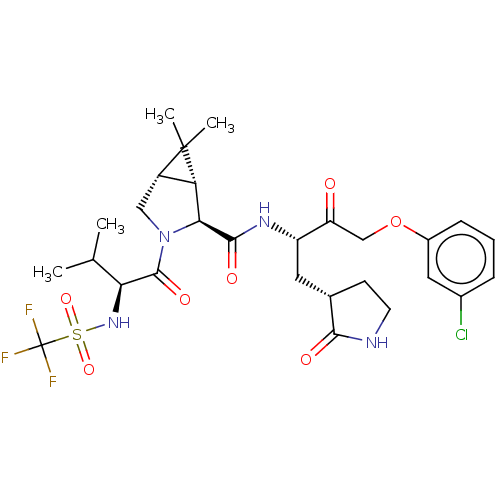 (WO2022208262, Example 40) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 4.22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50160613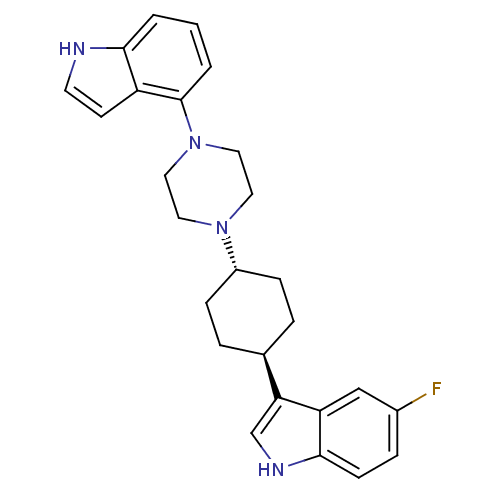 (5-Fluoro-3-[trans-4-[4-(1H-indol-4-yl)-1-piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity determined against human 5-hydroxytryptamine 1A receptors transfected into CHO cells. | Bioorg Med Chem Lett 15: 911-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.064 BindingDB Entry DOI: 10.7270/Q2VD6XZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50137730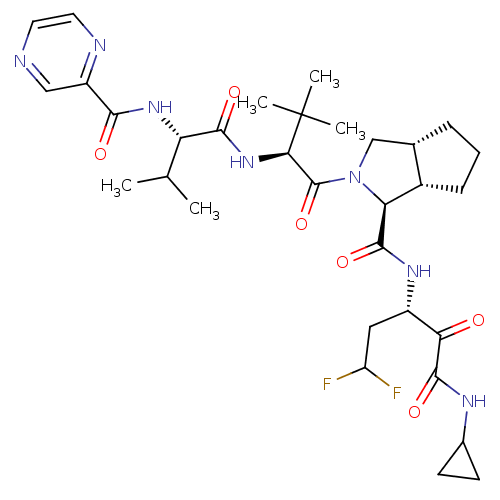 ((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity of the compound against cathepsin B | Bioorg Med Chem Lett 14: 257-61 (2003) BindingDB Entry DOI: 10.7270/Q20P0ZDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50160609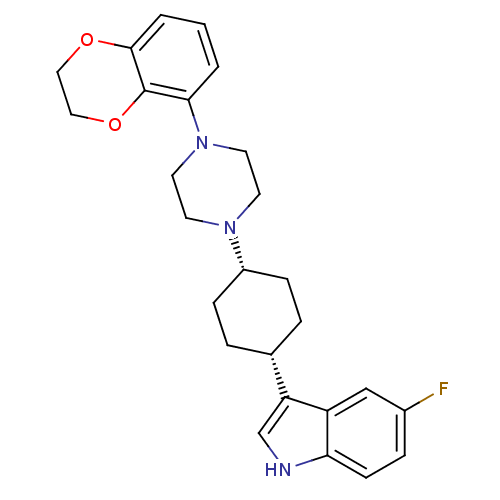 (3-{4-[4-(2,3-Dihydro-benzo[1,4]dioxin-5-yl)-pipera...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity determined against Serotonin transporter determined bydisplacement of [3H]-paroxetine from rat cortical membranes | Bioorg Med Chem Lett 15: 911-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.064 BindingDB Entry DOI: 10.7270/Q2VD6XZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50160603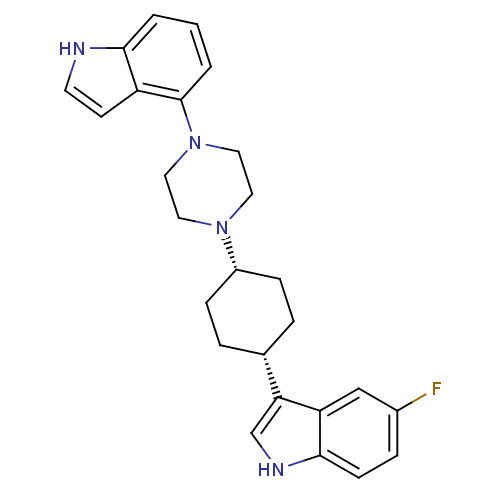 (5-Fluoro-3-[cis-4-[4-(1H-indol-4-yl)-1-piperazinyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity determined against Serotonin transporter determined bydisplacement of [3H]-paroxetine from rat cortical membranes | Bioorg Med Chem Lett 15: 911-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.064 BindingDB Entry DOI: 10.7270/Q2VD6XZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581865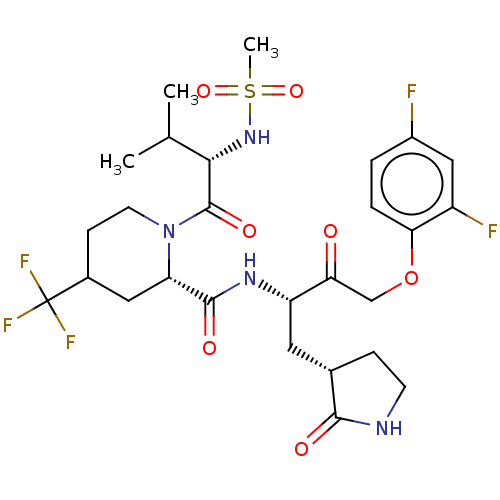 (WO2022208262, Example 41) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 5.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535159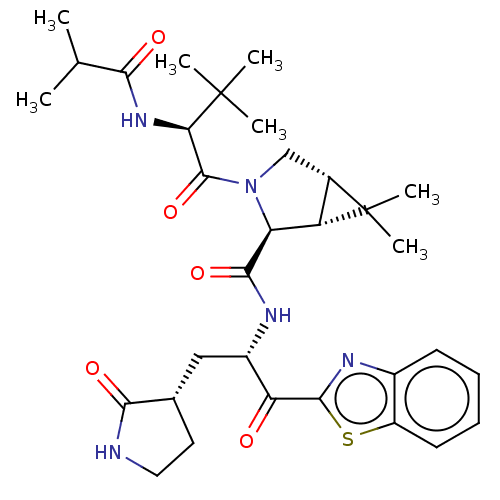 (WO2022013684, Example 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 6.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50150593 (3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory potency against HCV NS3 protease | Bioorg Med Chem Lett 14: 4333-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.078 BindingDB Entry DOI: 10.7270/Q2TX3DVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535160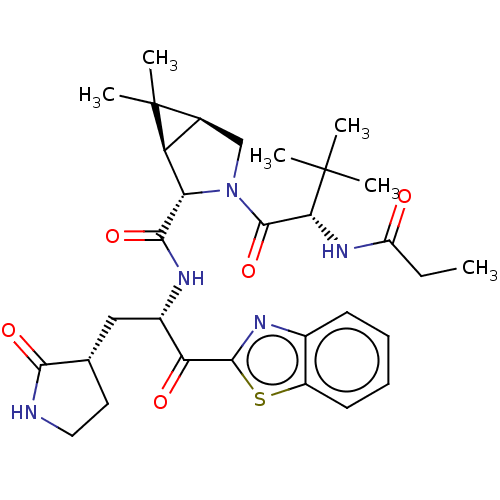 (WO2022013684, Example 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 6.14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581849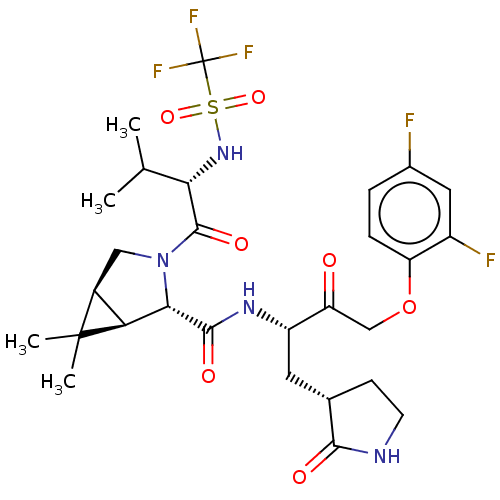 (WO2022208262, Example 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 6.34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50160605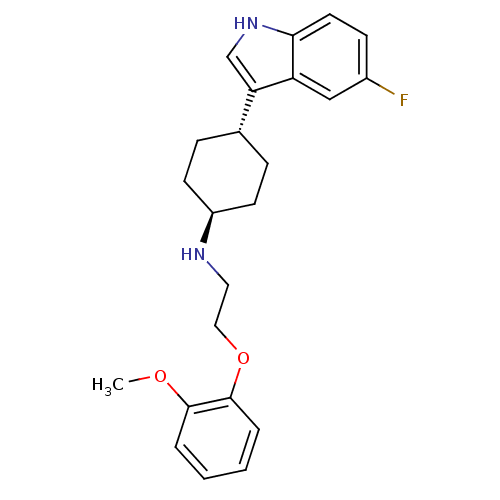 (CHEMBL359505 | [4-(5-Fluoro-1H-indol-3-yl)-cyclohe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity determined against Serotonin transporter determined bydisplacement of [3H]-paroxetine from rat cortical membranes | Bioorg Med Chem Lett 15: 911-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.064 BindingDB Entry DOI: 10.7270/Q2VD6XZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581866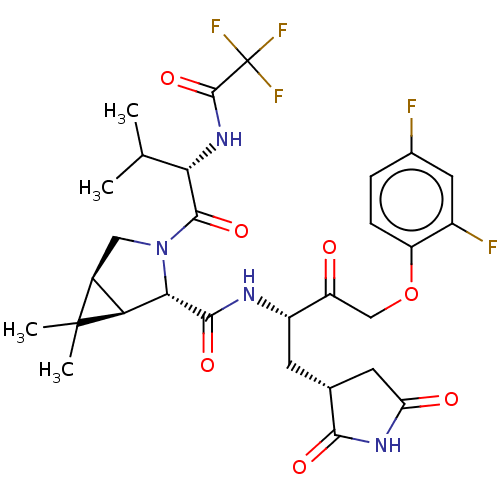 (WO2022208262, Example 42) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 7.05 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535126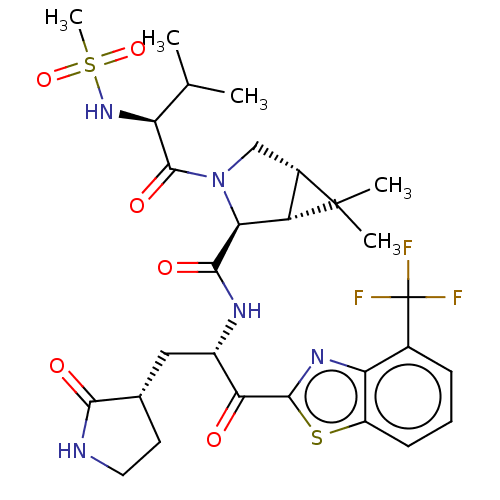 ((1R,2S,5S)-6,6-Dimethyl-3-[N-(methylsulfonyl)-L-va...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 7.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581867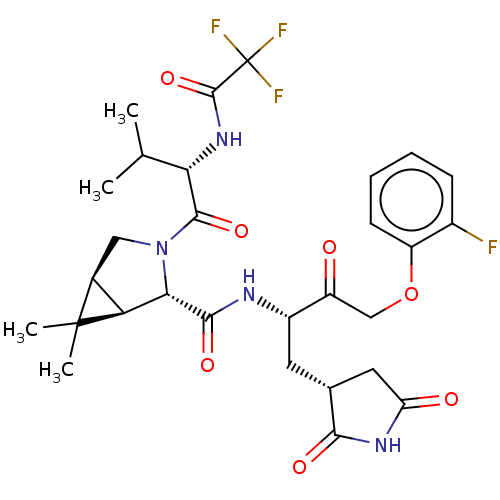 (WO2022208262, Example 48) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 7.87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581844 (WO2022208262, Example 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 8.34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581854 (WO2022208262, Example 27) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 8.84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535168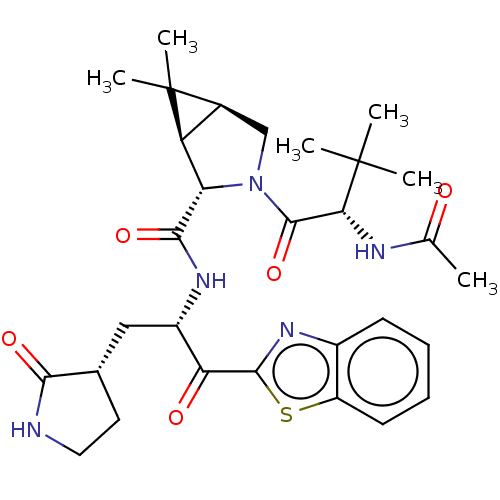 (WO2022013684, Example 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 9.77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535164 (WO2022013684, Example 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535123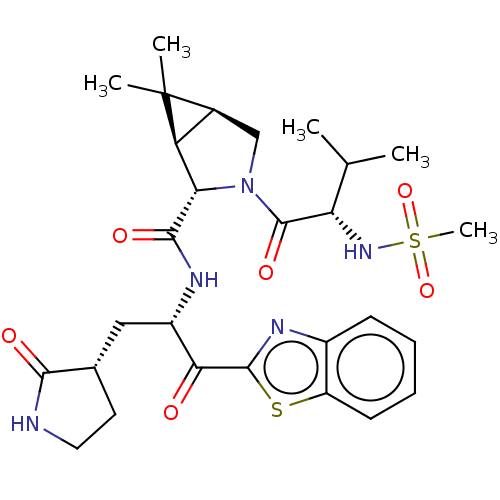 ((1R,2S,5S)-N-{(2S)-1-(1,3-Benzothiazol-2-yl)-1-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM581841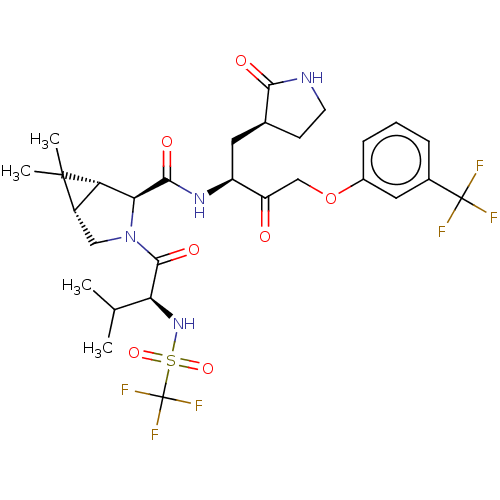 (WO2022208262, Example 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022208262 | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compounds to prevent SARS-CoV-2 coronavirus-induced cell death or cytopathic effect can be assessed via cell viability, using an assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50160606 (3-{4-[4-(2,3-Dihydro-benzo[1,4]dioxin-5-yl)-pipera...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity determined against human 5-hydroxytryptamine 1A receptors transfected into CHO cells. | Bioorg Med Chem Lett 15: 911-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.064 BindingDB Entry DOI: 10.7270/Q2VD6XZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50137720 ((1S,3aR,6aS)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity of the compound against cathepsin L | Bioorg Med Chem Lett 14: 257-61 (2003) BindingDB Entry DOI: 10.7270/Q20P0ZDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50160610 (CHEMBL178304 | [2-(2,3-Dihydro-benzo[1,4]dioxin-5-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity determined against Serotonin transporter determined bydisplacement of [3H]-paroxetine from rat cortical membranes | Bioorg Med Chem Lett 15: 911-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.064 BindingDB Entry DOI: 10.7270/Q2VD6XZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50160607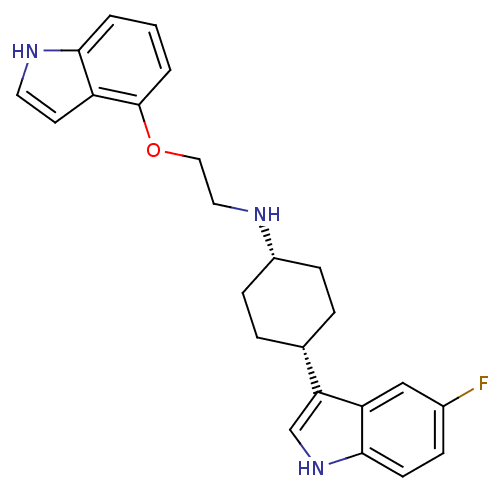 (CHEMBL178467 | [4-(5-Fluoro-1H-indol-3-yl)-cyclohe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity determined against Serotonin transporter determined bydisplacement of [3H]-paroxetine from rat cortical membranes | Bioorg Med Chem Lett 15: 911-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.064 BindingDB Entry DOI: 10.7270/Q2VD6XZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3140 total ) | Next | Last >> |