

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucan 1,4-alpha-glucosidase (Shiitake mushroom) | BDBM546721 (US11292789, MDPX-V2021) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using the samples prepared according to Example 1 and Comparative Examples 2 and 3, inhibitory activity against α-glucosidase was evaluated to a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucan 1,4-alpha-glucosidase (Shiitake mushroom) | BDBM23406 ((3R,4R,5S,6R)-5-{[(2R,3R,4R,5S,6R)-5-{[(2R,3R,4S,5...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using the samples prepared according to Example 1 and Comparative Examples 2 and 3, inhibitory activity against α-glucosidase was evaluated to a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucan 1,4-alpha-glucosidase (Shiitake mushroom) | BDBM50049572 (1,3-benzodiazine | 1,3-diazanaphthalene | 5,6-benz...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | US Patent | n/a | n/a | 278 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using the samples prepared according to Example 1 and Comparative Examples 2 and 3, inhibitory activity against α-glucosidase was evaluated to a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50144206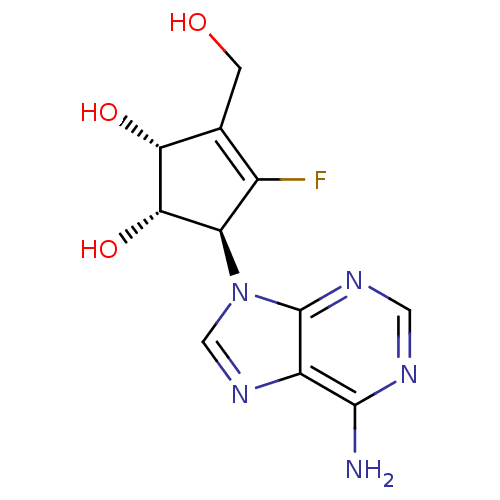 ((1S,2R,5S)-5-(6-Amino-purin-9-yl)-4-fluoro-3-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against recombinant S-adenosyl-L-homocysteine hydrolase obtained from human placenta | Bioorg Med Chem Lett 14: 2091-3 (2004) Article DOI: 10.1016/j.bmcl.2004.02.039 BindingDB Entry DOI: 10.7270/Q2DR2TXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006222 ((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against recombinant S-adenosyl-L-homocysteine hydrolase obtained from human placenta | Bioorg Med Chem Lett 14: 2091-3 (2004) Article DOI: 10.1016/j.bmcl.2004.02.039 BindingDB Entry DOI: 10.7270/Q2DR2TXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006215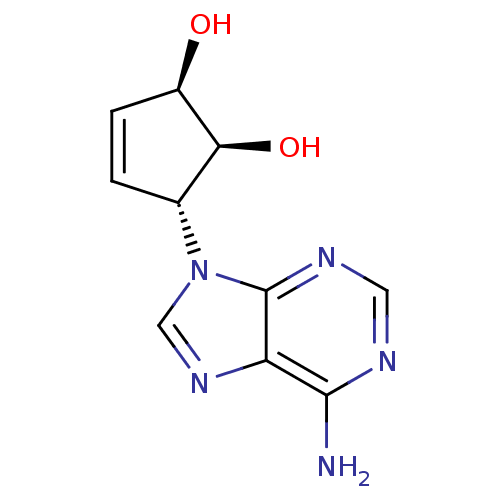 ((1'R,2'S,3'R)-9-(2',3'-dihydroxycyclopent-4'-enyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against recombinant S-adenosyl-L-homocysteine hydrolase obtained from human placenta | Bioorg Med Chem Lett 14: 2091-3 (2004) Article DOI: 10.1016/j.bmcl.2004.02.039 BindingDB Entry DOI: 10.7270/Q2DR2TXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50144205 ((1S,2R,5S)-5-(6-Amino-purin-9-yl)-4-fluoro-cyclope...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against recombinant S-adenosyl-L-homocysteine hydrolase obtained from human placenta | Bioorg Med Chem Lett 14: 2091-3 (2004) Article DOI: 10.1016/j.bmcl.2004.02.039 BindingDB Entry DOI: 10.7270/Q2DR2TXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||