

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nicotinamide N-methyltransferase (Mus musculus) | BDBM50566781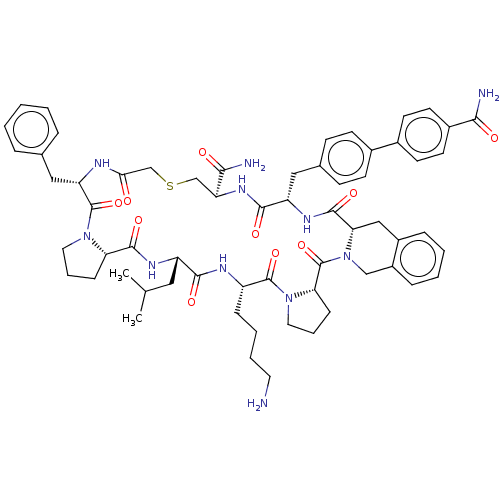 (CHEMBL4867273) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50566781 (CHEMBL4867273) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50566783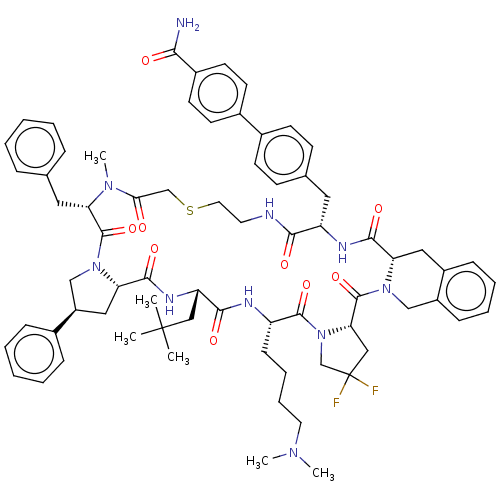 (CHEMBL4859806) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50566776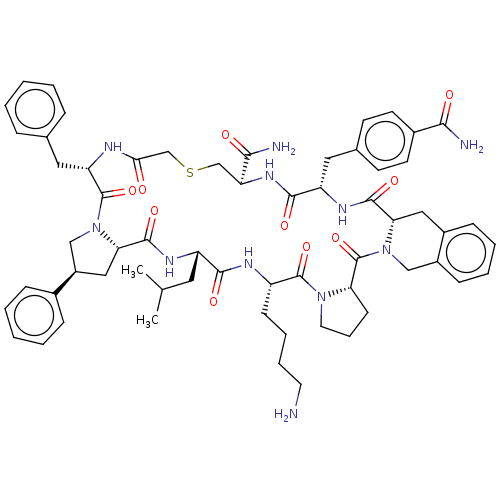 (CHEMBL4847740) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Mus musculus) | BDBM50566783 (CHEMBL4859806) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50566782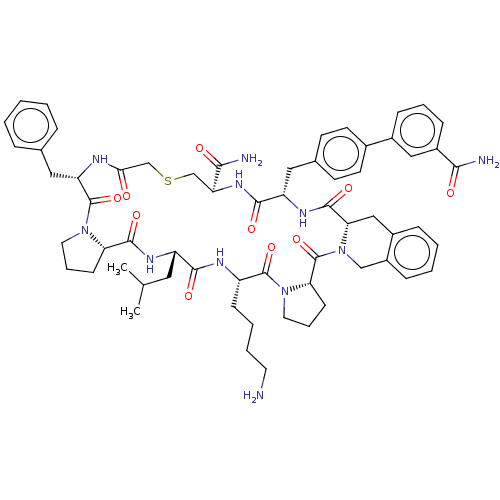 (CHEMBL4857394) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Mus musculus) | BDBM50566782 (CHEMBL4857394) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50598881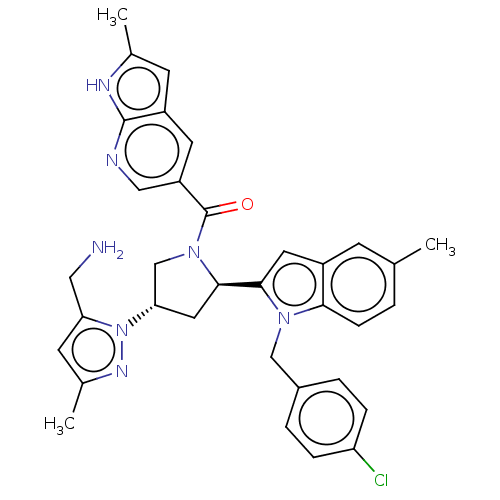 (CHEMBL5194905) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00919 BindingDB Entry DOI: 10.7270/Q2KS6WJ1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50566772 (CHEMBL4878738) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Mus musculus) | BDBM50566776 (CHEMBL4847740) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Mus musculus) | BDBM50566772 (CHEMBL4878738) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50566784 (CHEMBL4872872) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Mus musculus) | BDBM50566784 (CHEMBL4872872) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50566775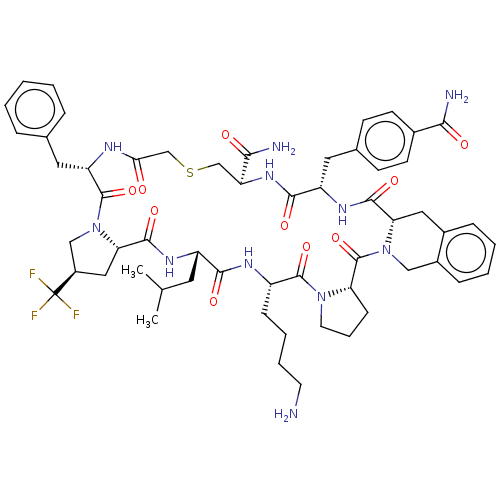 (CHEMBL4856854) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50566786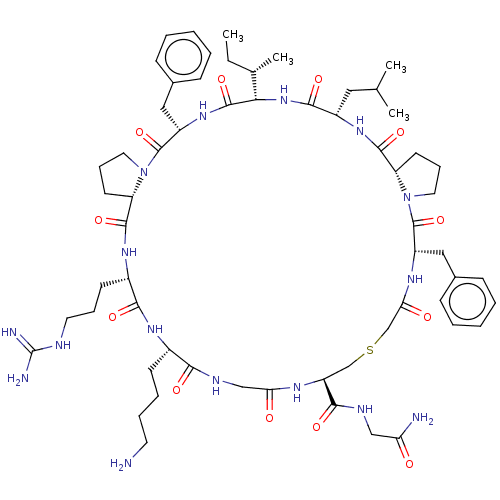 (CHEMBL4866114) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Mus musculus) | BDBM50566777 (CHEMBL4848464) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50566773 (CHEMBL4853328) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50566777 (CHEMBL4848464) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50598880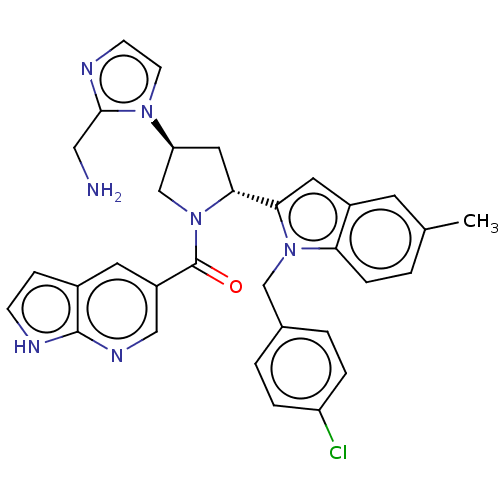 (CHEMBL5187248) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00919 BindingDB Entry DOI: 10.7270/Q2KS6WJ1 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nicotinamide N-methyltransferase (Mus musculus) | BDBM50566775 (CHEMBL4856854) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Mus musculus) | BDBM50566773 (CHEMBL4853328) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Mus musculus) | BDBM50566771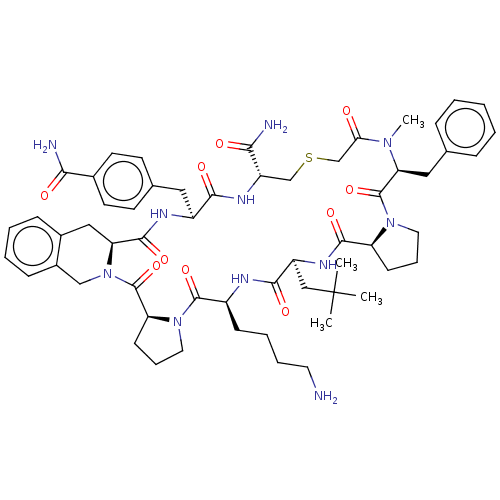 (CHEMBL4859039) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Mus musculus) | BDBM50566759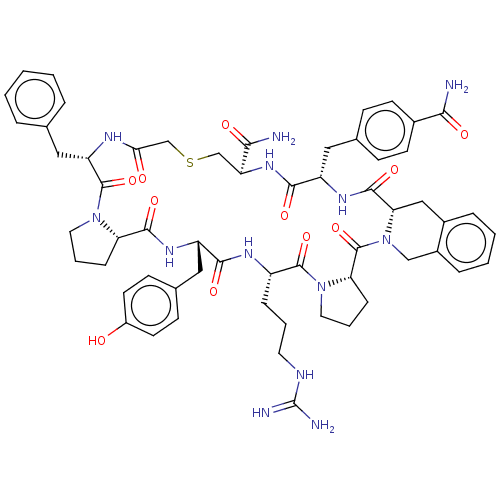 (CHEMBL4868588) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50566759 (CHEMBL4868588) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50566771 (CHEMBL4859039) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Mus musculus) | BDBM50566785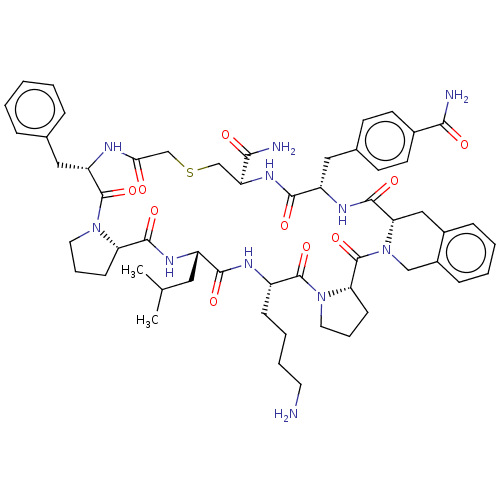 (CHEMBL4868364) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50566765 (CHEMBL4849636) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50566774 (CHEMBL4858283) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50566785 (CHEMBL4868364) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Mus musculus) | BDBM50566770 (CHEMBL4863816) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50566770 (CHEMBL4863816) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Mus musculus) | BDBM50566780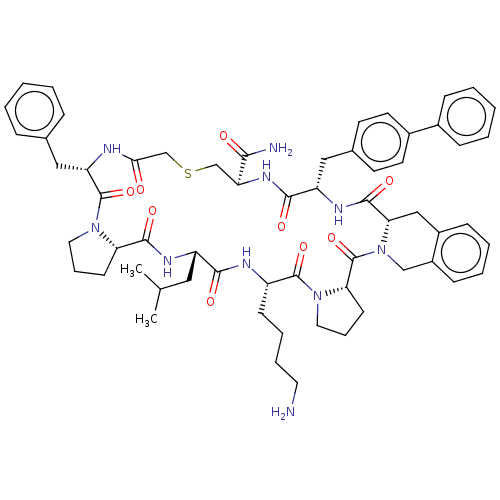 (CHEMBL4860372) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Mus musculus) | BDBM50566765 (CHEMBL4849636) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50598882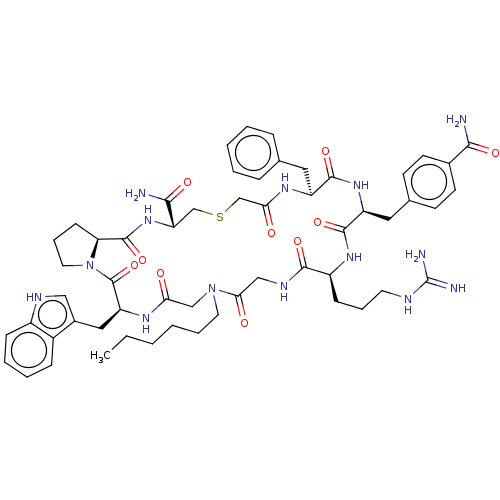 (CHEMBL5178360) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00919 BindingDB Entry DOI: 10.7270/Q2KS6WJ1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50598878 (CHEMBL5174146) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00919 BindingDB Entry DOI: 10.7270/Q2KS6WJ1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50566766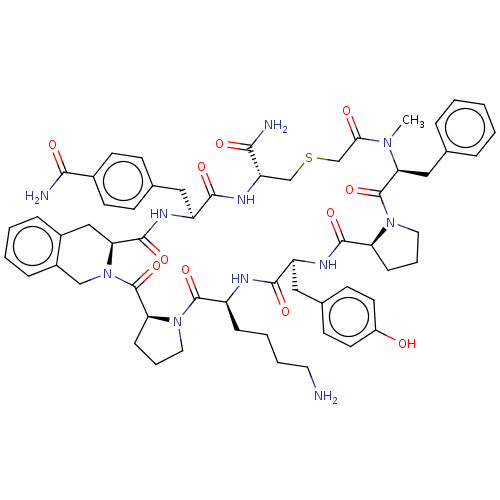 (CHEMBL4875414) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Mus musculus (Mouse)) | BDBM194635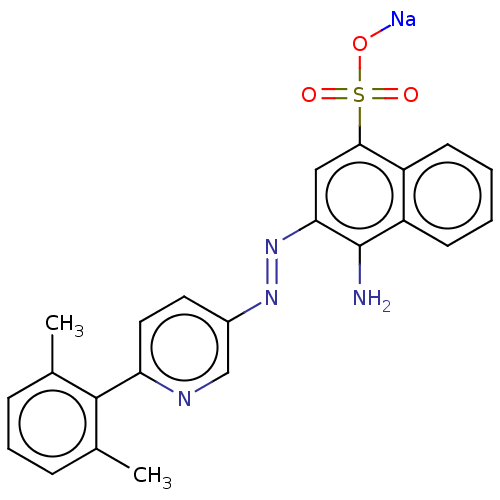 (US9206129, 51) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 112 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Daito Chemix Corporation; Kyoto University US Patent | Assay Description Mouse VCP cDNA was added with a DNA sequence corresponding to histidine tag at the amino-terminal, subcloned into a baculovirus vector pVL1392 (BD Bi... | US Patent US9206129 (2015) BindingDB Entry DOI: 10.7270/Q29P30GQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Mus musculus (Mouse)) | BDBM194589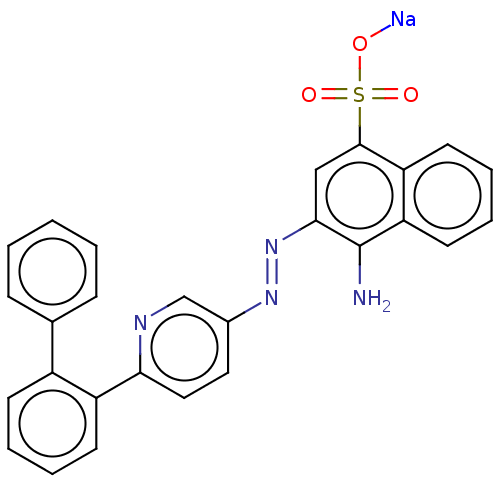 (US9206129, 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 116 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Daito Chemix Corporation; Kyoto University US Patent | Assay Description Mouse VCP cDNA was added with a DNA sequence corresponding to histidine tag at the amino-terminal, subcloned into a baculovirus vector pVL1392 (BD Bi... | US Patent US9206129 (2015) BindingDB Entry DOI: 10.7270/Q29P30GQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50566760 (CHEMBL4870987) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Mus musculus (Mouse)) | BDBM194615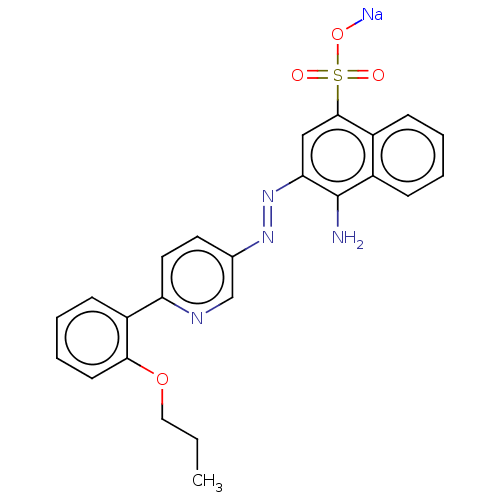 (US9206129, 31) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 127 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Daito Chemix Corporation; Kyoto University US Patent | Assay Description Mouse VCP cDNA was added with a DNA sequence corresponding to histidine tag at the amino-terminal, subcloned into a baculovirus vector pVL1392 (BD Bi... | US Patent US9206129 (2015) BindingDB Entry DOI: 10.7270/Q29P30GQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Mus musculus (Mouse)) | BDBM194629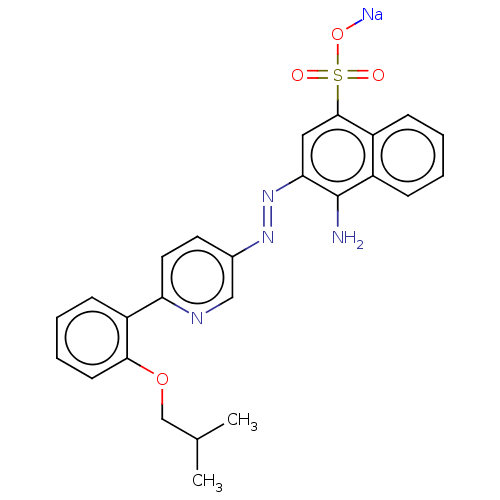 (US9206129, 45) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Daito Chemix Corporation; Kyoto University US Patent | Assay Description Mouse VCP cDNA was added with a DNA sequence corresponding to histidine tag at the amino-terminal, subcloned into a baculovirus vector pVL1392 (BD Bi... | US Patent US9206129 (2015) BindingDB Entry DOI: 10.7270/Q29P30GQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Mus musculus (Mouse)) | BDBM194592 (US9206129, 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 136 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Daito Chemix Corporation; Kyoto University US Patent | Assay Description Mouse VCP cDNA was added with a DNA sequence corresponding to histidine tag at the amino-terminal, subcloned into a baculovirus vector pVL1392 (BD Bi... | US Patent US9206129 (2015) BindingDB Entry DOI: 10.7270/Q29P30GQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Mus musculus (Mouse)) | BDBM194617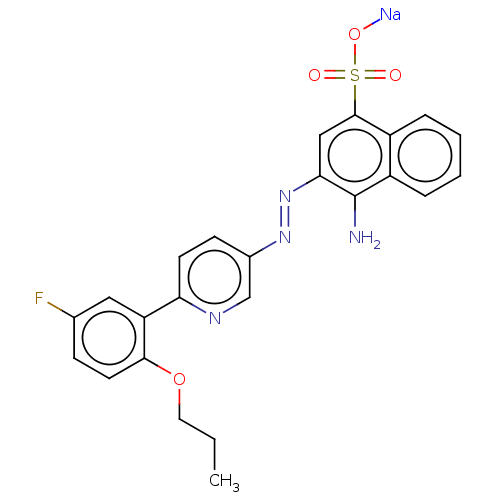 (US9206129, 33) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 139 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Daito Chemix Corporation; Kyoto University US Patent | Assay Description Mouse VCP cDNA was added with a DNA sequence corresponding to histidine tag at the amino-terminal, subcloned into a baculovirus vector pVL1392 (BD Bi... | US Patent US9206129 (2015) BindingDB Entry DOI: 10.7270/Q29P30GQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50566780 (CHEMBL4860372) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Mus musculus (Mouse)) | BDBM194601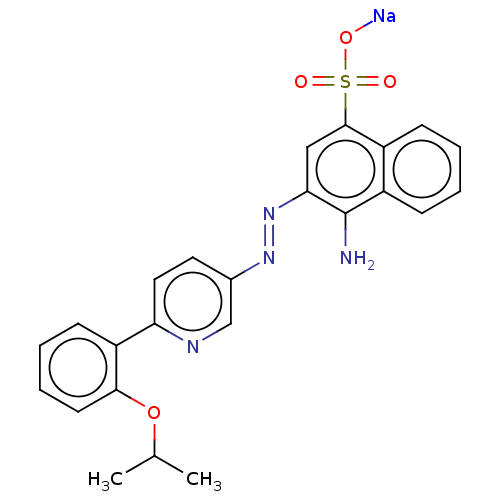 (US9206129, 17) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 145 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Daito Chemix Corporation; Kyoto University US Patent | Assay Description Mouse VCP cDNA was added with a DNA sequence corresponding to histidine tag at the amino-terminal, subcloned into a baculovirus vector pVL1392 (BD Bi... | US Patent US9206129 (2015) BindingDB Entry DOI: 10.7270/Q29P30GQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50566764 (CHEMBL4873940) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant NNMT using nicotinamide and SAM as substrate assessed as reduction in 1-methyl-nicotinamide formation incubated for 2... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00134 BindingDB Entry DOI: 10.7270/Q21J9FJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Mus musculus (Mouse)) | BDBM194588 (US9206129, 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 149 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Daito Chemix Corporation; Kyoto University US Patent | Assay Description Mouse VCP cDNA was added with a DNA sequence corresponding to histidine tag at the amino-terminal, subcloned into a baculovirus vector pVL1392 (BD Bi... | US Patent US9206129 (2015) BindingDB Entry DOI: 10.7270/Q29P30GQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50598884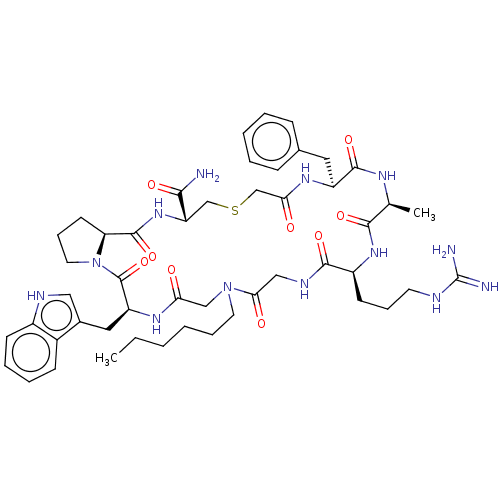 (CHEMBL5191549) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00919 BindingDB Entry DOI: 10.7270/Q2KS6WJ1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Mus musculus (Mouse)) | BDBM194637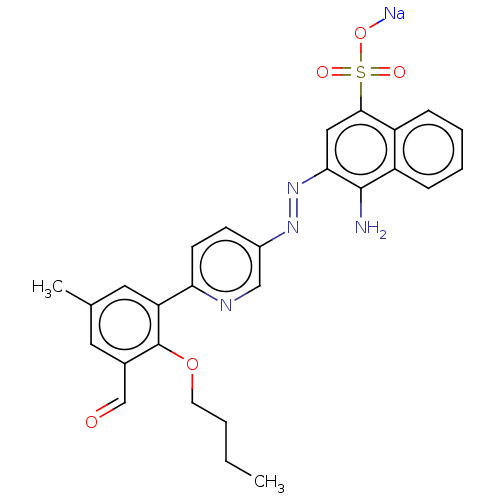 (US9206129, 53) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 161 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Daito Chemix Corporation; Kyoto University US Patent | Assay Description Mouse VCP cDNA was added with a DNA sequence corresponding to histidine tag at the amino-terminal, subcloned into a baculovirus vector pVL1392 (BD Bi... | US Patent US9206129 (2015) BindingDB Entry DOI: 10.7270/Q29P30GQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Mus musculus (Mouse)) | BDBM194594 (US9206129, 10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Daito Chemix Corporation; Kyoto University US Patent | Assay Description Mouse VCP cDNA was added with a DNA sequence corresponding to histidine tag at the amino-terminal, subcloned into a baculovirus vector pVL1392 (BD Bi... | US Patent US9206129 (2015) BindingDB Entry DOI: 10.7270/Q29P30GQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 143 total ) | Next | Last >> |