 Found 4599 hits with Last Name = 'yu' and Initial = 'a'
Found 4599 hits with Last Name = 'yu' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50366620

(RESINIFERATOXIN)Show SMILES COc1cc(CC(=O)OCC2=C[C@H]3[C@H]4OC5(Cc6ccccc6)O[C@@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)ccc1O |r,t:10,35,TLB:23:15:12:24.25.26,THB:16:15:12:24.25.26| Show InChI InChI=1S/C37H40O9/c1-21(2)35-17-23(4)37-27(33(35)44-36(45-35,46-37)19-24-9-7-6-8-10-24)14-26(18-34(41)30(37)13-22(3)32(34)40)20-43-31(39)16-25-11-12-28(38)29(15-25)42-5/h6-15,23,27,30,33,38,41H,1,16-20H2,2-5H3/t23-,27+,30-,33-,34-,35+,36?,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0426 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system |
Bioorg Med Chem 17: 690-8 (2009)
Article DOI: 10.1016/j.bmc.2008.11.085
BindingDB Entry DOI: 10.7270/Q2CJ8FCW |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50247744
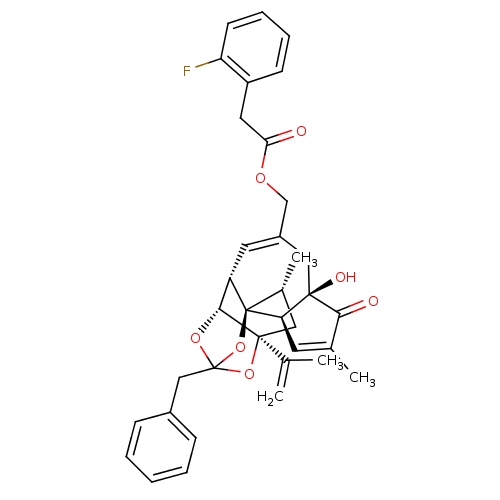
(CHEMBL504725 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...)Show SMILES C[C@@H]1C[C@]2(OC3(Cc4ccccc4)O[C@@H]2[C@@H]2C=C(COC(=O)Cc4ccccc4F)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]12O3)C(C)=C |r,t:18,37,TLB:6:5:15:3.2.1,THB:4:5:15:3.2.1| Show InChI InChI=1S/C36H37FO7/c1-21(2)34-17-23(4)36-27(32(34)42-35(43-34,44-36)19-24-10-6-5-7-11-24)15-25(18-33(40)29(36)14-22(3)31(33)39)20-41-30(38)16-26-12-8-9-13-28(26)37/h5-15,23,27,29,32,40H,1,16-20H2,2-4H3/t23-,27+,29-,32-,33-,34+,35?,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system |
Bioorg Med Chem 17: 690-8 (2009)
Article DOI: 10.1016/j.bmc.2008.11.085
BindingDB Entry DOI: 10.7270/Q2CJ8FCW |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM4994

((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...)Show SMILES CCC(CC)O[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:7| Show InChI InChI=1S/C14H24N2O4/c1-4-10(5-2)20-12-7-9(14(18)19)6-11(15)13(12)16-8(3)17/h7,10-13H,4-6,15H2,1-3H3,(H,16,17)(H,18,19)/t11-,12+,13+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/Thailand/1(KAN-1)/2004(H5N1)) neuraminidase by by Michaelis Menten equation analysis |
Antimicrob Agents Chemother 53: 3088-96 (2009)
Article DOI: 10.1128/AAC.01667-08
BindingDB Entry DOI: 10.7270/Q2VM4CHC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50247741
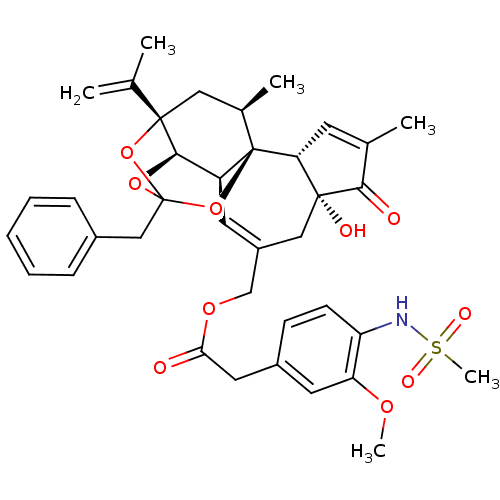
(CHEMBL503101 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...)Show SMILES COc1cc(CC(=O)OCC2=C[C@H]3[C@H]4OC5(Cc6ccccc6)O[C@@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)ccc1NS(C)(=O)=O |r,t:10,35,TLB:23:15:12:24.25.26,THB:16:15:12:24.25.26| Show InChI InChI=1S/C38H43NO10S/c1-22(2)36-18-24(4)38-28(34(36)47-37(48-36,49-38)20-25-10-8-7-9-11-25)15-27(19-35(42)31(38)14-23(3)33(35)41)21-46-32(40)17-26-12-13-29(30(16-26)45-5)39-50(6,43)44/h7-16,24,28,31,34,39,42H,1,17-21H2,2-6H3/t24-,28+,31-,34-,35-,36+,37?,38-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.228 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system |
Bioorg Med Chem 17: 690-8 (2009)
Article DOI: 10.1016/j.bmc.2008.11.085
BindingDB Entry DOI: 10.7270/Q2CJ8FCW |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50247749
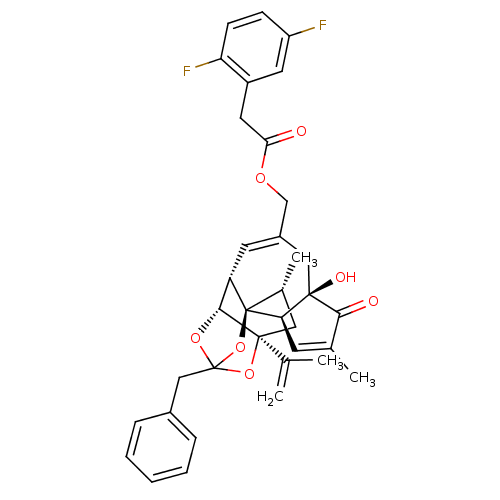
(CHEMBL510583 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...)Show SMILES C[C@@H]1C[C@]2(OC3(Cc4ccccc4)O[C@@H]2[C@@H]2C=C(COC(=O)Cc4cc(F)ccc4F)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]12O3)C(C)=C |r,t:18,38,TLB:6:5:15:3.2.1,THB:4:5:15:3.2.1| Show InChI InChI=1S/C36H36F2O7/c1-20(2)34-16-22(4)36-27(32(34)43-35(44-34,45-36)18-23-8-6-5-7-9-23)13-24(17-33(41)29(36)12-21(3)31(33)40)19-42-30(39)15-25-14-26(37)10-11-28(25)38/h5-14,22,27,29,32,41H,1,15-19H2,2-4H3/t22-,27+,29-,32-,33-,34+,35?,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system |
Bioorg Med Chem 17: 690-8 (2009)
Article DOI: 10.1016/j.bmc.2008.11.085
BindingDB Entry DOI: 10.7270/Q2CJ8FCW |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50330326

((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/chicken/Yogjakarta/BBVet-IX/2004(H5N1)) neuraminidase by Michaelis Menten equation analysis |
Antimicrob Agents Chemother 53: 3088-96 (2009)
Article DOI: 10.1128/AAC.01667-08
BindingDB Entry DOI: 10.7270/Q2VM4CHC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
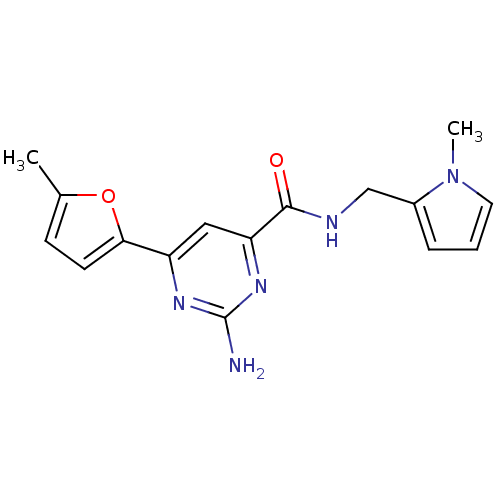
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50385670
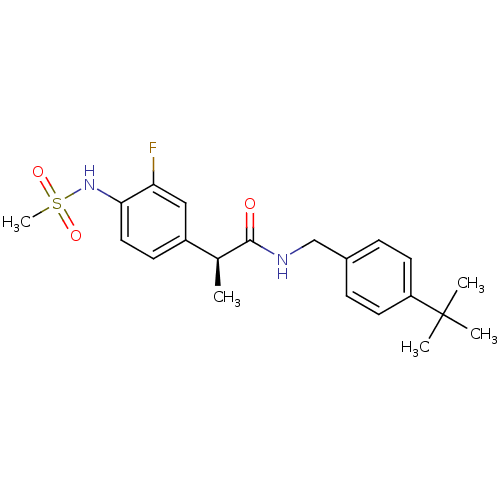
(CHEMBL2042399)Show SMILES C[C@H](C(=O)NCc1ccc(cc1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C21H27FN2O3S/c1-14(16-8-11-19(18(22)12-16)24-28(5,26)27)20(25)23-13-15-6-9-17(10-7-15)21(2,3)4/h6-12,14,24H,13H2,1-5H3,(H,23,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 receptor expressed in CHO cells assessed as decrease in capsaicin-induced intracellular 45Ca2+ uptake after 1 hr b... |
Bioorg Med Chem 20: 215-24 (2011)
Article DOI: 10.1016/j.bmc.2011.11.008
BindingDB Entry DOI: 10.7270/Q2MG7QJJ |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50330326

((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/Thailand/1(KAN-1)/2004(H5N1)) neuraminidase by by Michaelis Menten equation analysis |
Antimicrob Agents Chemother 53: 3088-96 (2009)
Article DOI: 10.1128/AAC.01667-08
BindingDB Entry DOI: 10.7270/Q2VM4CHC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuraminidase
(Influenza A virus) | BDBM50330326

((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/duck/Laos/25/2006(H5N1)) neuraminidase by Michaelis Menten equation analysis |
Antimicrob Agents Chemother 53: 3088-96 (2009)
Article DOI: 10.1128/AAC.01667-08
BindingDB Entry DOI: 10.7270/Q2VM4CHC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50539860
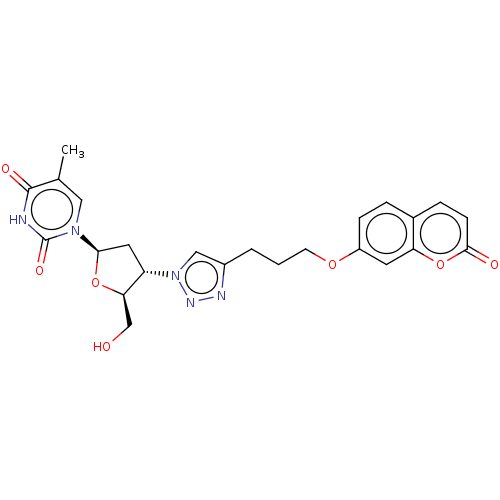
(CHEMBL4644555)Show SMILES Cc1cn([C@H]2C[C@@H]([C@@H](CO)O2)n2cc(CCCOc3ccc4ccc(=O)oc4c3)nn2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C24H25N5O7/c1-14-11-28(24(33)25-23(14)32)21-10-18(20(13-30)35-21)29-12-16(26-27-29)3-2-8-34-17-6-4-15-5-7-22(31)36-19(15)9-17/h4-7,9,11-12,18,20-21,30H,2-3,8,10,13H2,1H3,(H,25,32,33)/t18-,20+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human CA7 pre-incubated for 15 mins by stopped flow CO2 hydrase assay |
J Med Chem 63: 7392-7409 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00636
BindingDB Entry DOI: 10.7270/Q2F47SNQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50539861

(CHEMBL4638601)Show SMILES Cc1cn([C@H]2C[C@@H]([C@@H](CO)O2)n2cc(CCCCOc3ccc4ccc(=O)oc4c3)nn2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C25H27N5O7/c1-15-12-29(25(34)26-24(15)33)22-11-19(21(14-31)36-22)30-13-17(27-28-30)4-2-3-9-35-18-7-5-16-6-8-23(32)37-20(16)10-18/h5-8,10,12-13,19,21-22,31H,2-4,9,11,14H2,1H3,(H,26,33,34)/t19-,21+,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human CA7 pre-incubated for 15 mins by stopped flow CO2 hydrase assay |
J Med Chem 63: 7392-7409 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00636
BindingDB Entry DOI: 10.7270/Q2F47SNQ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20286
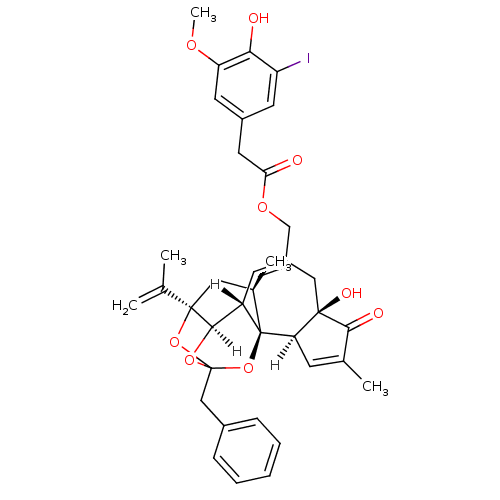
(5-I-RTX | 5-iodoresiniferatoxin | [(1R,2R,6R,10S,1...)Show SMILES [H][C@]12OC3(Cc4ccccc4)O[C@]1(C[C@@H](C)[C@]1(O3)[C@]3([H])C=C(C)C(=O)[C@@]3(O)CC(COC(=O)Cc3cc(I)c(O)c(OC)c3)=C[C@@]21[H])C(C)=C |c:48,t:23,TLB:11:3:12.14.13:45,THB:4:3:12.14.13:45| Show InChI InChI=1S/C37H39IO9/c1-20(2)35-16-22(4)37-26(33(35)45-36(46-35,47-37)18-23-9-7-6-8-10-23)12-25(17-34(42)29(37)11-21(3)32(34)41)19-44-30(39)15-24-13-27(38)31(40)28(14-24)43-5/h6-14,22,26,29,33,40,42H,1,15-19H2,2-5H3/t22-,26+,29-,33-,34-,35-,36?,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.610 | -54.7 | n/a | n/a | 12.2 | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
J Med Chem 51: 57-67 (2008)
Article DOI: 10.1021/jm701049p
BindingDB Entry DOI: 10.7270/Q2222S1N |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50539856

(CHEMBL4649280)Show SMILES Cc1cn([C@H]2C[C@@H]([C@@H](CO)O2)n2cc(CCCCOc3cc(=O)oc4ccccc34)nn2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C25H27N5O7/c1-15-12-29(25(34)26-24(15)33)22-10-18(21(14-31)36-22)30-13-16(27-28-30)6-4-5-9-35-20-11-23(32)37-19-8-3-2-7-17(19)20/h2-3,7-8,11-13,18,21-22,31H,4-6,9-10,14H2,1H3,(H,26,33,34)/t18-,21+,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human CA7 pre-incubated for 15 mins by stopped flow CO2 hydrase assay |
J Med Chem 63: 7392-7409 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00636
BindingDB Entry DOI: 10.7270/Q2F47SNQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50539858
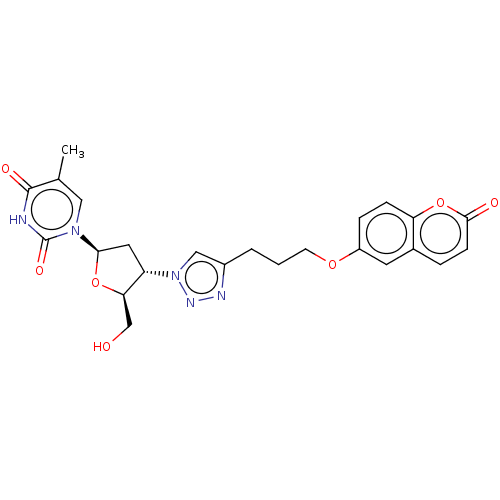
(CHEMBL4639447)Show SMILES Cc1cn([C@H]2C[C@@H]([C@@H](CO)O2)n2cc(CCCOc3ccc4oc(=O)ccc4c3)nn2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C24H25N5O7/c1-14-11-28(24(33)25-23(14)32)21-10-18(20(13-30)35-21)29-12-16(26-27-29)3-2-8-34-17-5-6-19-15(9-17)4-7-22(31)36-19/h4-7,9,11-12,18,20-21,30H,2-3,8,10,13H2,1H3,(H,25,32,33)/t18-,20+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human CA7 pre-incubated for 15 mins by stopped flow CO2 hydrase assay |
J Med Chem 63: 7392-7409 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00636
BindingDB Entry DOI: 10.7270/Q2F47SNQ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50247742
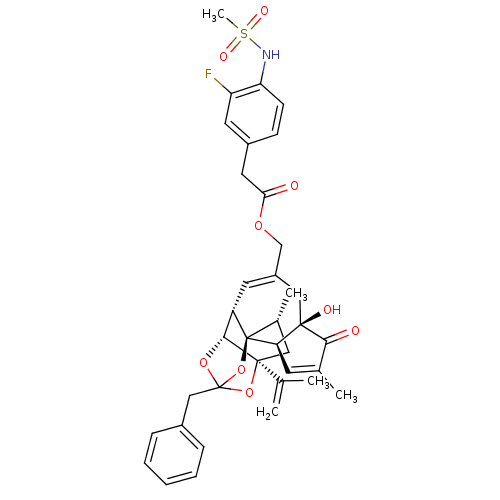
(CHEMBL509154 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...)Show SMILES C[C@@H]1C[C@]2(OC3(Cc4ccccc4)O[C@@H]2[C@@H]2C=C(COC(=O)Cc4ccc(NS(C)(=O)=O)c(F)c4)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]12O3)C(C)=C |r,t:18,42,TLB:6:5:15:3.2.1,THB:4:5:15:3.2.1| Show InChI InChI=1S/C37H40FNO9S/c1-21(2)35-17-23(4)37-27(33(35)46-36(47-35,48-37)19-24-9-7-6-8-10-24)14-26(18-34(42)30(37)13-22(3)32(34)41)20-45-31(40)16-25-11-12-29(28(38)15-25)39-49(5,43)44/h6-15,23,27,30,33,39,42H,1,16-20H2,2-5H3/t23-,27+,30-,33-,34-,35+,36?,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system |
Bioorg Med Chem 17: 690-8 (2009)
Article DOI: 10.1016/j.bmc.2008.11.085
BindingDB Entry DOI: 10.7270/Q2CJ8FCW |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50247748
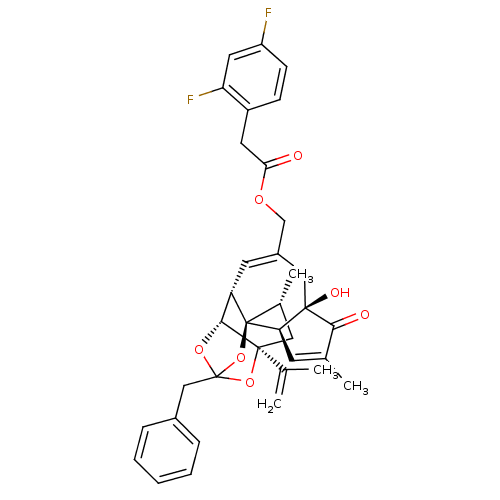
(CHEMBL510228 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...)Show SMILES C[C@@H]1C[C@]2(OC3(Cc4ccccc4)O[C@@H]2[C@@H]2C=C(COC(=O)Cc4ccc(F)cc4F)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]12O3)C(C)=C |r,t:18,38,TLB:6:5:15:3.2.1,THB:4:5:15:3.2.1| Show InChI InChI=1S/C36H36F2O7/c1-20(2)34-16-22(4)36-27(32(34)43-35(44-34,45-36)18-23-8-6-5-7-9-23)13-24(17-33(41)29(36)12-21(3)31(33)40)19-42-30(39)14-25-10-11-26(37)15-28(25)38/h5-13,15,22,27,29,32,41H,1,14,16-19H2,2-4H3/t22-,27+,29-,32-,33-,34+,35?,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system |
Bioorg Med Chem 17: 690-8 (2009)
Article DOI: 10.1016/j.bmc.2008.11.085
BindingDB Entry DOI: 10.7270/Q2CJ8FCW |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50247751
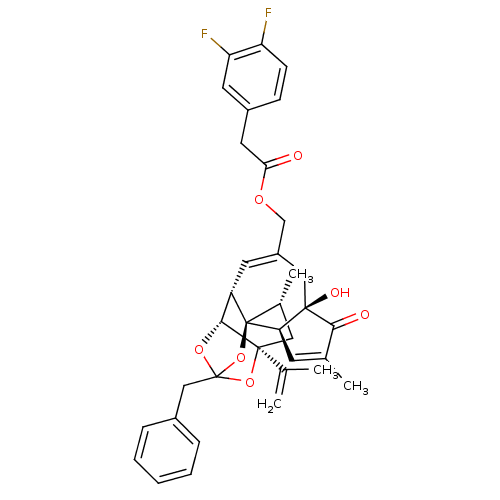
(CHEMBL449201 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...)Show SMILES C[C@@H]1C[C@]2(OC3(Cc4ccccc4)O[C@@H]2[C@@H]2C=C(COC(=O)Cc4ccc(F)c(F)c4)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]12O3)C(C)=C |r,t:18,38,TLB:6:5:15:3.2.1,THB:4:5:15:3.2.1| Show InChI InChI=1S/C36H36F2O7/c1-20(2)34-16-22(4)36-26(32(34)43-35(44-34,45-36)18-23-8-6-5-7-9-23)13-25(17-33(41)29(36)12-21(3)31(33)40)19-42-30(39)15-24-10-11-27(37)28(38)14-24/h5-14,22,26,29,32,41H,1,15-19H2,2-4H3/t22-,26+,29-,32-,33-,34+,35?,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system |
Bioorg Med Chem 17: 690-8 (2009)
Article DOI: 10.1016/j.bmc.2008.11.085
BindingDB Entry DOI: 10.7270/Q2CJ8FCW |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM35836

(pyrimidine-4-carboxamide, 118)Show InChI InChI=1S/C14H14N6O2/c1-8-2-3-11(22-8)9-6-10(20-14(15)19-9)13(21)18-7-12-16-4-5-17-12/h2-6H,7H2,1H3,(H,16,17)(H,18,21)(H2,15,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
| Assay Description
Binding assays were performed in a total volume of 250 uL, containing radioligand, membranes and test compounds. Following 60 (A2B, A3) or 90 (A1, A2... |
Bioorg Med Chem 17: 6590-605 (2009)
Article DOI: 10.1016/j.bmc.2009.07.078
BindingDB Entry DOI: 10.7270/Q27M069H |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM50330326

((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/Turkey/651242/2006(H5N1)) neuraminidase by Michaelis Menten equation analysis |
Antimicrob Agents Chemother 53: 3088-96 (2009)
Article DOI: 10.1128/AAC.01667-08
BindingDB Entry DOI: 10.7270/Q2VM4CHC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuraminidase
(Influenza A virus) | BDBM4994

((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...)Show SMILES CCC(CC)O[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:7| Show InChI InChI=1S/C14H24N2O4/c1-4-10(5-2)20-12-7-9(14(18)19)6-11(15)13(12)16-8(3)17/h7,10-13H,4-6,15H2,1-3H3,(H,16,17)(H,18,19)/t11-,12+,13+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/duck/Laos/25/2006(H5N1)) neuraminidase by Michaelis Menten equation analysis |
Antimicrob Agents Chemother 53: 3088-96 (2009)
Article DOI: 10.1128/AAC.01667-08
BindingDB Entry DOI: 10.7270/Q2VM4CHC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50215615

(1-((3R,6S)-6-(3-cyanophenyl)bicyclo[4.1.0]heptan-3...)Show SMILES CC1CC[C@@H](CO)N1CCN([C@@H]1CC[C@@]2(CC2C1)c1cccc(c1)C#N)C(=O)Nc1cc(Cl)nc(Cl)c1 |w:16.18,1.0| Show InChI InChI=1S/C28H33Cl2N5O2/c1-18-5-6-24(17-36)34(18)9-10-35(27(37)32-22-13-25(29)33-26(30)14-22)23-7-8-28(15-21(28)12-23)20-4-2-3-19(11-20)16-31/h2-4,11,13-14,18,21,23-24,36H,5-10,12,15,17H2,1H3,(H,32,33,37)/t18?,21?,23-,24+,28-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Cartagena
Curated by ChEMBL
| Assay Description
Antagonist activity at MCH1 receptor |
Eur J Med Chem 45: 4509-22 (2010)
Article DOI: 10.1016/j.ejmech.2010.07.011
BindingDB Entry DOI: 10.7270/Q2FT8N9V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50539849
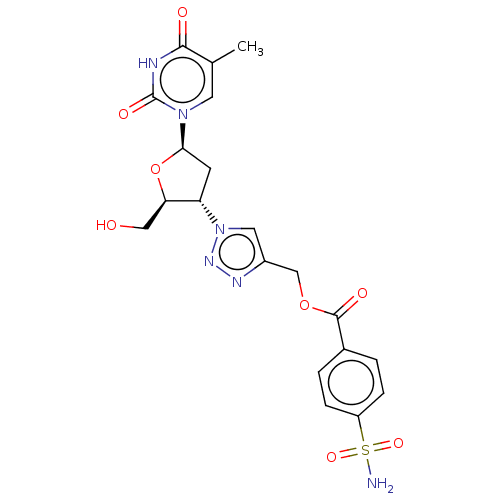
(CHEMBL4632724)Show SMILES Cc1cn([C@H]2C[C@@H]([C@@H](CO)O2)n2cc(COC(=O)c3ccc(cc3)S(N)(=O)=O)nn2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C20H22N6O8S/c1-11-7-25(20(30)22-18(11)28)17-6-15(16(9-27)34-17)26-8-13(23-24-26)10-33-19(29)12-2-4-14(5-3-12)35(21,31)32/h2-5,7-8,15-17,27H,6,9-10H2,1H3,(H2,21,31,32)(H,22,28,30)/t15-,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 pre-incubated for 15 mins by stopped flow CO2 hydrase assay |
J Med Chem 63: 7392-7409 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00636
BindingDB Entry DOI: 10.7270/Q2F47SNQ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50215655

(1-((3R,6S)-6-(3-cyanophenyl)bicyclo[4.1.0]heptan-3...)Show SMILES CC(C)N(CCO)CCN([C@@H]1CC[C@@]2(CC2C1)c1cccc(c1)C#N)C(=O)Nc1cc(Cl)nc(Cl)c1 |w:15.16| Show InChI InChI=1S/C27H33Cl2N5O2/c1-18(2)33(10-11-35)8-9-34(26(36)31-22-14-24(28)32-25(29)15-22)23-6-7-27(16-21(27)13-23)20-5-3-4-19(12-20)17-30/h3-5,12,14-15,18,21,23,35H,6-11,13,16H2,1-2H3,(H,31,32,36)/t21?,23-,27-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Cartagena
Curated by ChEMBL
| Assay Description
Antagonist activity at MCH1 receptor |
Eur J Med Chem 45: 4509-22 (2010)
Article DOI: 10.1016/j.ejmech.2010.07.011
BindingDB Entry DOI: 10.7270/Q2FT8N9V |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50215583
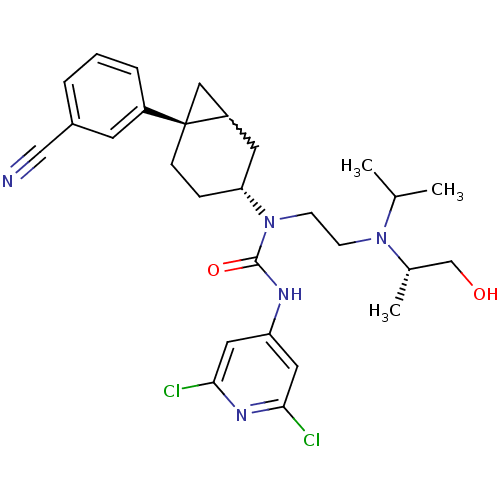
(1-((3R,6S)-6-(3-cyanophenyl)bicyclo[4.1.0]heptan-3...)Show SMILES CC(C)N(CCN([C@@H]1CC[C@@]2(CC2C1)c1cccc(c1)C#N)C(=O)Nc1cc(Cl)nc(Cl)c1)[C@@H](C)CO |w:12.13| Show InChI InChI=1S/C28H35Cl2N5O2/c1-18(2)34(19(3)17-36)9-10-35(27(37)32-23-13-25(29)33-26(30)14-23)24-7-8-28(15-22(28)12-24)21-6-4-5-20(11-21)16-31/h4-6,11,13-14,18-19,22,24,36H,7-10,12,15,17H2,1-3H3,(H,32,33,37)/t19-,22?,24+,28+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Cartagena
Curated by ChEMBL
| Assay Description
Antagonist activity at MCH1 receptor |
Eur J Med Chem 45: 4509-22 (2010)
Article DOI: 10.1016/j.ejmech.2010.07.011
BindingDB Entry DOI: 10.7270/Q2FT8N9V |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20314
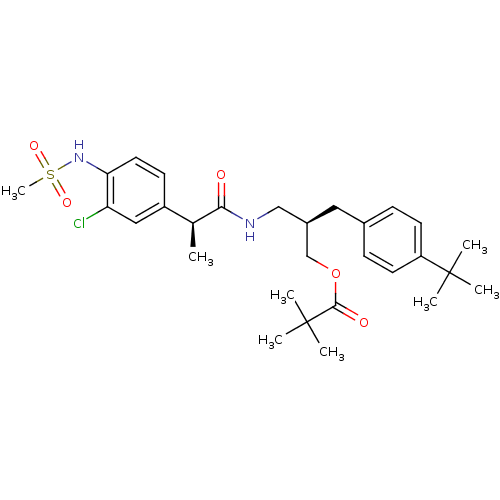
((2R)-2-[(4-tert-butylphenyl)methyl]-3-[(2S)-2-(3-c...)Show SMILES C[C@H](C(=O)NC[C@H](COC(=O)C(C)(C)C)Cc1ccc(cc1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(Cl)c1 |r| Show InChI InChI=1S/C29H41ClN2O5S/c1-19(22-11-14-25(24(30)16-22)32-38(8,35)36)26(33)31-17-21(18-37-27(34)29(5,6)7)15-20-9-12-23(13-10-20)28(2,3)4/h9-14,16,19,21,32H,15,17-18H2,1-8H3,(H,31,33)/t19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.83 | -51.9 | n/a | n/a | 5.20 | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
J Med Chem 51: 57-67 (2008)
Article DOI: 10.1021/jm701049p
BindingDB Entry DOI: 10.7270/Q2222S1N |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50215645

(1-((3R,6S)-6-(3-cyanophenyl)bicyclo[4.1.0]heptan-3...)Show SMILES CC(C)NCCN([C@@H]1CC[C@@]2(CC2C1)c1cccc(c1)C#N)C(=O)Nc1cc(Cl)nc(Cl)c1 |w:12.13| Show InChI InChI=1S/C25H29Cl2N5O/c1-16(2)29-8-9-32(24(33)30-20-12-22(26)31-23(27)13-20)21-6-7-25(14-19(25)11-21)18-5-3-4-17(10-18)15-28/h3-5,10,12-13,16,19,21,29H,6-9,11,14H2,1-2H3,(H,30,31,33)/t19?,21-,25-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Cartagena
Curated by ChEMBL
| Assay Description
Antagonist activity at MCH1 receptor |
Eur J Med Chem 45: 4509-22 (2010)
Article DOI: 10.1016/j.ejmech.2010.07.011
BindingDB Entry DOI: 10.7270/Q2FT8N9V |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM35834
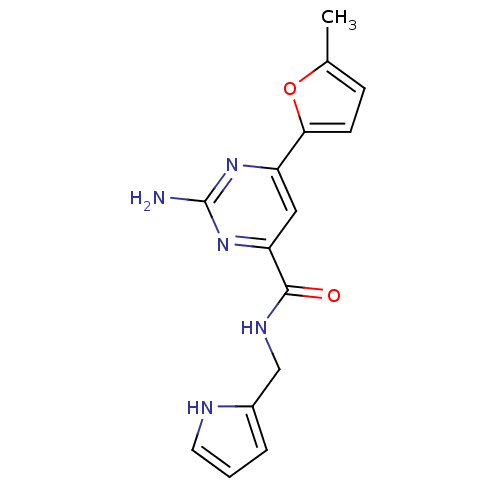
(pyrimidine-4-carboxamide, 116)Show InChI InChI=1S/C15H15N5O2/c1-9-4-5-13(22-9)11-7-12(20-15(16)19-11)14(21)18-8-10-3-2-6-17-10/h2-7,17H,8H2,1H3,(H,18,21)(H2,16,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
| Assay Description
Binding assays were performed in a total volume of 250 uL, containing radioligand, membranes and test compounds. Following 60 (A2B, A3) or 90 (A1, A2... |
Bioorg Med Chem 17: 6590-605 (2009)
Article DOI: 10.1016/j.bmc.2009.07.078
BindingDB Entry DOI: 10.7270/Q27M069H |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50215579

(1-((3R,6S)-6-(3-cyanophenyl)bicyclo[4.1.0]heptan-3...)Show SMILES CC(C)N(CCN([C@@H]1CC[C@@]2(CC2C1)c1cccc(c1)C#N)C(=O)Nc1cccc(F)c1)C(C)C |w:12.13| Show InChI InChI=1S/C29H37FN4O/c1-20(2)33(21(3)4)13-14-34(28(35)32-26-10-6-9-25(30)17-26)27-11-12-29(18-24(29)16-27)23-8-5-7-22(15-23)19-31/h5-10,15,17,20-21,24,27H,11-14,16,18H2,1-4H3,(H,32,35)/t24?,27-,29-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Cartagena
Curated by ChEMBL
| Assay Description
Antagonist activity at MCH1 receptor |
Eur J Med Chem 45: 4509-22 (2010)
Article DOI: 10.1016/j.ejmech.2010.07.011
BindingDB Entry DOI: 10.7270/Q2FT8N9V |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50017594
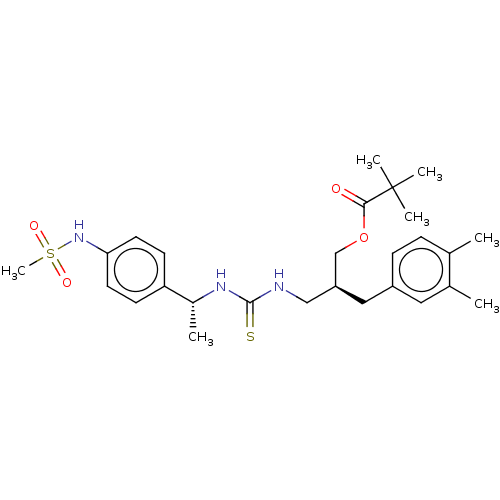
(CHEMBL3288626)Show SMILES C[C@@H](NC(=S)NC[C@@H](COC(=O)C(C)(C)C)Cc1ccc(C)c(C)c1)c1ccc(NS(C)(=O)=O)cc1 |r| Show InChI InChI=1S/C27H39N3O4S2/c1-18-8-9-21(14-19(18)2)15-22(17-34-25(31)27(4,5)6)16-28-26(35)29-20(3)23-10-12-24(13-11-23)30-36(7,32)33/h8-14,20,22,30H,15-17H2,1-7H3,(H2,28,29,35)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from rat TRPV1 after 60 mins by competitive binding assay |
Bioorg Med Chem Lett 24: 2685-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.054
BindingDB Entry DOI: 10.7270/Q26111VT |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50247745
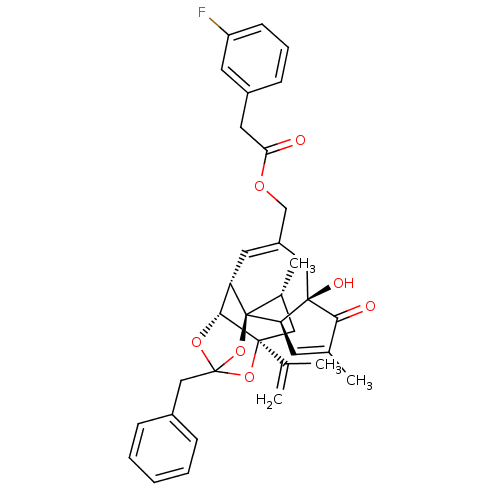
(CHEMBL455555 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...)Show SMILES C[C@@H]1C[C@]2(OC3(Cc4ccccc4)O[C@@H]2[C@@H]2C=C(COC(=O)Cc4cccc(F)c4)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]12O3)C(C)=C |r,t:18,37,TLB:6:5:15:3.2.1,THB:4:5:15:3.2.1| Show InChI InChI=1S/C36H37FO7/c1-21(2)34-17-23(4)36-28(32(34)42-35(43-34,44-36)19-24-9-6-5-7-10-24)15-26(18-33(40)29(36)13-22(3)31(33)39)20-41-30(38)16-25-11-8-12-27(37)14-25/h5-15,23,28-29,32,40H,1,16-20H2,2-4H3/t23-,28+,29-,32-,33-,34+,35?,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system |
Bioorg Med Chem 17: 690-8 (2009)
Article DOI: 10.1016/j.bmc.2008.11.085
BindingDB Entry DOI: 10.7270/Q2CJ8FCW |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50215607

(1-((3R,6S)-6-(3-cyanophenyl)bicyclo[4.1.0]heptan-3...)Show SMILES CC(C)N(CCN([C@@H]1CC[C@@]2(CC2C1)c1cccc(c1)C#N)C(=O)Nc1cc(Cl)nc(Cl)c1)C(C)C |w:12.13| Show InChI InChI=1S/C28H35Cl2N5O/c1-18(2)34(19(3)4)10-11-35(27(36)32-23-14-25(29)33-26(30)15-23)24-8-9-28(16-22(28)13-24)21-7-5-6-20(12-21)17-31/h5-7,12,14-15,18-19,22,24H,8-11,13,16H2,1-4H3,(H,32,33,36)/t22?,24-,28-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Cartagena
Curated by ChEMBL
| Assay Description
Antagonist activity at MCH1 receptor |
Eur J Med Chem 45: 4509-22 (2010)
Article DOI: 10.1016/j.ejmech.2010.07.011
BindingDB Entry DOI: 10.7270/Q2FT8N9V |
More data for this
Ligand-Target Pair | |
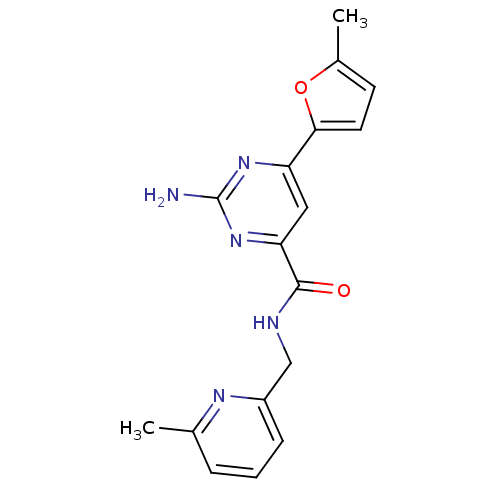
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM35833
(pyrimidine-4-carboxamide, 115)Show InChI InChI=1S/C16H17N5O2/c1-10-5-6-14(23-10)12-8-13(20-16(17)19-12)15(22)18-9-11-4-3-7-21(11)2/h3-8H,9H2,1-2H3,(H,18,22)(H2,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
| Assay Description
Binding assays were performed in a total volume of 250 uL, containing radioligand, membranes and test compounds. Following 60 (A2B, A3) or 90 (A1, A2... |
Bioorg Med Chem 17: 6590-605 (2009)
Article DOI: 10.1016/j.bmc.2009.07.078
BindingDB Entry DOI: 10.7270/Q27M069H |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM35829
(pyrimidine-4-carboxamide, 111)Show InChI InChI=1S/C17H17N5O2/c1-10-4-3-5-12(20-10)9-19-16(23)14-8-13(21-17(18)22-14)15-7-6-11(2)24-15/h3-8H,9H2,1-2H3,(H,19,23)(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
| Assay Description
Binding assays were performed in a total volume of 250 uL, containing radioligand, membranes and test compounds. Following 60 (A2B, A3) or 90 (A1, A2... |
Bioorg Med Chem 17: 6590-605 (2009)
Article DOI: 10.1016/j.bmc.2009.07.078
BindingDB Entry DOI: 10.7270/Q27M069H |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human CA7 pre-incubated for 15 mins by stopped flow CO2 hydrase assay |
J Med Chem 63: 7392-7409 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00636
BindingDB Entry DOI: 10.7270/Q2F47SNQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50247750
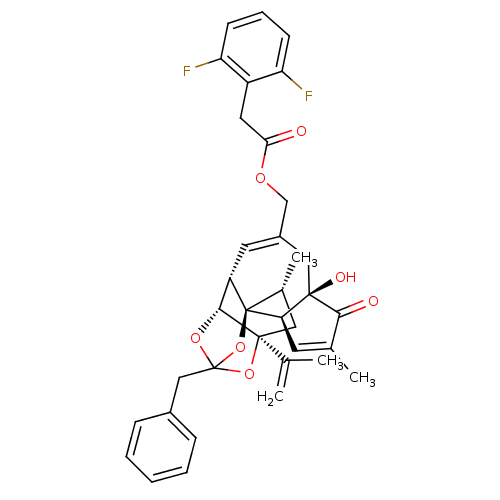
(CHEMBL455155 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...)Show SMILES C[C@@H]1C[C@]2(OC3(Cc4ccccc4)O[C@@H]2[C@@H]2C=C(COC(=O)Cc4c(F)cccc4F)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]12O3)C(C)=C |r,t:18,38,TLB:6:5:15:3.2.1,THB:4:5:15:3.2.1| Show InChI InChI=1S/C36H36F2O7/c1-20(2)34-16-22(4)36-26(32(34)43-35(44-34,45-36)18-23-9-6-5-7-10-23)14-24(17-33(41)29(36)13-21(3)31(33)40)19-42-30(39)15-25-27(37)11-8-12-28(25)38/h5-14,22,26,29,32,41H,1,15-19H2,2-4H3/t22-,26+,29-,32-,33-,34+,35?,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system |
Bioorg Med Chem 17: 690-8 (2009)
Article DOI: 10.1016/j.bmc.2008.11.085
BindingDB Entry DOI: 10.7270/Q2CJ8FCW |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50215629
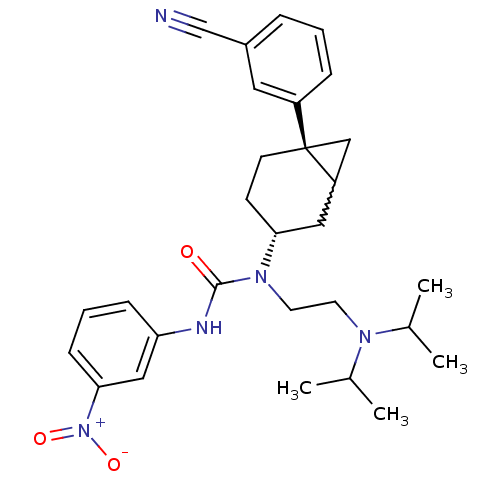
(1-((3R,6S)-6-(3-cyanophenyl)bicyclo[4.1.0]heptan-3...)Show SMILES CC(C)N(CCN([C@@H]1CC[C@@]2(CC2C1)c1cccc(c1)C#N)C(=O)Nc1cccc(c1)[N+]([O-])=O)C(C)C |w:12.13| Show InChI InChI=1S/C29H37N5O3/c1-20(2)32(21(3)4)13-14-33(28(35)31-25-9-6-10-27(17-25)34(36)37)26-11-12-29(18-24(29)16-26)23-8-5-7-22(15-23)19-30/h5-10,15,17,20-21,24,26H,11-14,16,18H2,1-4H3,(H,31,35)/t24?,26-,29-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Cartagena
Curated by ChEMBL
| Assay Description
Antagonist activity at MCH1 receptor |
Eur J Med Chem 45: 4509-22 (2010)
Article DOI: 10.1016/j.ejmech.2010.07.011
BindingDB Entry DOI: 10.7270/Q2FT8N9V |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50330657
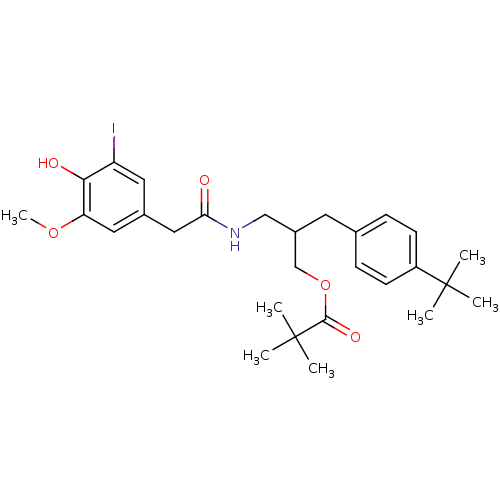
(2-(4-tert-Butylbenzyl)-3-[2-(4-hydroxy-3-iodo-5-me...)Show SMILES COc1cc(CC(=O)NCC(COC(=O)C(C)(C)C)Cc2ccc(cc2)C(C)(C)C)cc(I)c1O Show InChI InChI=1S/C28H38INO5/c1-27(2,3)21-10-8-18(9-11-21)12-20(17-35-26(33)28(4,5)6)16-30-24(31)15-19-13-22(29)25(32)23(14-19)34-7/h8-11,13-14,20,32H,12,15-17H2,1-7H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced calcium uptake |
Bioorg Med Chem 18: 8092-105 (2010)
Article DOI: 10.1016/j.bmc.2010.09.001
BindingDB Entry DOI: 10.7270/Q2Q81D98 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50215578

(1-((3R,6S)-6-(3-cyanophenyl)bicyclo[4.1.0]heptan-3...)Show SMILES CC(C)N(CCN([C@@H]1CC[C@@]2(CC2C1)c1cccc(c1)C#N)C(=O)Nc1ccc(F)c(F)c1)C(C)C |w:12.13| Show InChI InChI=1S/C29H36F2N4O/c1-19(2)34(20(3)4)12-13-35(28(36)33-24-8-9-26(30)27(31)16-24)25-10-11-29(17-23(29)15-25)22-7-5-6-21(14-22)18-32/h5-9,14,16,19-20,23,25H,10-13,15,17H2,1-4H3,(H,33,36)/t23?,25-,29-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Cartagena
Curated by ChEMBL
| Assay Description
Antagonist activity at MCH1 receptor |
Eur J Med Chem 45: 4509-22 (2010)
Article DOI: 10.1016/j.ejmech.2010.07.011
BindingDB Entry DOI: 10.7270/Q2FT8N9V |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM35784
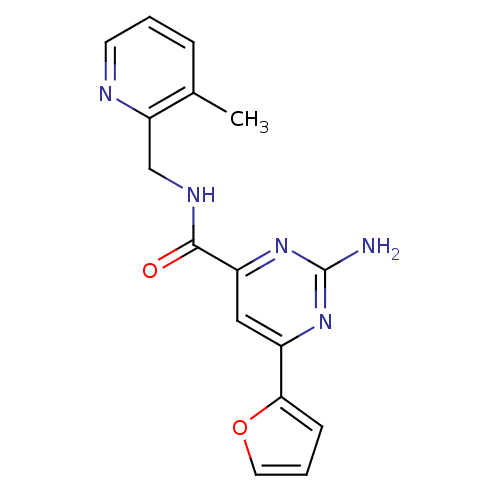
(pyrimidine-4-carboxamide, 19 | pyrimidine-4-carbox...)Show InChI InChI=1S/C16H15N5O2/c1-10-4-2-6-18-13(10)9-19-15(22)12-8-11(20-16(17)21-12)14-5-3-7-23-14/h2-8H,9H2,1H3,(H,19,22)(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
| Assay Description
Binding assays were performed in a total volume of 250 uL, containing radioligand, membranes and test compounds. Following 60 (A2B, A3) or 90 (A1, A2... |
Bioorg Med Chem 17: 6590-605 (2009)
Article DOI: 10.1016/j.bmc.2009.07.078
BindingDB Entry DOI: 10.7270/Q27M069H |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM35784

(pyrimidine-4-carboxamide, 19 | pyrimidine-4-carbox...)Show InChI InChI=1S/C16H15N5O2/c1-10-4-2-6-18-13(10)9-19-15(22)12-8-11(20-16(17)21-12)14-5-3-7-23-14/h2-8H,9H2,1H3,(H,19,22)(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
| Assay Description
Binding assays were performed in a total volume of 250 uL, containing radioligand, membranes and test compounds. Following 60 (A2B, A3) or 90 (A1, A2... |
Bioorg Med Chem 17: 6590-605 (2009)
Article DOI: 10.1016/j.bmc.2009.07.078
BindingDB Entry DOI: 10.7270/Q27M069H |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50539866

(CHEMBL4648357)Show SMILES Cc1cn([C@H]2C[C@@H]([C@@H](CO)O2)n2cc(COc3ccc4OS(=O)(=O)C=Cc4c3)nn2)c(=O)[nH]c1=O |r,c:25| Show InChI InChI=1S/C21H21N5O8S/c1-12-8-25(21(29)22-20(12)28)19-7-16(18(10-27)33-19)26-9-14(23-24-26)11-32-15-2-3-17-13(6-15)4-5-35(30,31)34-17/h2-6,8-9,16,18-19,27H,7,10-11H2,1H3,(H,22,28,29)/t16-,18+,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human CA12 pre-incubated for 15 mins by stopped flow CO2 hydrase assay |
J Med Chem 63: 7392-7409 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00636
BindingDB Entry DOI: 10.7270/Q2F47SNQ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM35843
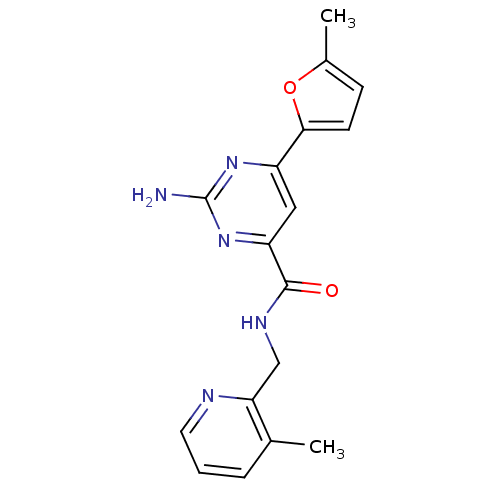
(pyrimidine-4-carboxamide, 28)Show InChI InChI=1S/C17H17N5O2/c1-10-4-3-7-19-14(10)9-20-16(23)13-8-12(21-17(18)22-13)15-6-5-11(2)24-15/h3-8H,9H2,1-2H3,(H,20,23)(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
| Assay Description
Binding assays were performed in a total volume of 250 uL, containing radioligand, membranes and test compounds. Following 60 (A2B, A3) or 90 (A1, A2... |
Bioorg Med Chem 17: 6590-605 (2009)
Article DOI: 10.1016/j.bmc.2009.07.078
BindingDB Entry DOI: 10.7270/Q27M069H |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM35832
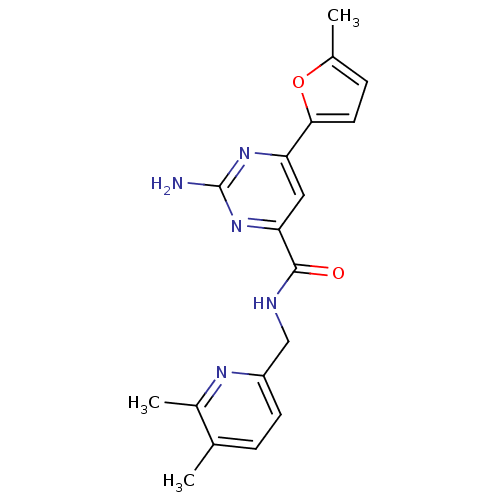
(pyrimidine-4-carboxamide, 114)Show SMILES Cc1ccc(o1)-c1cc(nc(N)n1)C(=O)NCc1ccc(C)c(C)n1 Show InChI InChI=1S/C18H19N5O2/c1-10-4-6-13(21-12(10)3)9-20-17(24)15-8-14(22-18(19)23-15)16-7-5-11(2)25-16/h4-8H,9H2,1-3H3,(H,20,24)(H2,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
| Assay Description
Binding assays were performed in a total volume of 250 uL, containing radioligand, membranes and test compounds. Following 60 (A2B, A3) or 90 (A1, A2... |
Bioorg Med Chem 17: 6590-605 (2009)
Article DOI: 10.1016/j.bmc.2009.07.078
BindingDB Entry DOI: 10.7270/Q27M069H |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM35830
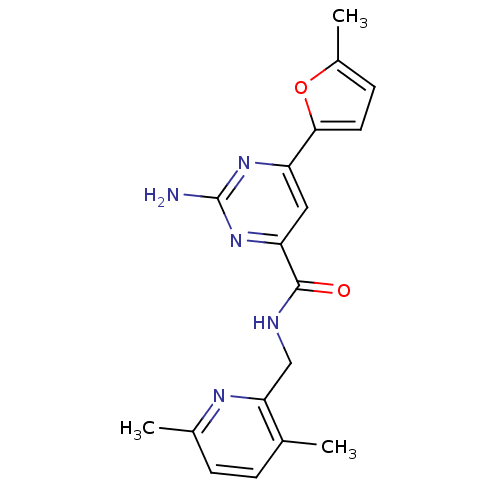
(pyrimidine-4-carboxamide, 112)Show SMILES Cc1ccc(o1)-c1cc(nc(N)n1)C(=O)NCc1nc(C)ccc1C Show InChI InChI=1S/C18H19N5O2/c1-10-4-5-11(2)21-15(10)9-20-17(24)14-8-13(22-18(19)23-14)16-7-6-12(3)25-16/h4-8H,9H2,1-3H3,(H,20,24)(H2,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd
| Assay Description
Binding assays were performed in a total volume of 250 uL, containing radioligand, membranes and test compounds. Following 60 (A2B, A3) or 90 (A1, A2... |
Bioorg Med Chem 17: 6590-605 (2009)
Article DOI: 10.1016/j.bmc.2009.07.078
BindingDB Entry DOI: 10.7270/Q27M069H |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50247746
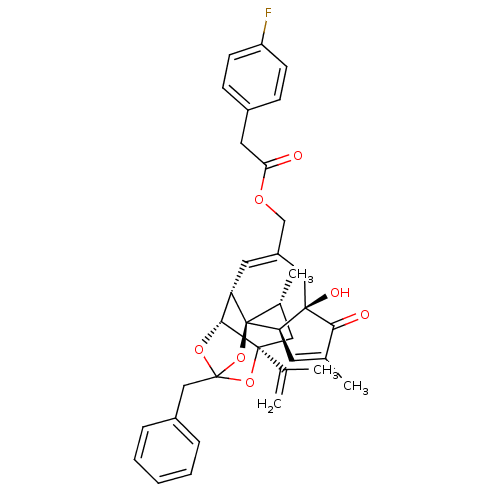
(CHEMBL502769 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...)Show SMILES C[C@@H]1C[C@]2(OC3(Cc4ccccc4)O[C@@H]2[C@@H]2C=C(COC(=O)Cc4ccc(F)cc4)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]12O3)C(C)=C |r,t:18,37,TLB:6:5:15:3.2.1,THB:4:5:15:3.2.1| Show InChI InChI=1S/C36H37FO7/c1-21(2)34-17-23(4)36-28(32(34)42-35(43-34,44-36)19-25-8-6-5-7-9-25)15-26(18-33(40)29(36)14-22(3)31(33)39)20-41-30(38)16-24-10-12-27(37)13-11-24/h5-15,23,28-29,32,40H,1,16-20H2,2-4H3/t23-,28+,29-,32-,33-,34+,35?,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system |
Bioorg Med Chem 17: 690-8 (2009)
Article DOI: 10.1016/j.bmc.2008.11.085
BindingDB Entry DOI: 10.7270/Q2CJ8FCW |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20311
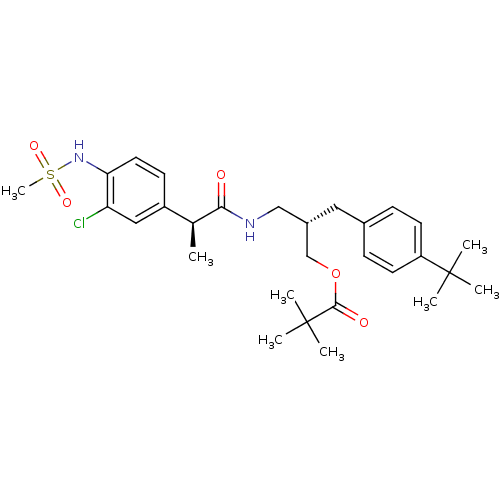
((2S)-2-[(4-tert-butylphenyl)methyl]-3-[(2S)-2-(3-c...)Show SMILES C[C@H](C(=O)NC[C@@H](COC(=O)C(C)(C)C)Cc1ccc(cc1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(Cl)c1 |r| Show InChI InChI=1S/C29H41ClN2O5S/c1-19(22-11-14-25(24(30)16-22)32-38(8,35)36)26(33)31-17-21(18-37-27(34)29(5,6)7)15-20-9-12-23(13-10-20)28(2,3)4/h9-14,16,19,21,32H,15,17-18H2,1-8H3,(H,31,33)/t19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.29 | -50.4 | n/a | n/a | 12.1 | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
J Med Chem 51: 57-67 (2008)
Article DOI: 10.1021/jm701049p
BindingDB Entry DOI: 10.7270/Q2222S1N |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50215613

(3-(2,6-dichloropyridin-4-yl)-1-((3R,6S)-6-(3-((dim...)Show SMILES CN(C)Cc1cccc(c1)[C@@]12CC1C[C@@H](CC2)N(CC1CCN(CC1)S(C)(=O)=O)C(=O)Nc1cc(Cl)nc(Cl)c1 |w:12.12| Show InChI InChI=1S/C29H39Cl2N5O3S/c1-34(2)18-21-5-4-6-22(13-21)29-10-7-25(14-23(29)17-29)36(19-20-8-11-35(12-9-20)40(3,38)39)28(37)32-24-15-26(30)33-27(31)16-24/h4-6,13,15-16,20,23,25H,7-12,14,17-19H2,1-3H3,(H,32,33,37)/t23?,25-,29-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Cartagena
Curated by ChEMBL
| Assay Description
Antagonist activity at MCH1 receptor |
Eur J Med Chem 45: 4509-22 (2010)
Article DOI: 10.1016/j.ejmech.2010.07.011
BindingDB Entry DOI: 10.7270/Q2FT8N9V |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20330
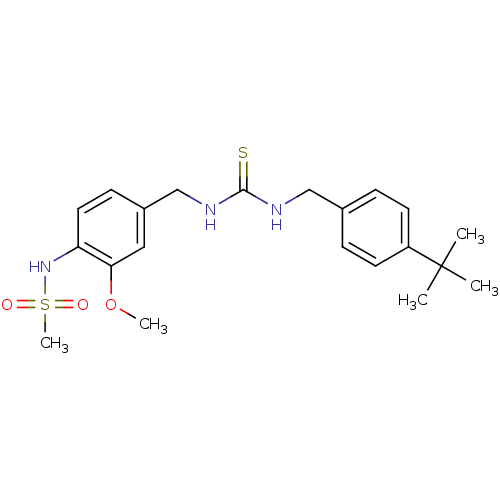
(3-[(4-tert-butylphenyl)methyl]-1-[(4-methanesulfon...)Show SMILES COc1cc(CNC(=S)NCc2ccc(cc2)C(C)(C)C)ccc1NS(C)(=O)=O Show InChI InChI=1S/C21H29N3O3S2/c1-21(2,3)17-9-6-15(7-10-17)13-22-20(28)23-14-16-8-11-18(19(12-16)27-4)24-29(5,25)26/h6-12,24H,13-14H2,1-5H3,(H2,22,23,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity towards rat TRPV1 expressed in CHO cells |
Bioorg Med Chem Lett 15: 4143-50 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.006
BindingDB Entry DOI: 10.7270/Q2JH3KQB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20330

(3-[(4-tert-butylphenyl)methyl]-1-[(4-methanesulfon...)Show SMILES COc1cc(CNC(=S)NCc2ccc(cc2)C(C)(C)C)ccc1NS(C)(=O)=O Show InChI InChI=1S/C21H29N3O3S2/c1-21(2,3)17-9-6-15(7-10-17)13-22-20(28)23-14-16-8-11-18(19(12-16)27-4)24-29(5,25)26/h6-12,24H,13-14H2,1-5H3,(H2,22,23,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity for rat TRPV1 expressed in CHO cells |
Bioorg Med Chem Lett 15: 4136-42 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.009
BindingDB Entry DOI: 10.7270/Q23B5ZN0 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50330677
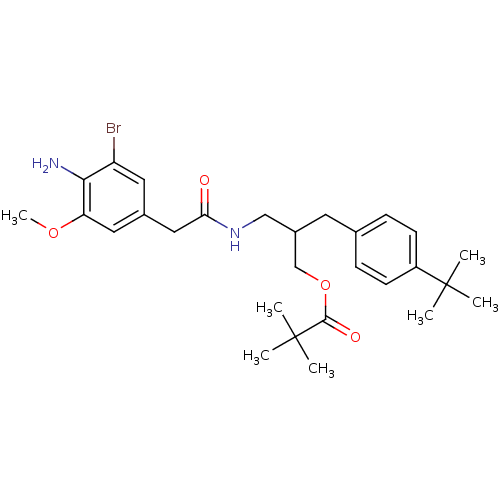
(2-(4-tert-Butylbenzyl)-3-[2-(4-amino-3-bromo-5-met...)Show SMILES COc1cc(CC(=O)NCC(COC(=O)C(C)(C)C)Cc2ccc(cc2)C(C)(C)C)cc(Br)c1N Show InChI InChI=1S/C28H39BrN2O4/c1-27(2,3)21-10-8-18(9-11-21)12-20(17-35-26(33)28(4,5)6)16-31-24(32)15-19-13-22(29)25(30)23(14-19)34-7/h8-11,13-14,20H,12,15-17,30H2,1-7H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced calcium uptake |
Bioorg Med Chem 18: 8092-105 (2010)
Article DOI: 10.1016/j.bmc.2010.09.001
BindingDB Entry DOI: 10.7270/Q2Q81D98 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data