 Found 100 hits with Last Name = 'yuan' and Initial = 'g'
Found 100 hits with Last Name = 'yuan' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM403460

(US10335399, Example 2-5 | US10806724, Example 2-5)Show InChI InChI=1S/C17H16F2N2O2/c1-17(2)6-5-11-12(10-4-3-9(18)7-13(10)19)8-14(15(20)22)21-16(11)23-17/h3-4,7-8H,5-6H2,1-2H3,(H2,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human recombinant mGlur2 assessed as inhibition constant by cAMP Glosensor assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02004
BindingDB Entry DOI: 10.7270/Q2TQ65FG |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM403460

(US10335399, Example 2-5 | US10806724, Example 2-5)Show InChI InChI=1S/C17H16F2N2O2/c1-17(2)6-5-11-12(10-4-3-9(18)7-13(10)19)8-14(15(20)22)21-16(11)23-17/h3-4,7-8H,5-6H2,1-2H3,(H2,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human recombinant mGlur2 assessed as inhibition constant by cAMP Glosensor assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02004
BindingDB Entry DOI: 10.7270/Q2TQ65FG |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50584759

(CHEMBL5089623)Show SMILES COc1ccc(c(F)c1)-c1cc(nc2OC(C)(C)CCc12)C(N)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human recombinant mGlur2 assessed as inhibition constant by cAMP Glosensor assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02004
BindingDB Entry DOI: 10.7270/Q2TQ65FG |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50584759

(CHEMBL5089623)Show SMILES COc1ccc(c(F)c1)-c1cc(nc2OC(C)(C)CCc12)C(N)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human recombinant mGlur2 assessed as inhibition constant by cAMP Glosensor assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02004
BindingDB Entry DOI: 10.7270/Q2TQ65FG |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM403460

(US10335399, Example 2-5 | US10806724, Example 2-5)Show InChI InChI=1S/C17H16F2N2O2/c1-17(2)6-5-11-12(10-4-3-9(18)7-13(10)19)8-14(15(20)22)21-16(11)23-17/h3-4,7-8H,5-6H2,1-2H3,(H2,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Negative allosteric modulation activity at human recombinant mGlur2 expressed in CHO cells in presence of cAMP by chemiluminescence based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02004
BindingDB Entry DOI: 10.7270/Q2TQ65FG |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM393885
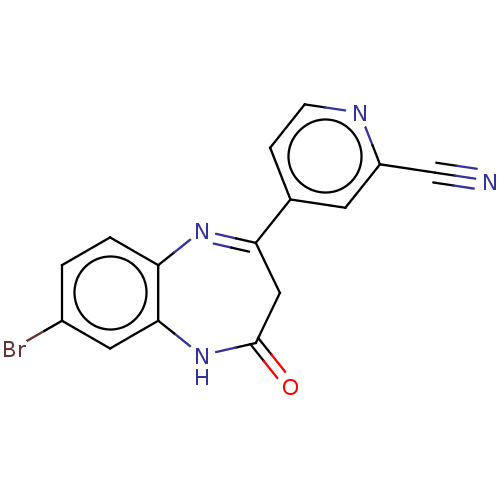
(US10597367, Example 182 | US9969726, Example 182)Show SMILES Brc1ccc2N=C(CC(=O)Nc2c1)c1ccnc(c1)C#N |c:5| Show InChI InChI=1S/C15H9BrN4O/c16-10-1-2-12-14(6-10)20-15(21)7-13(19-12)9-3-4-18-11(5-9)8-17/h1-6H,7H2,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Negative allosteric modulator activity at human recombinant mGlur2 expressed in HEK293 cells by calcium assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02004
BindingDB Entry DOI: 10.7270/Q2TQ65FG |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11695

((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...)Show SMILES OC12CC3CC(C1)CC(C3)(C2)NCC(=O)N1CCC[C@H]1C#N |r,TLB:9:8:6:3.2.4,4:3:10:7.6.5,4:5:10:3.2.9,THB:9:3:6:10.7.8,11:8:6:3.2.4| Show InChI InChI=1S/C17H25N3O2/c18-9-14-2-1-3-20(14)15(21)10-19-16-5-12-4-13(6-16)8-17(22,7-12)11-16/h12-14,19,22H,1-8,10-11H2/t12?,13?,14-,16?,17?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 20: 3565-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.120
BindingDB Entry DOI: 10.7270/Q2P55NQQ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50320285

((3aR,5beta,6aS)-5-(2-((S)-2-cyanopyrrolidin-1-yl)-...)Show SMILES CN(C)C(=O)N1C[C@@H]2C[C@@H](C[C@@H]2C1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C17H27N5O2/c1-20(2)17(24)21-10-12-6-14(7-13(12)11-21)19-9-16(23)22-5-3-4-15(22)8-18/h12-15,19H,3-7,9-11H2,1-2H3/t12-,13+,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 20: 3565-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.120
BindingDB Entry DOI: 10.7270/Q2P55NQQ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50320293
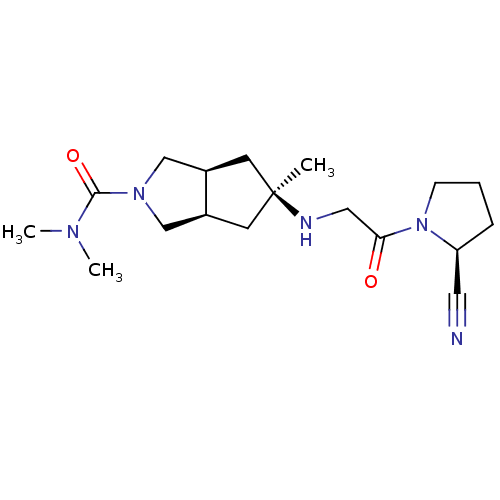
((3aR,5alpha,6aS)-5-(2-((S)-2-cyanopyrrolidin-1-yl)...)Show SMILES CN(C)C(=O)N1C[C@H]2C[C@](C)(C[C@H]2C1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C18H29N5O2/c1-18(20-10-16(24)23-6-4-5-15(23)9-19)7-13-11-22(12-14(13)8-18)17(25)21(2)3/h13-15,20H,4-8,10-12H2,1-3H3/t13-,14+,15-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 20: 3565-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.120
BindingDB Entry DOI: 10.7270/Q2P55NQQ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50320294

((3aR,5beta,6aS)-5-(2-((S)-2-cyanopyrrolidin-1-yl)-...)Show SMILES CN(C)C(=O)N1C[C@H]2C[C@@](C)(C[C@H]2C1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C18H29N5O2/c1-18(20-10-16(24)23-6-4-5-15(23)9-19)7-13-11-22(12-14(13)8-18)17(25)21(2)3/h13-15,20H,4-8,10-12H2,1-3H3/t13-,14+,15-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 20: 3565-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.120
BindingDB Entry DOI: 10.7270/Q2P55NQQ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50320291
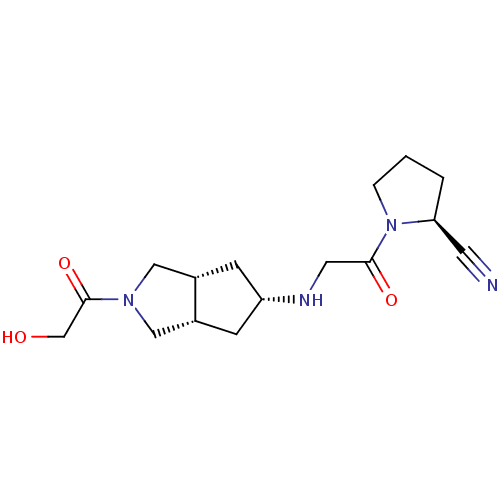
((S)-1-(2-((3aR,5beta,6aS)-2-(2-hydroxyacetyl)octah...)Show SMILES OCC(=O)N1C[C@@H]2C[C@@H](C[C@@H]2C1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C16H24N4O3/c17-6-14-2-1-3-20(14)15(22)7-18-13-4-11-8-19(16(23)10-21)9-12(11)5-13/h11-14,18,21H,1-5,7-10H2/t11-,12+,13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 20: 3565-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.120
BindingDB Entry DOI: 10.7270/Q2P55NQQ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50320290

((3aR,5beta,6aS)-methyl 5-(2-((S)-2-cyanopyrrolidin...)Show SMILES COC(=O)N1C[C@@H]2C[C@@H](C[C@@H]2C1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C16H24N4O3/c1-23-16(22)19-9-11-5-13(6-12(11)10-19)18-8-15(21)20-4-2-3-14(20)7-17/h11-14,18H,2-6,8-10H2,1H3/t11-,12+,13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 20: 3565-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.120
BindingDB Entry DOI: 10.7270/Q2P55NQQ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50320287
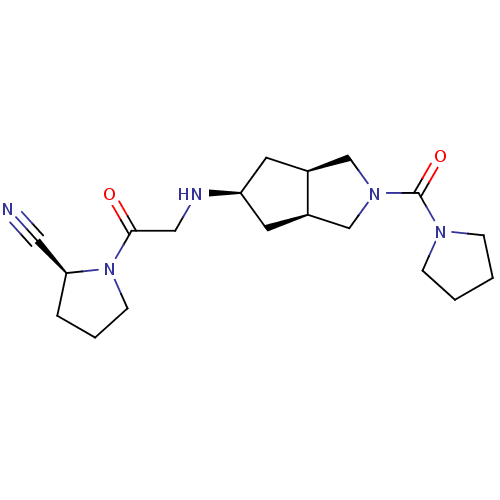
((3aR,5beta,6aS)-N-butyl-5-(2-((S)-2-cyanopyrrolidi...)Show SMILES O=C(CN[C@H]1C[C@H]2CN(C[C@H]2C1)C(=O)N1CCCC1)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C19H29N5O2/c20-10-17-4-3-7-24(17)18(25)11-21-16-8-14-12-23(13-15(14)9-16)19(26)22-5-1-2-6-22/h14-17,21H,1-9,11-13H2/t14-,15+,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 20: 3565-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.120
BindingDB Entry DOI: 10.7270/Q2P55NQQ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50320289
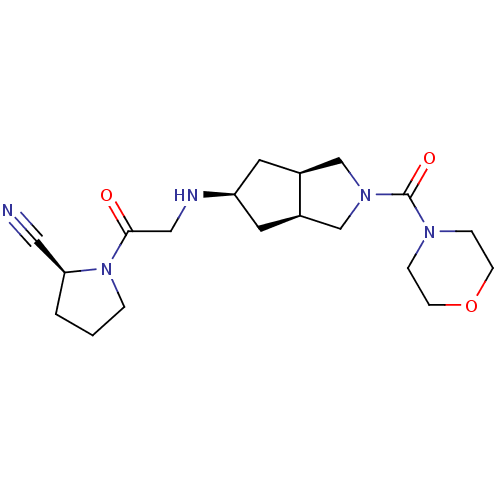
((3aR,5beta,6aS)-5-(2-((S)-2-cyanopyrrolidin-1-yl)-...)Show SMILES O=C(CN[C@H]1C[C@H]2CN(C[C@H]2C1)C(=O)N1CCOCC1)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C19H29N5O3/c20-10-17-2-1-3-24(17)18(25)11-21-16-8-14-12-23(13-15(14)9-16)19(26)22-4-6-27-7-5-22/h14-17,21H,1-9,11-13H2/t14-,15+,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 20: 3565-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.120
BindingDB Entry DOI: 10.7270/Q2P55NQQ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50320288
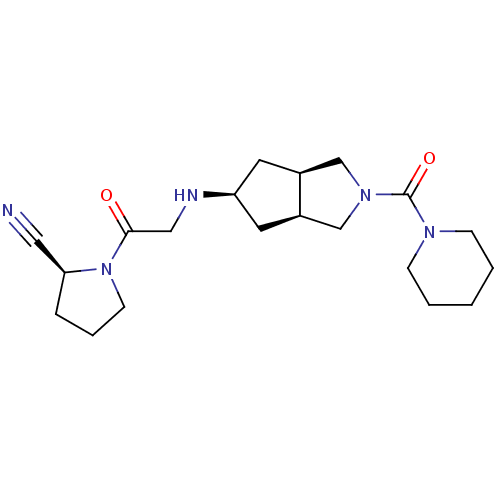
((3aR,5beta,6aS)-5-(2-((S)-2-cyanopyrrolidin-1-yl)-...)Show SMILES O=C(CN[C@H]1C[C@H]2CN(C[C@H]2C1)C(=O)N1CCCCC1)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C20H31N5O2/c21-11-18-5-4-8-25(18)19(26)12-22-17-9-15-13-24(14-16(15)10-17)20(27)23-6-2-1-3-7-23/h15-18,22H,1-10,12-14H2/t15-,16+,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 20: 3565-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.120
BindingDB Entry DOI: 10.7270/Q2P55NQQ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50320292
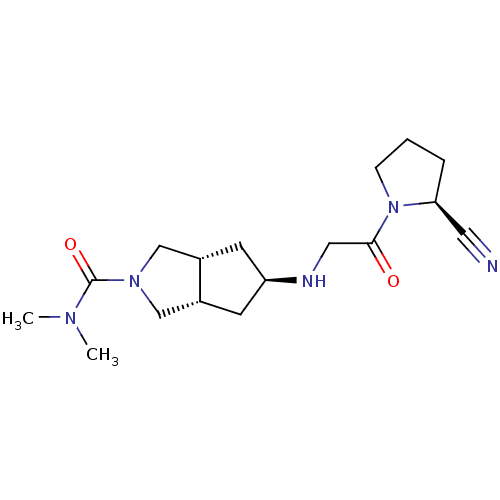
((3aR,5alpha,6aS)-5-(2-((S)-2-cyanopyrrolidin-1-yl)...)Show SMILES CN(C)C(=O)N1C[C@@H]2C[C@H](C[C@@H]2C1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C17H27N5O2/c1-20(2)17(24)21-10-12-6-14(7-13(12)11-21)19-9-16(23)22-5-3-4-15(22)8-18/h12-15,19H,3-7,9-11H2,1-2H3/t12-,13+,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 20: 3565-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.120
BindingDB Entry DOI: 10.7270/Q2P55NQQ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50584759

(CHEMBL5089623)Show SMILES COc1ccc(c(F)c1)-c1cc(nc2OC(C)(C)CCc12)C(N)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Negative allosteric modulation activity at human recombinant mGlur2 expressed in CHO cells in presence of cAMP by chemiluminescence based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02004
BindingDB Entry DOI: 10.7270/Q2TQ65FG |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50584759

(CHEMBL5089623)Show SMILES COc1ccc(c(F)c1)-c1cc(nc2OC(C)(C)CCc12)C(N)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Negative allosteric modulation activity at human recombinant mGlur2 expressed in CHO cells in presence of cAMP by chemiluminescence based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02004
BindingDB Entry DOI: 10.7270/Q2TQ65FG |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50320286
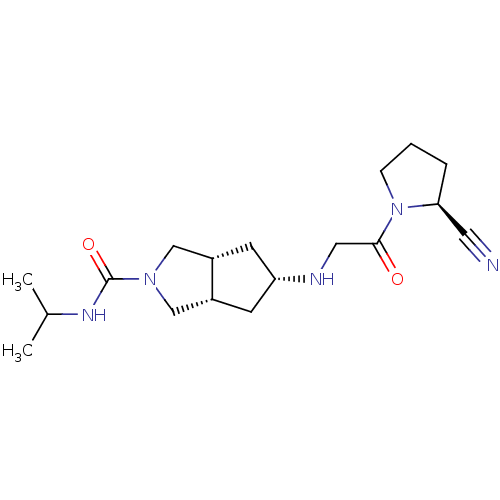
((3aR,5beta,6aS)-5-(2-((S)-2-cyanopyrrolidin-1-yl)-...)Show SMILES CC(C)NC(=O)N1C[C@@H]2C[C@@H](C[C@@H]2C1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C18H29N5O2/c1-12(2)21-18(25)22-10-13-6-15(7-14(13)11-22)20-9-17(24)23-5-3-4-16(23)8-19/h12-16,20H,3-7,9-11H2,1-2H3,(H,21,25)/t13-,14+,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 20: 3565-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.120
BindingDB Entry DOI: 10.7270/Q2P55NQQ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM11695

((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...)Show SMILES OC12CC3CC(C1)CC(C3)(C2)NCC(=O)N1CCC[C@H]1C#N |r,TLB:9:8:6:3.2.4,4:3:10:7.6.5,4:5:10:3.2.9,THB:9:3:6:10.7.8,11:8:6:3.2.4| Show InChI InChI=1S/C17H25N3O2/c18-9-14-2-1-3-20(14)15(21)10-19-16-5-12-4-13(6-16)8-17(22,7-12)11-16/h12-14,19,22H,1-8,10-11H2/t12?,13?,14-,16?,17?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 |
Bioorg Med Chem Lett 20: 3565-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.120
BindingDB Entry DOI: 10.7270/Q2P55NQQ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50320294

((3aR,5beta,6aS)-5-(2-((S)-2-cyanopyrrolidin-1-yl)-...)Show SMILES CN(C)C(=O)N1C[C@H]2C[C@@](C)(C[C@H]2C1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C18H29N5O2/c1-18(20-10-16(24)23-6-4-5-15(23)9-19)7-13-11-22(12-14(13)8-18)17(25)21(2)3/h13-15,20H,4-8,10-12H2,1-3H3/t13-,14+,15-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 |
Bioorg Med Chem Lett 20: 3565-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.120
BindingDB Entry DOI: 10.7270/Q2P55NQQ |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50449051

(CHEMBL3126485)Show SMILES CN(C)CCCn1c(=O)c2ccc3c4ccc5c6c(ccc(c7ccc(c2c37)c1=O)c46)c(=O)n(CCCN(C)C)c5=O Show InChI InChI=1S/C34H32N4O4/c1-35(2)15-5-17-37-31(39)23-11-7-19-21-9-13-25-30-26(34(42)38(33(25)41)18-6-16-36(3)4)14-10-22(28(21)30)20-8-12-24(32(37)40)29(23)27(19)20/h7-14H,5-6,15-18H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HIF-1 alpha in human MDA-MB-468 cells by Western blotting analysis in hypoxia condition |
Bioorg Med Chem 22: 1496-505 (2014)
Article DOI: 10.1016/j.bmc.2013.10.018
BindingDB Entry DOI: 10.7270/Q2C53NB7 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50449051

(CHEMBL3126485)Show SMILES CN(C)CCCn1c(=O)c2ccc3c4ccc5c6c(ccc(c7ccc(c2c37)c1=O)c46)c(=O)n(CCCN(C)C)c5=O Show InChI InChI=1S/C34H32N4O4/c1-35(2)15-5-17-37-31(39)23-11-7-19-21-9-13-25-30-26(34(42)38(33(25)41)18-6-16-36(3)4)14-10-22(28(21)30)20-8-12-24(32(37)40)29(23)27(19)20/h7-14H,5-6,15-18H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HIF-1 alpha in human OVCAR3 cells by Western blotting analysis in hypoxia condition |
Bioorg Med Chem 22: 1496-505 (2014)
Article DOI: 10.1016/j.bmc.2013.10.018
BindingDB Entry DOI: 10.7270/Q2C53NB7 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50449051

(CHEMBL3126485)Show SMILES CN(C)CCCn1c(=O)c2ccc3c4ccc5c6c(ccc(c7ccc(c2c37)c1=O)c46)c(=O)n(CCCN(C)C)c5=O Show InChI InChI=1S/C34H32N4O4/c1-35(2)15-5-17-37-31(39)23-11-7-19-21-9-13-25-30-26(34(42)38(33(25)41)18-6-16-36(3)4)14-10-22(28(21)30)20-8-12-24(32(37)40)29(23)27(19)20/h7-14H,5-6,15-18H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HIF-1 alpha in human PANC1 cells by Western blotting analysis in hypoxia condition |
Bioorg Med Chem 22: 1496-505 (2014)
Article DOI: 10.1016/j.bmc.2013.10.018
BindingDB Entry DOI: 10.7270/Q2C53NB7 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50449051

(CHEMBL3126485)Show SMILES CN(C)CCCn1c(=O)c2ccc3c4ccc5c6c(ccc(c7ccc(c2c37)c1=O)c46)c(=O)n(CCCN(C)C)c5=O Show InChI InChI=1S/C34H32N4O4/c1-35(2)15-5-17-37-31(39)23-11-7-19-21-9-13-25-30-26(34(42)38(33(25)41)18-6-16-36(3)4)14-10-22(28(21)30)20-8-12-24(32(37)40)29(23)27(19)20/h7-14H,5-6,15-18H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HIF-1 alpha in human MDA-MB-231 cells by Western blotting analysis in hypoxia condition |
Bioorg Med Chem 22: 1496-505 (2014)
Article DOI: 10.1016/j.bmc.2013.10.018
BindingDB Entry DOI: 10.7270/Q2C53NB7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM11695

((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...)Show SMILES OC12CC3CC(C1)CC(C3)(C2)NCC(=O)N1CCC[C@H]1C#N |r,TLB:9:8:6:3.2.4,4:3:10:7.6.5,4:5:10:3.2.9,THB:9:3:6:10.7.8,11:8:6:3.2.4| Show InChI InChI=1S/C17H25N3O2/c18-9-14-2-1-3-20(14)15(21)10-19-16-5-12-4-13(6-16)8-17(22,7-12)11-16/h12-14,19,22H,1-8,10-11H2/t12?,13?,14-,16?,17?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of DPP8 |
Bioorg Med Chem Lett 20: 3565-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.120
BindingDB Entry DOI: 10.7270/Q2P55NQQ |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 3
(Homo sapiens (Human)) | BDBM50449051

(CHEMBL3126485)Show SMILES CN(C)CCCn1c(=O)c2ccc3c4ccc5c6c(ccc(c7ccc(c2c37)c1=O)c46)c(=O)n(CCCN(C)C)c5=O Show InChI InChI=1S/C34H32N4O4/c1-35(2)15-5-17-37-31(39)23-11-7-19-21-9-13-25-30-26(34(42)38(33(25)41)18-6-16-36(3)4)14-10-22(28(21)30)20-8-12-24(32(37)40)29(23)27(19)20/h7-14H,5-6,15-18H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Stat3 phosphorylation in human MDA-MB-468 cells by Western blotting analysis in hypoxia condition |
Bioorg Med Chem 22: 1496-505 (2014)
Article DOI: 10.1016/j.bmc.2013.10.018
BindingDB Entry DOI: 10.7270/Q2C53NB7 |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 3
(Homo sapiens (Human)) | BDBM50449051

(CHEMBL3126485)Show SMILES CN(C)CCCn1c(=O)c2ccc3c4ccc5c6c(ccc(c7ccc(c2c37)c1=O)c46)c(=O)n(CCCN(C)C)c5=O Show InChI InChI=1S/C34H32N4O4/c1-35(2)15-5-17-37-31(39)23-11-7-19-21-9-13-25-30-26(34(42)38(33(25)41)18-6-16-36(3)4)14-10-22(28(21)30)20-8-12-24(32(37)40)29(23)27(19)20/h7-14H,5-6,15-18H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Stat3 phosphorylation in human OVCAR3 cells by Western blotting analysis in normoxia condition |
Bioorg Med Chem 22: 1496-505 (2014)
Article DOI: 10.1016/j.bmc.2013.10.018
BindingDB Entry DOI: 10.7270/Q2C53NB7 |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 3
(Homo sapiens (Human)) | BDBM50449051

(CHEMBL3126485)Show SMILES CN(C)CCCn1c(=O)c2ccc3c4ccc5c6c(ccc(c7ccc(c2c37)c1=O)c46)c(=O)n(CCCN(C)C)c5=O Show InChI InChI=1S/C34H32N4O4/c1-35(2)15-5-17-37-31(39)23-11-7-19-21-9-13-25-30-26(34(42)38(33(25)41)18-6-16-36(3)4)14-10-22(28(21)30)20-8-12-24(32(37)40)29(23)27(19)20/h7-14H,5-6,15-18H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Stat3 phosphorylation in human OVCAR3 cells by Western blotting analysis in hypoxia condition |
Bioorg Med Chem 22: 1496-505 (2014)
Article DOI: 10.1016/j.bmc.2013.10.018
BindingDB Entry DOI: 10.7270/Q2C53NB7 |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 3
(Homo sapiens (Human)) | BDBM50449051

(CHEMBL3126485)Show SMILES CN(C)CCCn1c(=O)c2ccc3c4ccc5c6c(ccc(c7ccc(c2c37)c1=O)c46)c(=O)n(CCCN(C)C)c5=O Show InChI InChI=1S/C34H32N4O4/c1-35(2)15-5-17-37-31(39)23-11-7-19-21-9-13-25-30-26(34(42)38(33(25)41)18-6-16-36(3)4)14-10-22(28(21)30)20-8-12-24(32(37)40)29(23)27(19)20/h7-14H,5-6,15-18H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Stat3 phosphorylation in human MDA-MB-231 cells by Western blotting analysis in hypoxia condition |
Bioorg Med Chem 22: 1496-505 (2014)
Article DOI: 10.1016/j.bmc.2013.10.018
BindingDB Entry DOI: 10.7270/Q2C53NB7 |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 3
(Homo sapiens (Human)) | BDBM50449051

(CHEMBL3126485)Show SMILES CN(C)CCCn1c(=O)c2ccc3c4ccc5c6c(ccc(c7ccc(c2c37)c1=O)c46)c(=O)n(CCCN(C)C)c5=O Show InChI InChI=1S/C34H32N4O4/c1-35(2)15-5-17-37-31(39)23-11-7-19-21-9-13-25-30-26(34(42)38(33(25)41)18-6-16-36(3)4)14-10-22(28(21)30)20-8-12-24(32(37)40)29(23)27(19)20/h7-14H,5-6,15-18H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Stat3 phosphorylation in human PANC1 cells by Western blotting analysis in normoxia condition |
Bioorg Med Chem 22: 1496-505 (2014)
Article DOI: 10.1016/j.bmc.2013.10.018
BindingDB Entry DOI: 10.7270/Q2C53NB7 |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 3
(Homo sapiens (Human)) | BDBM50449051

(CHEMBL3126485)Show SMILES CN(C)CCCn1c(=O)c2ccc3c4ccc5c6c(ccc(c7ccc(c2c37)c1=O)c46)c(=O)n(CCCN(C)C)c5=O Show InChI InChI=1S/C34H32N4O4/c1-35(2)15-5-17-37-31(39)23-11-7-19-21-9-13-25-30-26(34(42)38(33(25)41)18-6-16-36(3)4)14-10-22(28(21)30)20-8-12-24(32(37)40)29(23)27(19)20/h7-14H,5-6,15-18H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Stat3 phosphorylation in human PANC1 cells by Western blotting analysis in hypoxia condition |
Bioorg Med Chem 22: 1496-505 (2014)
Article DOI: 10.1016/j.bmc.2013.10.018
BindingDB Entry DOI: 10.7270/Q2C53NB7 |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 3
(Homo sapiens (Human)) | BDBM50449051

(CHEMBL3126485)Show SMILES CN(C)CCCn1c(=O)c2ccc3c4ccc5c6c(ccc(c7ccc(c2c37)c1=O)c46)c(=O)n(CCCN(C)C)c5=O Show InChI InChI=1S/C34H32N4O4/c1-35(2)15-5-17-37-31(39)23-11-7-19-21-9-13-25-30-26(34(42)38(33(25)41)18-6-16-36(3)4)14-10-22(28(21)30)20-8-12-24(32(37)40)29(23)27(19)20/h7-14H,5-6,15-18H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Stat3 phosphorylation in human MDA-MB-231 cells by Western blotting analysis in normoxia condition |
Bioorg Med Chem 22: 1496-505 (2014)
Article DOI: 10.1016/j.bmc.2013.10.018
BindingDB Entry DOI: 10.7270/Q2C53NB7 |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 3
(Homo sapiens (Human)) | BDBM50449051

(CHEMBL3126485)Show SMILES CN(C)CCCn1c(=O)c2ccc3c4ccc5c6c(ccc(c7ccc(c2c37)c1=O)c46)c(=O)n(CCCN(C)C)c5=O Show InChI InChI=1S/C34H32N4O4/c1-35(2)15-5-17-37-31(39)23-11-7-19-21-9-13-25-30-26(34(42)38(33(25)41)18-6-16-36(3)4)14-10-22(28(21)30)20-8-12-24(32(37)40)29(23)27(19)20/h7-14H,5-6,15-18H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Stat3 phosphorylation in human MDA-MB-468 cells by Western blotting analysis in normoxia condition |
Bioorg Med Chem 22: 1496-505 (2014)
Article DOI: 10.1016/j.bmc.2013.10.018
BindingDB Entry DOI: 10.7270/Q2C53NB7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50320293

((3aR,5alpha,6aS)-5-(2-((S)-2-cyanopyrrolidin-1-yl)...)Show SMILES CN(C)C(=O)N1C[C@H]2C[C@](C)(C[C@H]2C1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C18H29N5O2/c1-18(20-10-16(24)23-6-4-5-15(23)9-19)7-13-11-22(12-14(13)8-18)17(25)21(2)3/h13-15,20H,4-8,10-12H2,1-3H3/t13-,14+,15-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of DPP8 |
Bioorg Med Chem Lett 20: 3565-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.120
BindingDB Entry DOI: 10.7270/Q2P55NQQ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50320290

((3aR,5beta,6aS)-methyl 5-(2-((S)-2-cyanopyrrolidin...)Show SMILES COC(=O)N1C[C@@H]2C[C@@H](C[C@@H]2C1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C16H24N4O3/c1-23-16(22)19-9-11-5-13(6-12(11)10-19)18-8-15(21)20-4-2-3-14(20)7-17/h11-14,18H,2-6,8-10H2,1H3/t11-,12+,13-,14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 |
Bioorg Med Chem Lett 20: 3565-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.120
BindingDB Entry DOI: 10.7270/Q2P55NQQ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50320285

((3aR,5beta,6aS)-5-(2-((S)-2-cyanopyrrolidin-1-yl)-...)Show SMILES CN(C)C(=O)N1C[C@@H]2C[C@@H](C[C@@H]2C1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C17H27N5O2/c1-20(2)17(24)21-10-12-6-14(7-13(12)11-21)19-9-16(23)22-5-3-4-15(22)8-18/h12-15,19H,3-7,9-11H2,1-2H3/t12-,13+,14-,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 |
Bioorg Med Chem Lett 20: 3565-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.120
BindingDB Entry DOI: 10.7270/Q2P55NQQ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM145862
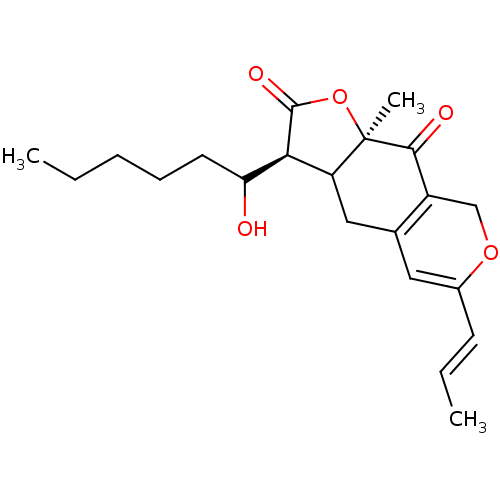
(US8957057, Monascuspiloin)Show SMILES CCCCCC(O)[C@@H]1C2CC3=C(COC(\C=C\C)=C3)C(=O)[C@]2(C)OC1=O |r,c:17,t:10| Show InChI InChI=1S/C21H28O5/c1-4-6-7-9-17(22)18-16-11-13-10-14(8-5-2)25-12-15(13)19(23)21(16,3)26-20(18)24/h5,8,10,16-18,22H,4,6-7,9,11-12H2,1-3H3/b8-5+/t16?,17?,18-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Food Industry Research and Development Institute
US Patent
| Assay Description
To conduct the assays, the 250 uM monasuspiloin solution was further diluted with 10% DMSO to prepare 25 uM, and 5 uM monasuspiloin samples, and the ... |
US Patent US8957057 (2015)
BindingDB Entry DOI: 10.7270/Q26Q1VZ0 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM145862

(US8957057, Monascuspiloin)Show SMILES CCCCCC(O)[C@@H]1C2CC3=C(COC(\C=C\C)=C3)C(=O)[C@]2(C)OC1=O |r,c:17,t:10| Show InChI InChI=1S/C21H28O5/c1-4-6-7-9-17(22)18-16-11-13-10-14(8-5-2)25-12-15(13)19(23)21(16,3)26-20(18)24/h5,8,10,16-18,22H,4,6-7,9,11-12H2,1-3H3/b8-5+/t16?,17?,18-,21+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Food Industry Research and Development Institute
US Patent
| Assay Description
To conduct the assays, the 250 uM monasuspiloin solution was further diluted with 10% DMSO to prepare 25 uM, and 5 uM monasuspiloin samples, and the ... |
US Patent US8957057 (2015)
BindingDB Entry DOI: 10.7270/Q26Q1VZ0 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50320293

((3aR,5alpha,6aS)-5-(2-((S)-2-cyanopyrrolidin-1-yl)...)Show SMILES CN(C)C(=O)N1C[C@H]2C[C@](C)(C[C@H]2C1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C18H29N5O2/c1-18(20-10-16(24)23-6-4-5-15(23)9-19)7-13-11-22(12-14(13)8-18)17(25)21(2)3/h13-15,20H,4-8,10-12H2,1-3H3/t13-,14+,15-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 |
Bioorg Med Chem Lett 20: 3565-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.120
BindingDB Entry DOI: 10.7270/Q2P55NQQ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50320285

((3aR,5beta,6aS)-5-(2-((S)-2-cyanopyrrolidin-1-yl)-...)Show SMILES CN(C)C(=O)N1C[C@@H]2C[C@@H](C[C@@H]2C1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C17H27N5O2/c1-20(2)17(24)21-10-12-6-14(7-13(12)11-21)19-9-16(23)22-5-3-4-15(22)8-18/h12-15,19H,3-7,9-11H2,1-2H3/t12-,13+,14-,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of DPP8 |
Bioorg Med Chem Lett 20: 3565-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.120
BindingDB Entry DOI: 10.7270/Q2P55NQQ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50320290

((3aR,5beta,6aS)-methyl 5-(2-((S)-2-cyanopyrrolidin...)Show SMILES COC(=O)N1C[C@@H]2C[C@@H](C[C@@H]2C1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C16H24N4O3/c1-23-16(22)19-9-11-5-13(6-12(11)10-19)18-8-15(21)20-4-2-3-14(20)7-17/h11-14,18H,2-6,8-10H2,1H3/t11-,12+,13-,14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of DPP8 |
Bioorg Med Chem Lett 20: 3565-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.120
BindingDB Entry DOI: 10.7270/Q2P55NQQ |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM145862

(US8957057, Monascuspiloin)Show SMILES CCCCCC(O)[C@@H]1C2CC3=C(COC(\C=C\C)=C3)C(=O)[C@]2(C)OC1=O |r,c:17,t:10| Show InChI InChI=1S/C21H28O5/c1-4-6-7-9-17(22)18-16-11-13-10-14(8-5-2)25-12-15(13)19(23)21(16,3)26-20(18)24/h5,8,10,16-18,22H,4,6-7,9,11-12H2,1-3H3/b8-5+/t16?,17?,18-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Food Industry Research and Development Institute
US Patent
| Assay Description
To conduct the assays, the 250 uM monasuspiloin solution was further diluted with 10% DMSO to prepare 25 uM, and 5 uM monasuspiloin samples, and the ... |
US Patent US8957057 (2015)
BindingDB Entry DOI: 10.7270/Q26Q1VZ0 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM145862

(US8957057, Monascuspiloin)Show SMILES CCCCCC(O)[C@@H]1C2CC3=C(COC(\C=C\C)=C3)C(=O)[C@]2(C)OC1=O |r,c:17,t:10| Show InChI InChI=1S/C21H28O5/c1-4-6-7-9-17(22)18-16-11-13-10-14(8-5-2)25-12-15(13)19(23)21(16,3)26-20(18)24/h5,8,10,16-18,22H,4,6-7,9,11-12H2,1-3H3/b8-5+/t16?,17?,18-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Food Industry Research and Development Institute
US Patent
| Assay Description
To conduct the assays, the 250 uM monasuspiloin solution was further diluted with 10% DMSO to prepare 25 uM, and 5 uM monasuspiloin samples, and the ... |
US Patent US8957057 (2015)
BindingDB Entry DOI: 10.7270/Q26Q1VZ0 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50320294

((3aR,5beta,6aS)-5-(2-((S)-2-cyanopyrrolidin-1-yl)-...)Show SMILES CN(C)C(=O)N1C[C@H]2C[C@@](C)(C[C@H]2C1)NCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C18H29N5O2/c1-18(20-10-16(24)23-6-4-5-15(23)9-19)7-13-11-22(12-14(13)8-18)17(25)21(2)3/h13-15,20H,4-8,10-12H2,1-3H3/t13-,14+,15-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of DPP8 |
Bioorg Med Chem Lett 20: 3565-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.120
BindingDB Entry DOI: 10.7270/Q2P55NQQ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50194617
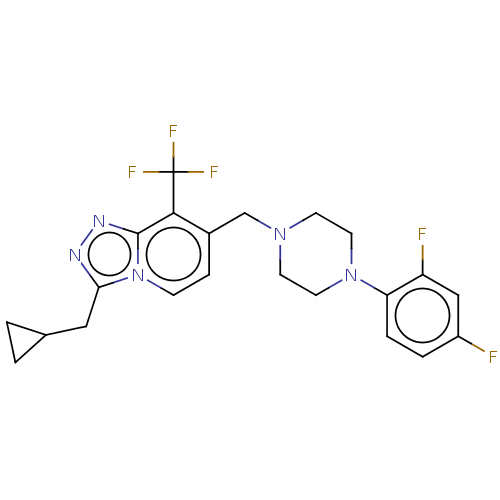
(CHEMBL3926416)Show SMILES Fc1ccc(N2CCN(Cc3ccn4c(CC5CC5)nnc4c3C(F)(F)F)CC2)c(F)c1 Show InChI InChI=1S/C22H22F5N5/c23-16-3-4-18(17(24)12-16)31-9-7-30(8-10-31)13-15-5-6-32-19(11-14-1-2-14)28-29-21(32)20(15)22(25,26)27/h3-6,12,14H,1-2,7-11,13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 166 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00593
BindingDB Entry DOI: 10.7270/Q2FB571B |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50194707
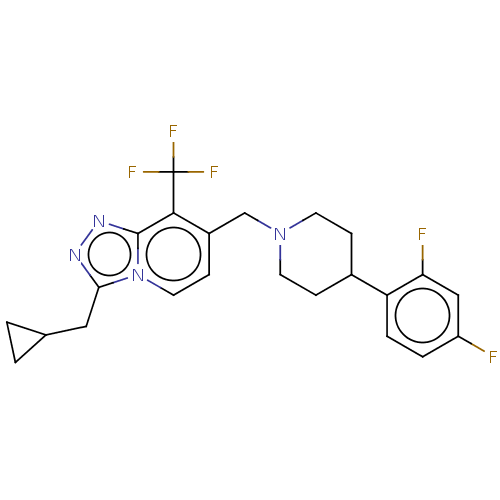
(CHEMBL3938796)Show SMILES Fc1ccc(C2CCN(Cc3ccn4c(CC5CC5)nnc4c3C(F)(F)F)CC2)c(F)c1 Show InChI InChI=1S/C23H23F5N4/c24-17-3-4-18(19(25)12-17)15-5-8-31(9-6-15)13-16-7-10-32-20(11-14-1-2-14)29-30-22(32)21(16)23(26,27)28/h3-4,7,10,12,14-15H,1-2,5-6,8-9,11,13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00593
BindingDB Entry DOI: 10.7270/Q2FB571B |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50601001

(CHEMBL5207431)Show SMILES Fc1ccc(N2CCN(Cc3ccn4c(CC5CC5)nnc4c3C(F)(F)F)CC2)c(Cl)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00593
BindingDB Entry DOI: 10.7270/Q2FB571B |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50601002
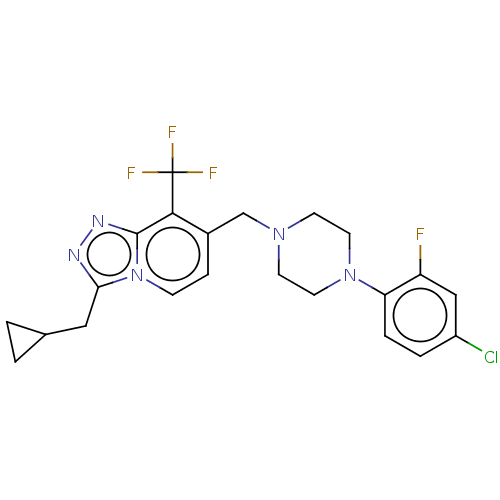
(CHEMBL5208947)Show SMILES Fc1cc(Cl)ccc1N1CCN(Cc2ccn3c(CC4CC4)nnc3c2C(F)(F)F)CC1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00593
BindingDB Entry DOI: 10.7270/Q2FB571B |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50601003
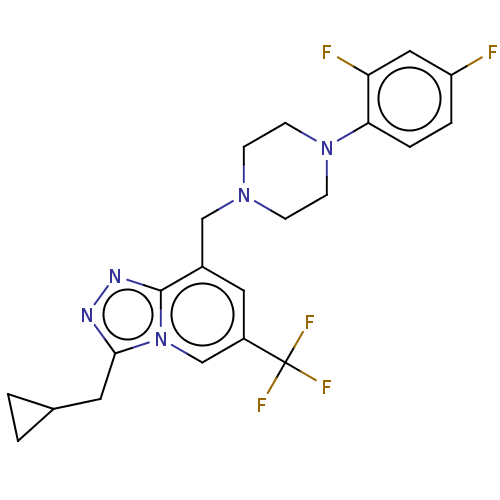
(CHEMBL5179002)Show SMILES Fc1ccc(N2CCN(Cc3cc(cn4c(CC5CC5)nnc34)C(F)(F)F)CC2)c(F)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 913 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00593
BindingDB Entry DOI: 10.7270/Q2FB571B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data