

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM388463 ((1aS,5aS)-2-(4-Oxy-pyrazin-2-yl)-1a,2,5,5a-tetrahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Preparation of Membranes: HEK293 cells stably expressing human CB2 receptor were collected, washed in ice cold PBS, and centrifuged at 48,000×g for 2... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q2B56N3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM532446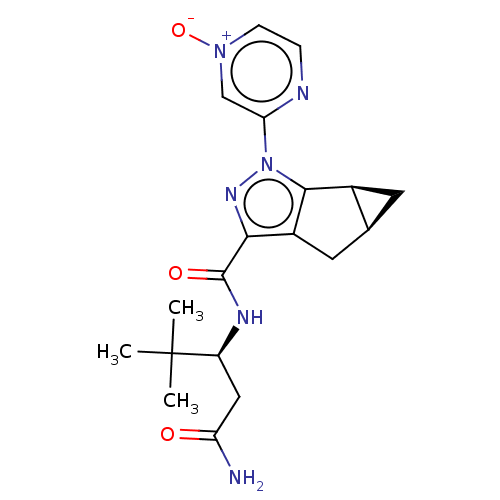 ((1aS,5aS)-2-(4-Oxy-pyrazin-2-yl)-1a,2,5,5a-tetrahy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table D: Radioligand binding assays for human CB2 receptors were performed using two different agonist radioligands, [3H]CP55,940 and [3H]WIN55,212-2... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BR8WC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM532448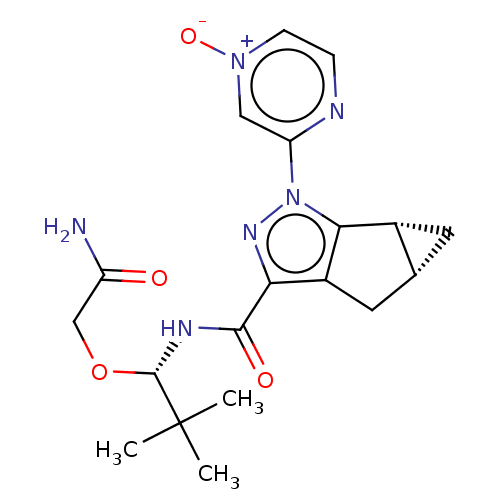 ((1aR,5aR)-2-(4-Oxy-pyrazin-2-yl)-1a,2,5,5a-tetrahy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table D: Radioligand binding assays for human CB2 receptors were performed using two different agonist radioligands, [3H]CP55,940 and [3H]WIN55,212-2... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BR8WC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM388466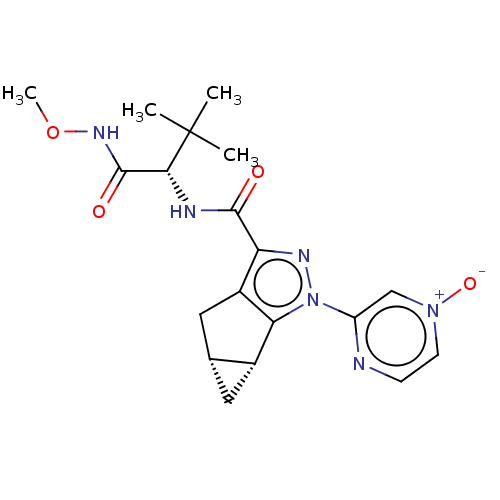 ((1aR,5aR)-2-(4-Oxy-pyrazin-2-yl)-1a,2,5,5a-tetrahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Preparation of Membranes: HEK293 cells stably expressing human CB2 receptor were collected, washed in ice cold PBS, and centrifuged at 48,000×g for 2... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q2B56N3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM388465 ((1aR,5aR)-2-(Tetrahydro-pyran-4-ylmethyl)-1a,2,5,5...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 58.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table D: Radioligand binding assays for human CB2 receptors were performed using two different agonist radioligands, [3H]CP55,940 and [3H]WIN55,212-2... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BR8WC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM388465 ((1aR,5aR)-2-(Tetrahydro-pyran-4-ylmethyl)-1a,2,5,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 58.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Preparation of Membranes: HEK293 cells stably expressing human CB2 receptor were collected, washed in ice cold PBS, and centrifuged at 48,000×g for 2... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q2B56N3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM388461 ((1aS,5aS)-2-(2,4-Difluoro-phenyl)-1a,2,5,5a-tetrah...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 97.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table D: Radioligand binding assays for human CB2 receptors were performed using two different agonist radioligands, [3H]CP55,940 and [3H]WIN55,212-2... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BR8WC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM388461 ((1aS,5aS)-2-(2,4-Difluoro-phenyl)-1a,2,5,5a-tetrah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 97.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Preparation of Membranes: HEK293 cells stably expressing human CB2 receptor were collected, washed in ice cold PBS, and centrifuged at 48,000×g for 2... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q2B56N3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM388462 ((1aR,5aR)-2-(4-Oxy-pyrazin-2-yl)-1a,2,5,5a-tetrahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 97.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Preparation of Membranes: HEK293 cells stably expressing human CB2 receptor were collected, washed in ice cold PBS, and centrifuged at 48,000×g for 2... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q2B56N3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM532445 ((1aR,5aR)-2-(4-Oxy-pyrazin-2-yl)-1a,2,5,5a-tetrahy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 97.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table D: Radioligand binding assays for human CB2 receptors were performed using two different agonist radioligands, [3H]CP55,940 and [3H]WIN55,212-2... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BR8WC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM388463 ((1aS,5aS)-2-(4-Oxy-pyrazin-2-yl)-1a,2,5,5a-tetrahy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Preparation of Membranes: HEK293 cells stably expressing human CB2 receptor were collected, washed in ice cold PBS, and centrifuged at 48,000×g for 2... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q2B56N3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM532446 ((1aS,5aS)-2-(4-Oxy-pyrazin-2-yl)-1a,2,5,5a-tetrahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table B: HTRF assay was carried out using a two-step protocol essentially according to the kit manufacturer's instructions, in 20 μL total v... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BR8WC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM388461 ((1aS,5aS)-2-(2,4-Difluoro-phenyl)-1a,2,5,5a-tetrah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 207 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table B: HTRF assay was carried out using a two-step protocol essentially according to the kit manufacturer's instructions, in 20 μL total v... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BR8WC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM388461 ((1aS,5aS)-2-(2,4-Difluoro-phenyl)-1a,2,5,5a-tetrah...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 207 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Preparation of Membranes: HEK293 cells stably expressing human CB2 receptor were collected, washed in ice cold PBS, and centrifuged at 48,000×g for 2... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q2B56N3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM388465 ((1aR,5aR)-2-(Tetrahydro-pyran-4-ylmethyl)-1a,2,5,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 568 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table B: HTRF assay was carried out using a two-step protocol essentially according to the kit manufacturer's instructions, in 20 μL total v... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BR8WC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM388465 ((1aR,5aR)-2-(Tetrahydro-pyran-4-ylmethyl)-1a,2,5,5...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 568 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Preparation of Membranes: HEK293 cells stably expressing human CB2 receptor were collected, washed in ice cold PBS, and centrifuged at 48,000×g for 2... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q2B56N3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM388466 ((1aR,5aR)-2-(4-Oxy-pyrazin-2-yl)-1a,2,5,5a-tetrahy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Preparation of Membranes: HEK293 cells stably expressing human CB2 receptor were collected, washed in ice cold PBS, and centrifuged at 48,000×g for 2... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q2B56N3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM532448 ((1aR,5aR)-2-(4-Oxy-pyrazin-2-yl)-1a,2,5,5a-tetrahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table B: HTRF assay was carried out using a two-step protocol essentially according to the kit manufacturer's instructions, in 20 μL total v... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BR8WC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50055992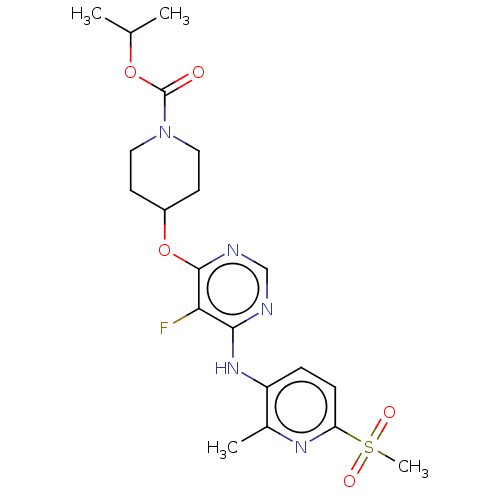 (CHEMBL3325842) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | Bioorg Med Chem Lett 24: 4332-5 (2014) Article DOI: 10.1016/j.bmcl.2014.06.071 BindingDB Entry DOI: 10.7270/Q2C82BZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50364559 (CHEMBL1951032) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | Bioorg Med Chem Lett 24: 4332-5 (2014) Article DOI: 10.1016/j.bmcl.2014.06.071 BindingDB Entry DOI: 10.7270/Q2C82BZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50056004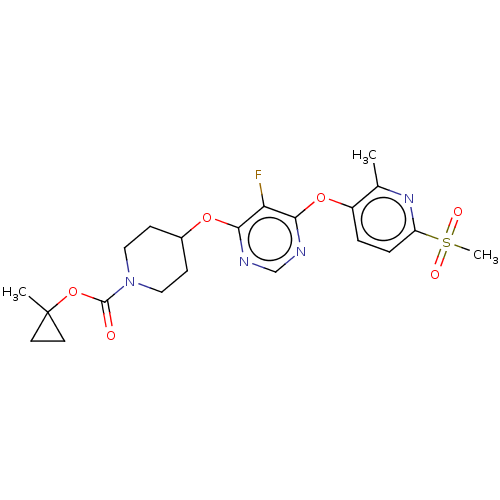 (CHEMBL3326667) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C19 (unknown origin) | Bioorg Med Chem Lett 24: 4332-5 (2014) Article DOI: 10.1016/j.bmcl.2014.06.071 BindingDB Entry DOI: 10.7270/Q2C82BZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50056004 (CHEMBL3326667) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | Bioorg Med Chem Lett 24: 4332-5 (2014) Article DOI: 10.1016/j.bmcl.2014.06.071 BindingDB Entry DOI: 10.7270/Q2C82BZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50106206 (CHEMBL3598099) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG by patch clamp assay | Bioorg Med Chem Lett 25: 3034-8 (2015) Article DOI: 10.1016/j.bmcl.2015.04.102 BindingDB Entry DOI: 10.7270/Q2SB47JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50040961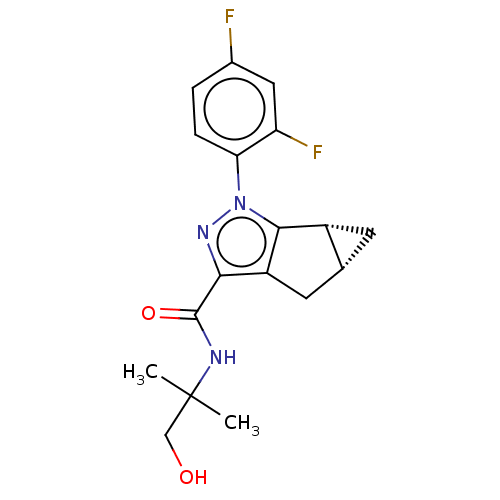 (CHEMBL3354952) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ERG channel by patch clamp technique | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50055993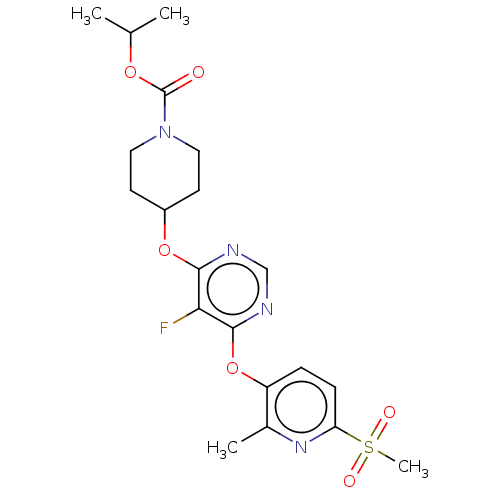 (CHEMBL3325843) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | Bioorg Med Chem Lett 24: 4332-5 (2014) Article DOI: 10.1016/j.bmcl.2014.06.071 BindingDB Entry DOI: 10.7270/Q2C82BZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50040961 (CHEMBL3354952) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]Astemizole from human ERG channel | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50106206 (CHEMBL3598099) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-Astemizole from human ERG | Bioorg Med Chem Lett 25: 3034-8 (2015) Article DOI: 10.1016/j.bmcl.2015.04.102 BindingDB Entry DOI: 10.7270/Q2SB47JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50040960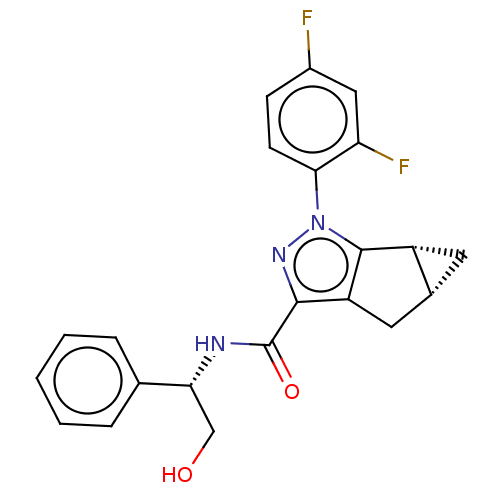 (CHEMBL3354956) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Inverse agonist activity at rat recombinant CB1 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50040962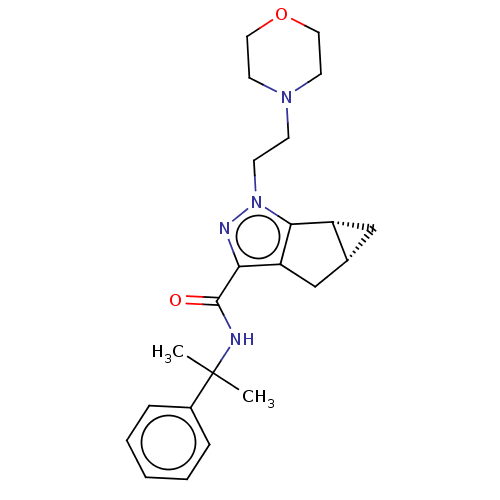 (CHEMBL3354940) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB1 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50040963 (CHEMBL3354939) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB1 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50040964 (CHEMBL3354938) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB1 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50040965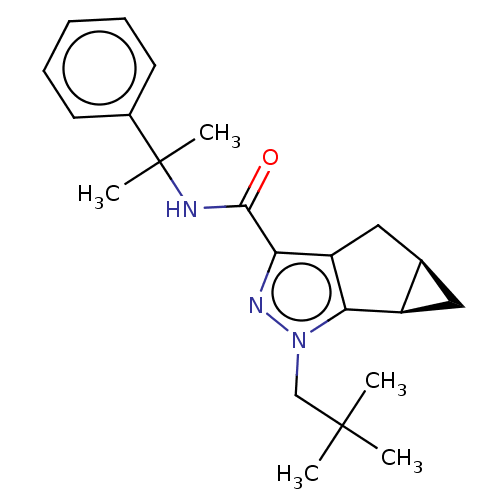 (CHEMBL3354937) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB1 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50040966 (CHEMBL3354936) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 51 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB1 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50040967 (CHEMBL3354935) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 86 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB1 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50040968 (CHEMBL3352843) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 420 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50040969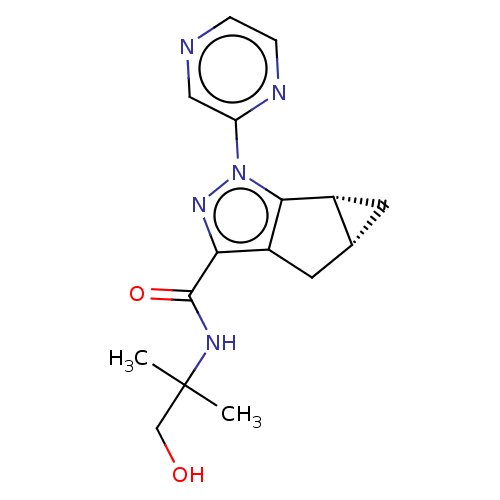 (CHEMBL3354976) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50040970 (CHEMBL3354975 | US11214548, Compound 269) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 809 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50040971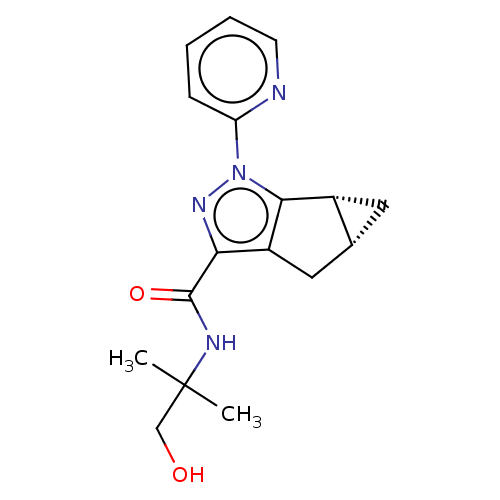 (CHEMBL3354974) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50040972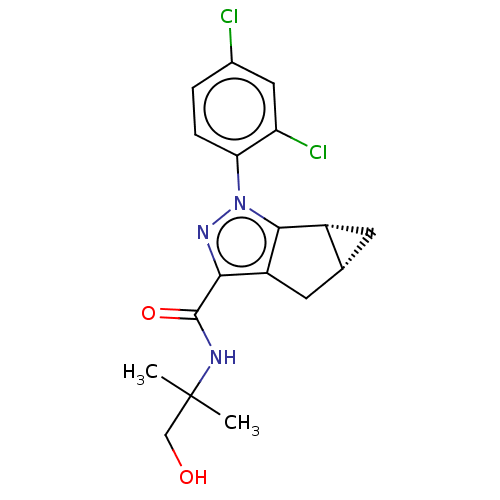 (CHEMBL3354973) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50040973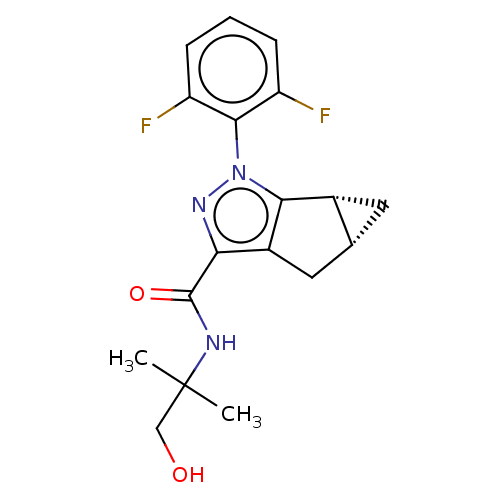 (CHEMBL3354972) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50040974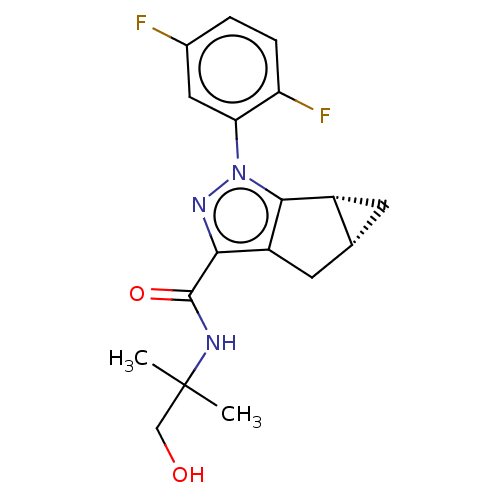 (CHEMBL3354971) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50040975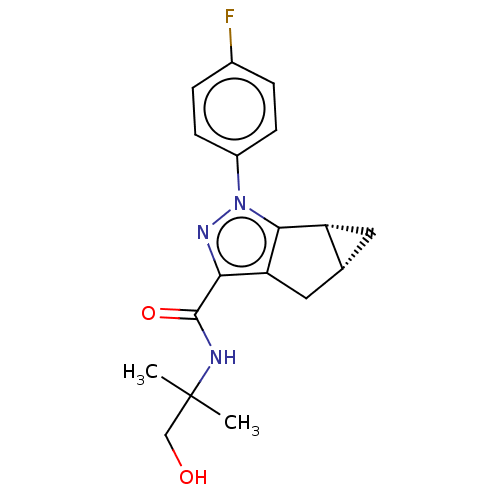 (CHEMBL3354970) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50040976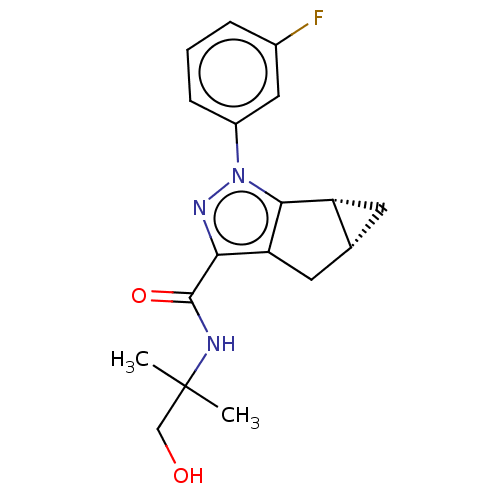 (CHEMBL3354969) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50040977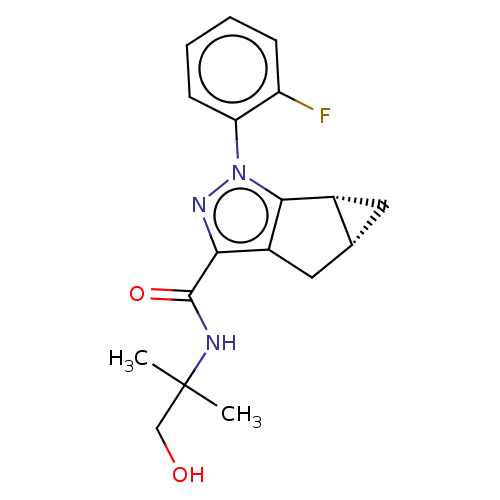 (CHEMBL3354968) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50040978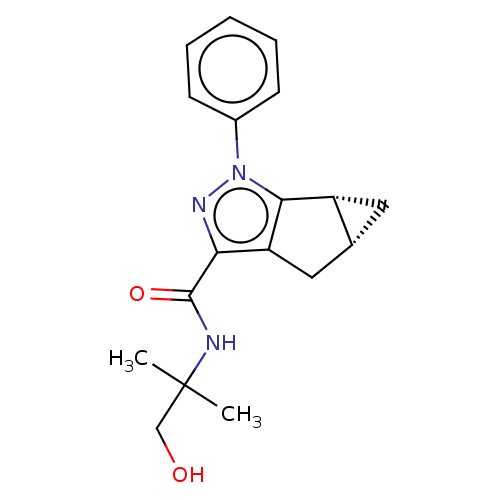 (CHEMBL3354967) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 101 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50040979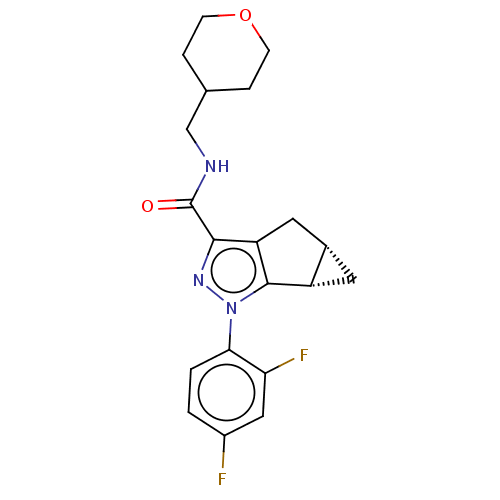 (CHEMBL3354966 | US11214548, Compound 408) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 76 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50040980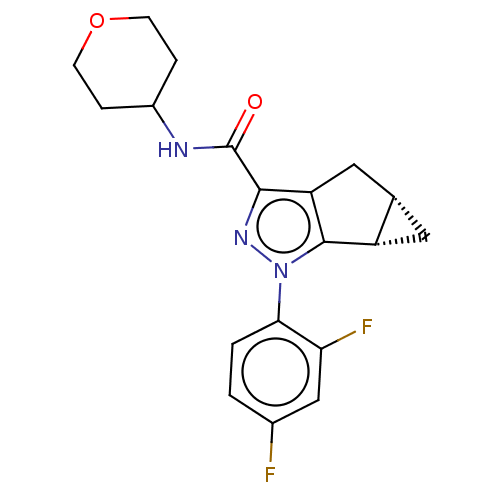 (CHEMBL3354965) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 39 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50040981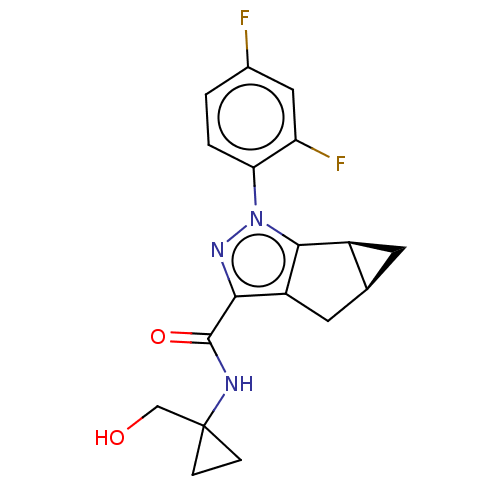 (CHEMBL3354964) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50040982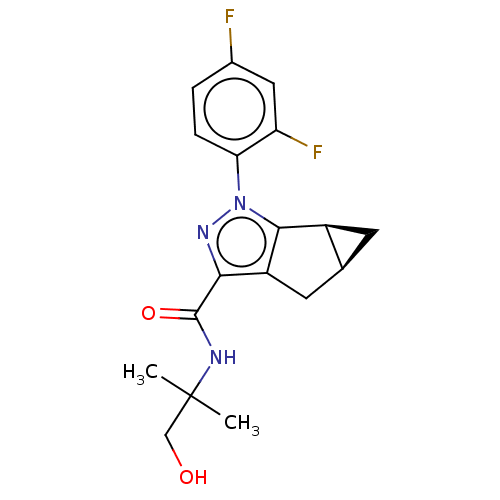 (CHEMBL3354963) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50040983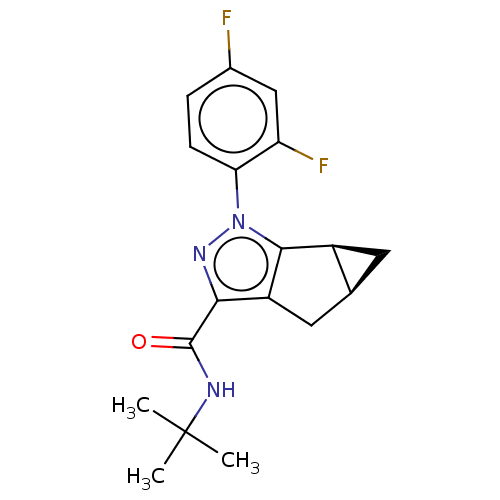 (CHEMBL3354962) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 359 total ) | Next | Last >> |