 Found 103 hits with Last Name = 'zhan' and Initial = 'zj'
Found 103 hits with Last Name = 'zhan' and Initial = 'zj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50288283
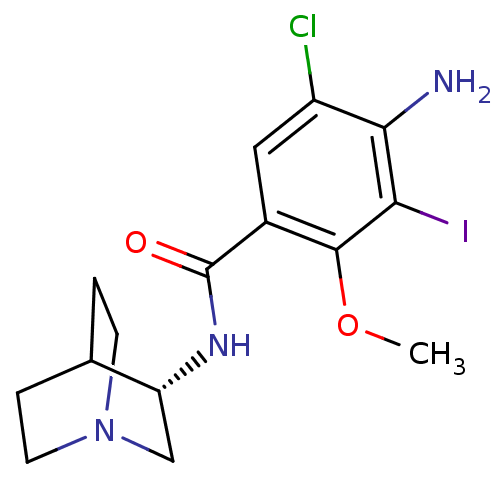
(4-Amino-N-(S)-1-aza-bicyclo[2.2.2]oct-3-yl-5-chlor...)Show SMILES COc1c(I)c(N)c(Cl)cc1C(=O)N[C@@H]1CN2CCC1CC2 |wU:14.14,(11.77,-27.01,;11.77,-25.48,;10.45,-24.71,;9.1,-25.48,;9.1,-27.01,;7.78,-24.71,;6.45,-25.48,;7.78,-23.17,;6.45,-22.4,;9.1,-22.4,;10.45,-23.17,;11.77,-22.4,;11.77,-20.86,;13.1,-23.17,;14.43,-22.4,;15.77,-23.18,;17.09,-22.42,;17.11,-20.88,;15.77,-20.1,;14.43,-20.86,;15.24,-22.25,;16.38,-21.1,)| Show InChI InChI=1S/C15H19ClIN3O2/c1-22-14-9(6-10(16)13(18)12(14)17)15(21)19-11-7-20-4-2-8(11)3-5-20/h6,8,11H,2-5,7,18H2,1H3,(H,19,21)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity towards 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 6: 2657-2662 (1996)
Article DOI: 10.1016/S0960-894X(96)00497-0
BindingDB Entry DOI: 10.7270/Q20V8CRP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50288283

(4-Amino-N-(S)-1-aza-bicyclo[2.2.2]oct-3-yl-5-chlor...)Show SMILES COc1c(I)c(N)c(Cl)cc1C(=O)N[C@@H]1CN2CCC1CC2 |wU:14.14,(11.77,-27.01,;11.77,-25.48,;10.45,-24.71,;9.1,-25.48,;9.1,-27.01,;7.78,-24.71,;6.45,-25.48,;7.78,-23.17,;6.45,-22.4,;9.1,-22.4,;10.45,-23.17,;11.77,-22.4,;11.77,-20.86,;13.1,-23.17,;14.43,-22.4,;15.77,-23.18,;17.09,-22.42,;17.11,-20.88,;15.77,-20.1,;14.43,-20.86,;15.24,-22.25,;16.38,-21.1,)| Show InChI InChI=1S/C15H19ClIN3O2/c1-22-14-9(6-10(16)13(18)12(14)17)15(21)19-11-7-20-4-2-8(11)3-5-20/h6,8,11H,2-5,7,18H2,1H3,(H,19,21)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for its antagonistic activity against 5-hydroxytryptamine 3 receptor in rat CNS. |
Bioorg Med Chem Lett 6: 2657-2662 (1996)
Article DOI: 10.1016/S0960-894X(96)00497-0
BindingDB Entry DOI: 10.7270/Q20V8CRP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50288283

(4-Amino-N-(S)-1-aza-bicyclo[2.2.2]oct-3-yl-5-chlor...)Show SMILES COc1c(I)c(N)c(Cl)cc1C(=O)N[C@@H]1CN2CCC1CC2 |wU:14.14,(11.77,-27.01,;11.77,-25.48,;10.45,-24.71,;9.1,-25.48,;9.1,-27.01,;7.78,-24.71,;6.45,-25.48,;7.78,-23.17,;6.45,-22.4,;9.1,-22.4,;10.45,-23.17,;11.77,-22.4,;11.77,-20.86,;13.1,-23.17,;14.43,-22.4,;15.77,-23.18,;17.09,-22.42,;17.11,-20.88,;15.77,-20.1,;14.43,-20.86,;15.24,-22.25,;16.38,-21.1,)| Show InChI InChI=1S/C15H19ClIN3O2/c1-22-14-9(6-10(16)13(18)12(14)17)15(21)19-11-7-20-4-2-8(11)3-5-20/h6,8,11H,2-5,7,18H2,1H3,(H,19,21)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity towards 5-hydroxytryptamine 3 receptor in whole rat brain using [125I]-DAIZAC as the radioligand. |
Bioorg Med Chem Lett 6: 2657-2662 (1996)
Article DOI: 10.1016/S0960-894X(96)00497-0
BindingDB Entry DOI: 10.7270/Q20V8CRP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50288284
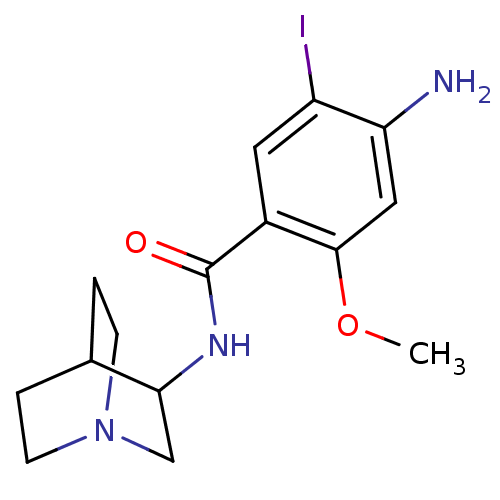
(4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl)-5-iodo-2-...)Show SMILES COc1cc(N)c(I)cc1C(=O)NC1CN2CCC1CC2 |(16.09,-14.57,;16.09,-13.02,;14.76,-12.26,;13.41,-13.02,;12.08,-12.26,;10.74,-13.02,;12.08,-10.71,;10.74,-9.94,;13.41,-9.94,;14.76,-10.71,;16.09,-9.94,;16.09,-8.4,;17.42,-10.71,;18.74,-9.94,;20.07,-10.71,;21.39,-9.95,;21.42,-8.42,;20.08,-7.64,;18.74,-8.41,;19.5,-9.73,;20.82,-8.97,)| Show InChI InChI=1S/C15H20IN3O2/c1-21-14-7-12(17)11(16)6-10(14)15(20)18-13-8-19-4-2-9(13)3-5-19/h6-7,9,13H,2-5,8,17H2,1H3,(H,18,20) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity towards 5-hydroxytryptamine 3 receptor in whole rat brain using (S)-[125I]-zacopride as the radioligand. |
Bioorg Med Chem Lett 6: 2657-2662 (1996)
Article DOI: 10.1016/S0960-894X(96)00497-0
BindingDB Entry DOI: 10.7270/Q20V8CRP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50288284

(4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl)-5-iodo-2-...)Show SMILES COc1cc(N)c(I)cc1C(=O)NC1CN2CCC1CC2 |(16.09,-14.57,;16.09,-13.02,;14.76,-12.26,;13.41,-13.02,;12.08,-12.26,;10.74,-13.02,;12.08,-10.71,;10.74,-9.94,;13.41,-9.94,;14.76,-10.71,;16.09,-9.94,;16.09,-8.4,;17.42,-10.71,;18.74,-9.94,;20.07,-10.71,;21.39,-9.95,;21.42,-8.42,;20.08,-7.64,;18.74,-8.41,;19.5,-9.73,;20.82,-8.97,)| Show InChI InChI=1S/C15H20IN3O2/c1-21-14-7-12(17)11(16)6-10(14)15(20)18-13-8-19-4-2-9(13)3-5-19/h6-7,9,13H,2-5,8,17H2,1H3,(H,18,20) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity towards 5-hydroxytryptamine 3 receptor in whole rat brain using [125I]-DAIZAC as the radioligand. |
Bioorg Med Chem Lett 6: 2657-2662 (1996)
Article DOI: 10.1016/S0960-894X(96)00497-0
BindingDB Entry DOI: 10.7270/Q20V8CRP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50342601

(CHEMBL1255901 | Huperzine A)Show SMILES C\C=C1/[C@@H]2Cc3[nH]c(=O)ccc3[C@]1(N)CC(C)=C2 |r,c:18,TLB:1:2:11.5.4:17.14.15| Show InChI InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as inhibition of acetylcholine hydrolysis after 30 mins by Ellmann's method |
J Nat Prod 77: 2054-9 (2014)
Article DOI: 10.1021/np500412f
BindingDB Entry DOI: 10.7270/Q2BC4147 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50313194
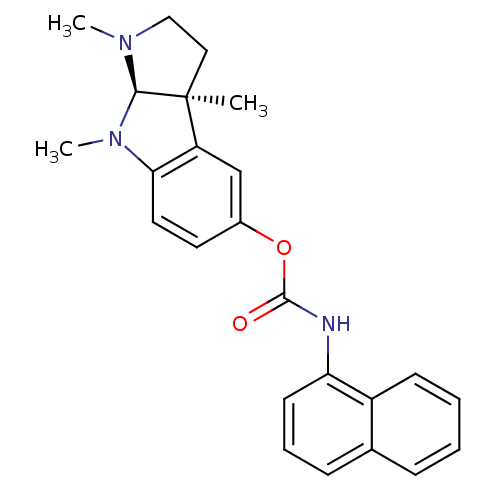
(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)Nc3cccc4ccccc34)cc21 |r| Show InChI InChI=1S/C24H25N3O2/c1-24-13-14-26(2)22(24)27(3)21-12-11-17(15-19(21)24)29-23(28)25-20-10-6-8-16-7-4-5-9-18(16)20/h4-12,15,22H,13-14H2,1-3H3,(H,25,28)/t22-,24+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Rattus norvegicus (rat)) | BDBM50014107
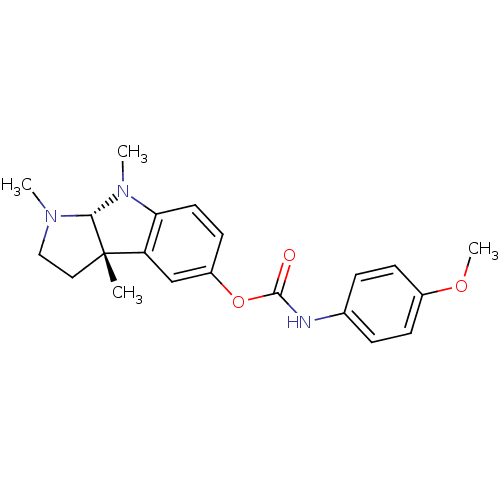
((-)-(4-Methoxy-phenyl)-carbamic acid 1,3a,8-trimet...)Show SMILES COc1ccc(NC(=O)Oc2ccc3N(C)[C@H]4N(C)CC[C@@]4(C)c3c2)cc1 Show InChI InChI=1S/C21H25N3O3/c1-21-11-12-23(2)19(21)24(3)18-10-9-16(13-17(18)21)27-20(25)22-14-5-7-15(26-4)8-6-14/h5-10,13,19H,11-12H2,1-4H3,(H,22,25)/t19-,21+/m1/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat plasma BChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50313190

(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)Nc3cccc(Cl)c3C)cc21 |r| Show InChI InChI=1S/C21H24ClN3O2/c1-13-16(22)6-5-7-17(13)23-20(26)27-14-8-9-18-15(12-14)21(2)10-11-24(3)19(21)25(18)4/h5-9,12,19H,10-11H2,1-4H3,(H,23,26)/t19-,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50313191
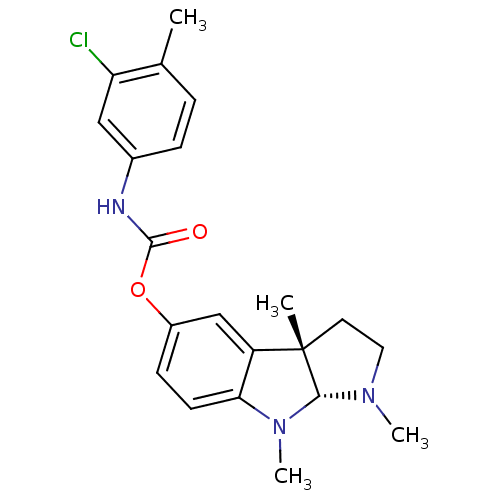
(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)Nc3ccc(C)c(Cl)c3)cc21 |r| Show InChI InChI=1S/C21H24ClN3O2/c1-13-5-6-14(11-17(13)22)23-20(26)27-15-7-8-18-16(12-15)21(2)9-10-24(3)19(21)25(18)4/h5-8,11-12,19H,9-10H2,1-4H3,(H,23,26)/t19-,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Rattus norvegicus (rat)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat plasma BChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Rattus norvegicus (rat)) | BDBM50313191

(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)Nc3ccc(C)c(Cl)c3)cc21 |r| Show InChI InChI=1S/C21H24ClN3O2/c1-13-5-6-14(11-17(13)22)23-20(26)27-15-7-8-18-16(12-15)21(2)9-10-24(3)19(21)25(18)4/h5-8,11-12,19H,9-10H2,1-4H3,(H,23,26)/t19-,21+/m1/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat plasma BChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50313195
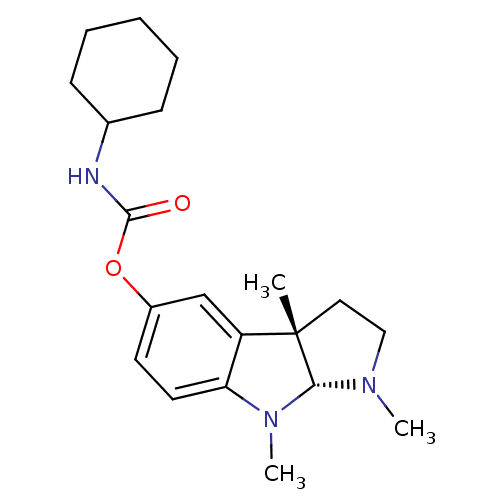
(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)NC3CCCCC3)cc21 |r| Show InChI InChI=1S/C20H29N3O2/c1-20-11-12-22(2)18(20)23(3)17-10-9-15(13-16(17)20)25-19(24)21-14-7-5-4-6-8-14/h9-10,13-14,18H,4-8,11-12H2,1-3H3,(H,21,24)/t18-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM10958
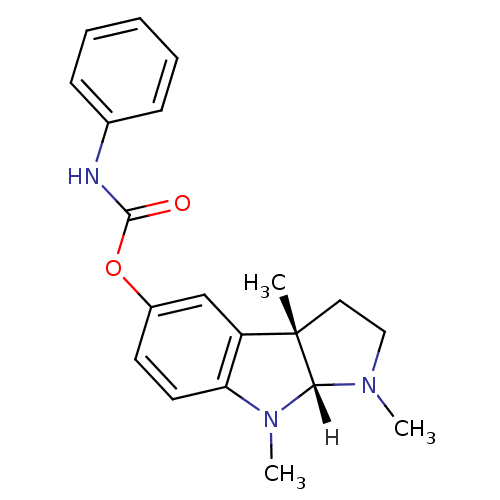
((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)Nc3ccccc3)ccc1N2C |r| Show InChI InChI=1S/C20H23N3O2/c1-20-11-12-22(2)18(20)23(3)17-10-9-15(13-16(17)20)25-19(24)21-14-7-5-4-6-8-14/h4-10,13,18H,11-12H2,1-3H3,(H,21,24)/t18-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Rattus norvegicus (rat)) | BDBM50313194

(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)Nc3cccc4ccccc34)cc21 |r| Show InChI InChI=1S/C24H25N3O2/c1-24-13-14-26(2)22(24)27(3)21-12-11-17(15-19(21)24)29-23(28)25-20-10-6-8-16-7-4-5-9-18(16)20/h4-12,15,22H,13-14H2,1-3H3,(H,25,28)/t22-,24+/m1/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat plasma BChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Rattus norvegicus (rat)) | BDBM50313184
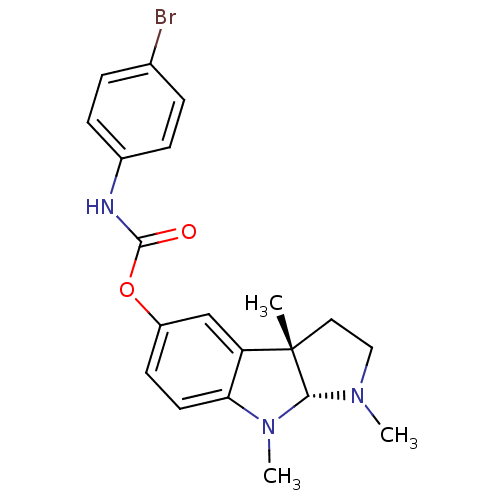
(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)Nc3ccc(Br)cc3)cc21 |r| Show InChI InChI=1S/C20H22BrN3O2/c1-20-10-11-23(2)18(20)24(3)17-9-8-15(12-16(17)20)26-19(25)22-14-6-4-13(21)5-7-14/h4-9,12,18H,10-11H2,1-3H3,(H,22,25)/t18-,20+/m1/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat plasma BChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50014107

((-)-(4-Methoxy-phenyl)-carbamic acid 1,3a,8-trimet...)Show SMILES COc1ccc(NC(=O)Oc2ccc3N(C)[C@H]4N(C)CC[C@@]4(C)c3c2)cc1 Show InChI InChI=1S/C21H25N3O3/c1-21-11-12-23(2)19(21)24(3)18-10-9-16(13-17(18)21)27-20(25)22-14-5-7-15(26-4)8-6-14/h5-10,13,19H,11-12H2,1-4H3,(H,22,25)/t19-,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50313185

(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)Nc3cccc(Cl)c3)cc21 |r| Show InChI InChI=1S/C20H22ClN3O2/c1-20-9-10-23(2)18(20)24(3)17-8-7-15(12-16(17)20)26-19(25)22-14-6-4-5-13(21)11-14/h4-8,11-12,18H,9-10H2,1-3H3,(H,22,25)/t18-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Rattus norvegicus (rat)) | BDBM50313188
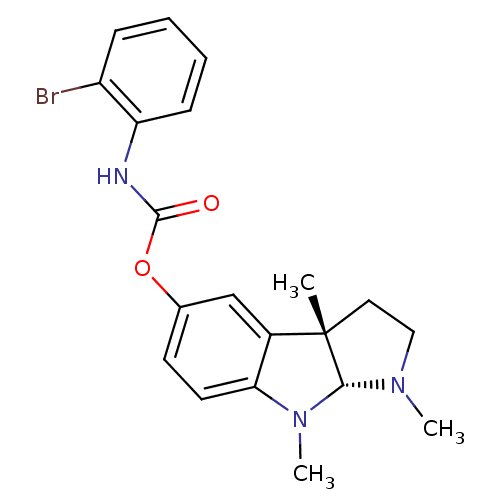
(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)Nc3ccccc3Br)cc21 |r| Show InChI InChI=1S/C20H22BrN3O2/c1-20-10-11-23(2)18(20)24(3)17-9-8-13(12-14(17)20)26-19(25)22-16-7-5-4-6-15(16)21/h4-9,12,18H,10-11H2,1-3H3,(H,22,25)/t18-,20+/m1/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat plasma BChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50027327
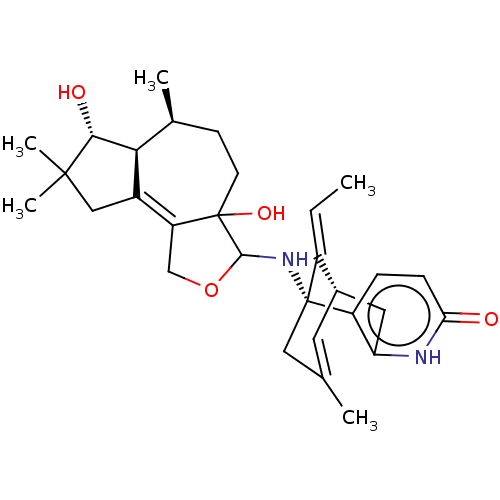
(CHEMBL3338389)Show SMILES [H][C@@]12[C@@H](O)C(C)(C)CC1=C1COC(N[C@@]34CC(C)=C[C@@]([H])(Cc5[nH]c(=O)ccc35)\C4=C/C)C1(O)CC[C@@H]2C |r,c:18,t:9,TLB:30:29:21.22.28:18.16.15| Show InChI InChI=1S/C30H40N2O4/c1-6-20-18-11-16(2)13-29(20,21-7-8-24(33)31-23(21)12-18)32-27-30(35)10-9-17(3)25-19(22(30)15-36-27)14-28(4,5)26(25)34/h6-8,11,17-18,25-27,32,34-35H,9-10,12-15H2,1-5H3,(H,31,33)/b20-6+/t17-,18-,25-,26+,27?,29+,30?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as inhibition of acetylcholine hydrolysis after 30 mins by Ellmann's method |
J Nat Prod 77: 2054-9 (2014)
Article DOI: 10.1021/np500412f
BindingDB Entry DOI: 10.7270/Q2BC4147 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Rattus norvegicus (rat)) | BDBM50313183

(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)Nc3ccc(Cl)cc3)cc21 |r| Show InChI InChI=1S/C20H22ClN3O2/c1-20-10-11-23(2)18(20)24(3)17-9-8-15(12-16(17)20)26-19(25)22-14-6-4-13(21)5-7-14/h4-9,12,18H,10-11H2,1-3H3,(H,22,25)/t18-,20+/m1/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat plasma BChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50313189
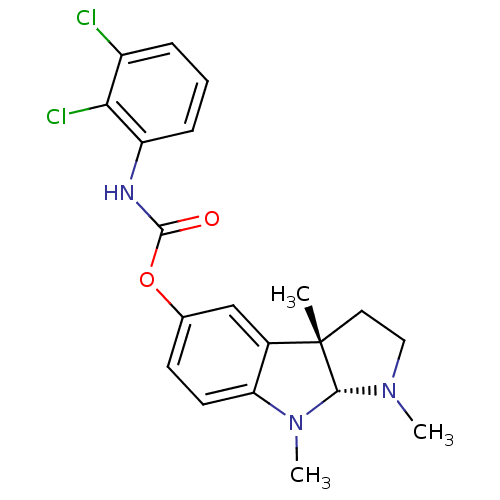
(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)Nc3cccc(Cl)c3Cl)cc21 |r| Show InChI InChI=1S/C20H21Cl2N3O2/c1-20-9-10-24(2)18(20)25(3)16-8-7-12(11-13(16)20)27-19(26)23-15-6-4-5-14(21)17(15)22/h4-8,11,18H,9-10H2,1-3H3,(H,23,26)/t18-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50313188

(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)Nc3ccccc3Br)cc21 |r| Show InChI InChI=1S/C20H22BrN3O2/c1-20-10-11-23(2)18(20)24(3)17-9-8-13(12-14(17)20)26-19(25)22-16-7-5-4-6-15(16)21/h4-9,12,18H,10-11H2,1-3H3,(H,22,25)/t18-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Rattus norvegicus (rat)) | BDBM50313187

(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)Nc3ccccc3Cl)cc21 |r| Show InChI InChI=1S/C20H22ClN3O2/c1-20-10-11-23(2)18(20)24(3)17-9-8-13(12-14(17)20)26-19(25)22-16-7-5-4-6-15(16)21/h4-9,12,18H,10-11H2,1-3H3,(H,22,25)/t18-,20+/m1/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 88.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat plasma BChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Rattus norvegicus (rat)) | BDBM10958

((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)Nc3ccccc3)ccc1N2C |r| Show InChI InChI=1S/C20H23N3O2/c1-20-11-12-22(2)18(20)23(3)17-10-9-15(13-16(17)20)25-19(24)21-14-7-5-4-6-8-14/h4-10,13,18H,11-12H2,1-3H3,(H,21,24)/t18-,20+/m1/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat plasma BChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Rattus norvegicus (rat)) | BDBM50313185

(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)Nc3cccc(Cl)c3)cc21 |r| Show InChI InChI=1S/C20H22ClN3O2/c1-20-9-10-23(2)18(20)24(3)17-8-7-15(12-16(17)20)26-19(25)22-14-6-4-5-13(21)11-14/h4-8,11-12,18H,9-10H2,1-3H3,(H,22,25)/t18-,20+/m1/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat plasma BChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50027339
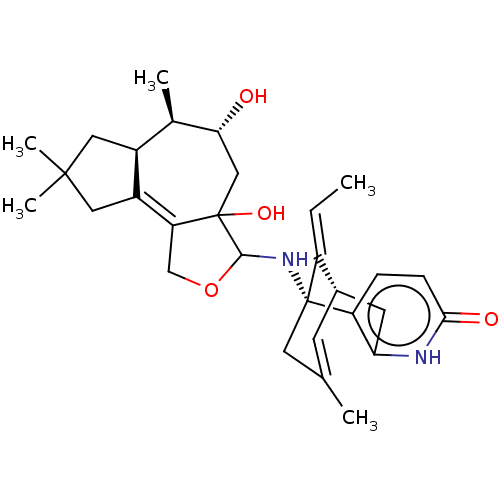
(CHEMBL3338388)Show SMILES [H][C@@]12CC(C)(C)CC1=C1COC(N[C@@]34CC(C)=C[C@@]([H])(Cc5[nH]c(=O)ccc35)\C4=C/C)C1(O)C[C@@H](O)[C@@H]2C |r,c:17,t:8,TLB:29:28:20.21.27:17.15.14| Show InChI InChI=1S/C30H40N2O4/c1-6-21-18-9-16(2)11-29(21,22-7-8-26(34)31-24(22)10-18)32-27-30(35)14-25(33)17(3)19-12-28(4,5)13-20(19)23(30)15-36-27/h6-9,17-19,25,27,32-33,35H,10-15H2,1-5H3,(H,31,34)/b21-6+/t17-,18+,19+,25-,27?,29-,30?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as inhibition of acetylcholine hydrolysis after 30 mins by Ellmann's method |
J Nat Prod 77: 2054-9 (2014)
Article DOI: 10.1021/np500412f
BindingDB Entry DOI: 10.7270/Q2BC4147 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50313192
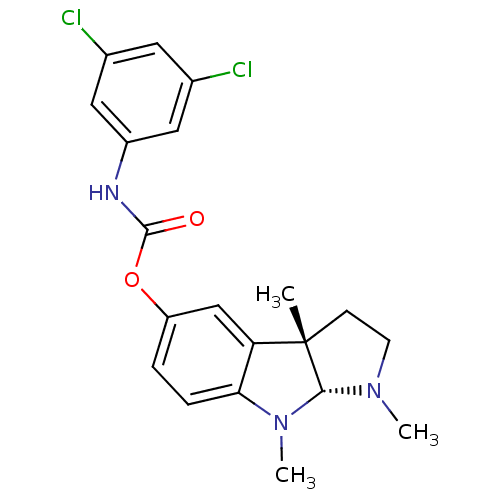
(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)Nc3cc(Cl)cc(Cl)c3)cc21 |r| Show InChI InChI=1S/C20H21Cl2N3O2/c1-20-6-7-24(2)18(20)25(3)17-5-4-15(11-16(17)20)27-19(26)23-14-9-12(21)8-13(22)10-14/h4-5,8-11,18H,6-7H2,1-3H3,(H,23,26)/t18-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Rattus norvegicus (rat)) | BDBM50313190

(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)Nc3cccc(Cl)c3C)cc21 |r| Show InChI InChI=1S/C21H24ClN3O2/c1-13-16(22)6-5-7-17(13)23-20(26)27-14-8-9-18-15(12-14)21(2)10-11-24(3)19(21)25(18)4/h5-9,12,19H,10-11H2,1-4H3,(H,23,26)/t19-,21+/m1/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat plasma BChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50313184

(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)Nc3ccc(Br)cc3)cc21 |r| Show InChI InChI=1S/C20H22BrN3O2/c1-20-10-11-23(2)18(20)24(3)17-9-8-15(12-16(17)20)26-19(25)22-14-6-4-13(21)5-7-14/h4-9,12,18H,10-11H2,1-3H3,(H,22,25)/t18-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 168 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50313187

(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)Nc3ccccc3Cl)cc21 |r| Show InChI InChI=1S/C20H22ClN3O2/c1-20-10-11-23(2)18(20)24(3)17-9-8-13(12-14(17)20)26-19(25)22-16-7-5-4-6-15(16)21/h4-9,12,18H,10-11H2,1-3H3,(H,22,25)/t18-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 264 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50313186
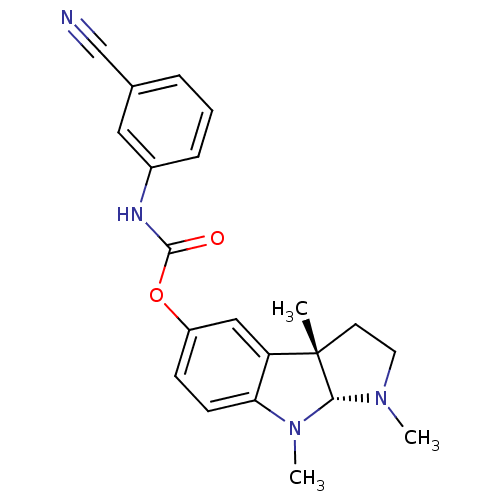
(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)Nc3cccc(c3)C#N)cc21 |r| Show InChI InChI=1S/C21H22N4O2/c1-21-9-10-24(2)19(21)25(3)18-8-7-16(12-17(18)21)27-20(26)23-15-6-4-5-14(11-15)13-22/h4-8,11-12,19H,9-10H2,1-3H3,(H,23,26)/t19-,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 442 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Rattus norvegicus (rat)) | BDBM50313189

(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)Nc3cccc(Cl)c3Cl)cc21 |r| Show InChI InChI=1S/C20H21Cl2N3O2/c1-20-9-10-24(2)18(20)25(3)16-8-7-12(11-13(16)20)27-19(26)23-15-6-4-5-14(21)17(15)22/h4-8,11,18H,9-10H2,1-3H3,(H,23,26)/t18-,20+/m1/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 517 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat plasma BChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50313193
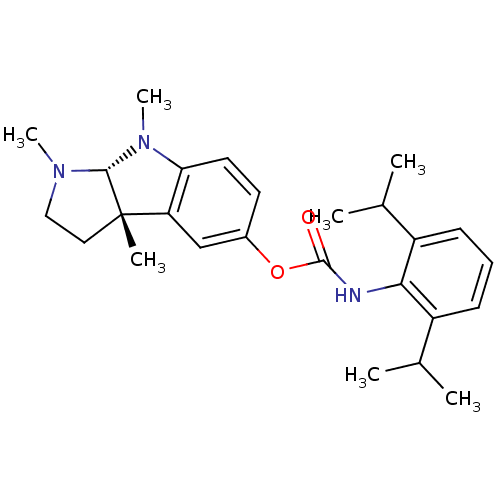
(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CC(C)c1cccc(C(C)C)c1NC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C26H35N3O2/c1-16(2)19-9-8-10-20(17(3)4)23(19)27-25(30)31-18-11-12-22-21(15-18)26(5)13-14-28(6)24(26)29(22)7/h8-12,15-17,24H,13-14H2,1-7H3,(H,27,30)/t24-,26+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 735 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Rattus norvegicus (rat)) | BDBM50313193

(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CC(C)c1cccc(C(C)C)c1NC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C26H35N3O2/c1-16(2)19-9-8-10-20(17(3)4)23(19)27-25(30)31-18-11-12-22-21(15-18)26(5)13-14-28(6)24(26)29(22)7/h8-12,15-17,24H,13-14H2,1-7H3,(H,27,30)/t24-,26+/m1/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat plasma BChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50313183

(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)Nc3ccc(Cl)cc3)cc21 |r| Show InChI InChI=1S/C20H22ClN3O2/c1-20-10-11-23(2)18(20)24(3)17-9-8-15(12-16(17)20)26-19(25)22-14-6-4-13(21)5-7-14/h4-9,12,18H,10-11H2,1-3H3,(H,22,25)/t18-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 914 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat brain AChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Rattus norvegicus (rat)) | BDBM50313192

(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)Nc3cc(Cl)cc(Cl)c3)cc21 |r| Show InChI InChI=1S/C20H21Cl2N3O2/c1-20-6-7-24(2)18(20)25(3)17-5-4-15(11-16(17)20)27-19(26)23-14-9-12(21)8-13(22)10-14/h4-5,8-11,18H,6-7H2,1-3H3,(H,23,26)/t18-,20+/m1/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 935 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat plasma BChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50027368
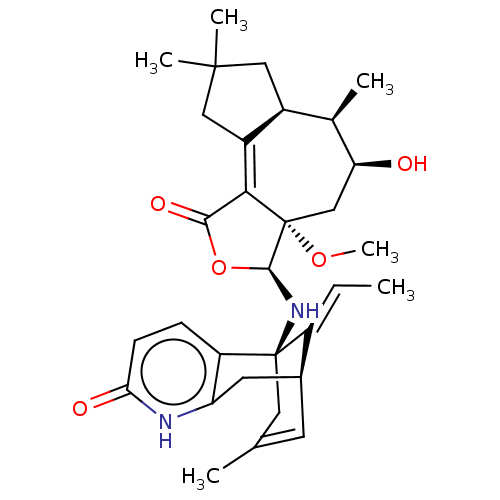
(CHEMBL3338386)Show SMILES [H][C@@]12CC(C)(C)CC1=C1C(=O)O[C@]([H])(N[C@@]34CC(C)=C[C@@]([H])(Cc5[nH]c(=O)ccc35)\C4=C/C)[C@@]1(C[C@H](O)[C@@H]2C)OC |r,c:19,t:8,TLB:31:30:22.23.29:19.17.16| Show InChI InChI=1S/C31H40N2O5/c1-7-21-18-10-16(2)12-30(21,22-8-9-25(35)32-23(22)11-18)33-28-31(37-6)15-24(34)17(3)19-13-29(4,5)14-20(19)26(31)27(36)38-28/h7-10,17-19,24,28,33-34H,11-15H2,1-6H3,(H,32,35)/b21-7+/t17-,18+,19+,24+,28+,30-,31+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as inhibition of acetylcholine hydrolysis after 30 mins by Ellmann's method |
J Nat Prod 77: 2054-9 (2014)
Article DOI: 10.1021/np500412f
BindingDB Entry DOI: 10.7270/Q2BC4147 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Rattus norvegicus (rat)) | BDBM50313186

(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)Nc3cccc(c3)C#N)cc21 |r| Show InChI InChI=1S/C21H22N4O2/c1-21-9-10-24(2)19(21)25(3)18-8-7-16(12-17(18)21)27-20(26)23-15-6-4-5-14(11-15)13-22/h4-8,11-12,19H,9-10H2,1-3H3,(H,23,26)/t19-,21+/m1/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat plasma BChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Rattus norvegicus (rat)) | BDBM50313195

(1,3alpha,8-trimethyl-1,2,3,3alpha,8,8alpha-hexahyd...)Show SMILES CN1CC[C@]2(C)[C@H]1N(C)c1ccc(OC(=O)NC3CCCCC3)cc21 |r| Show InChI InChI=1S/C20H29N3O2/c1-20-11-12-22(2)18(20)23(3)17-10-9-15(13-16(17)20)25-19(24)21-14-7-5-4-6-8-14/h9-10,13-14,18H,4-8,11-12H2,1-3H3,(H,21,24)/t18-,20+/m1/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of rat plasma BChE by Ellman's method |
Bioorg Med Chem Lett 20: 1532-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.097
BindingDB Entry DOI: 10.7270/Q29K4BCV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50027367
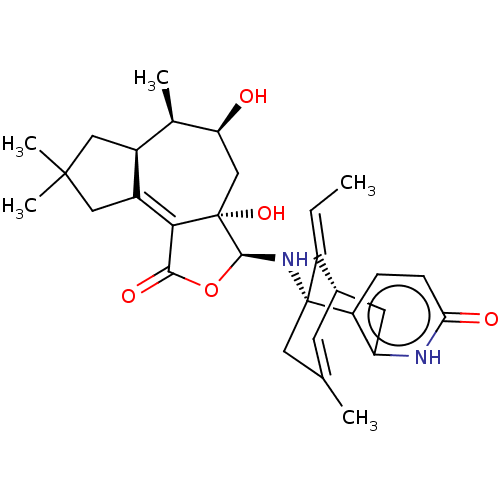
(CHEMBL3338387)Show SMILES [H][C@@]12CC(C)(C)CC1=C1C(=O)O[C@]([H])(N[C@@]34CC(C)=C[C@@]([H])(Cc5[nH]c(=O)ccc35)\C4=C/C)[C@]1(O)C[C@H](O)[C@@H]2C |r,c:19,t:8,TLB:31:30:22.23.29:19.17.16| Show InChI InChI=1S/C30H38N2O5/c1-6-20-17-9-15(2)11-29(20,21-7-8-24(34)31-22(21)10-17)32-27-30(36)14-23(33)16(3)18-12-28(4,5)13-19(18)25(30)26(35)37-27/h6-9,16-18,23,27,32-33,36H,10-14H2,1-5H3,(H,31,34)/b20-6+/t16-,17+,18+,23+,27+,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as inhibition of acetylcholine hydrolysis after 30 mins by Ellmann's method |
J Nat Prod 77: 2054-9 (2014)
Article DOI: 10.1021/np500412f
BindingDB Entry DOI: 10.7270/Q2BC4147 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem Lett 20: 6185-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.123
BindingDB Entry DOI: 10.7270/Q28053KV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50329104

(CHEMBL182120 | Indole alkaloid derivative | voacan...)Show SMILES CC[C@H]1C[C@@H]2CN3CCc4c([nH]c5ccc(OC)cc45)[C@](C2)([C@H]13)C(=O)OC |r,THB:2:22:10.9.8.7:21.4.5,1:2:20.21:6.5| Show InChI InChI=1S/C22H28N2O3/c1-4-14-9-13-11-22(21(25)27-3)19-16(7-8-24(12-13)20(14)22)17-10-15(26-2)5-6-18(17)23-19/h5-6,10,13-14,20,23H,4,7-9,11-12H2,1-3H3/t13-,14-,20-,22+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem Lett 20: 6185-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.123
BindingDB Entry DOI: 10.7270/Q28053KV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50329102

(CHEMBL364613 | Indole alkaloid derivative | corona...)Show SMILES CC[C@H]1C[C@@H]2CN3CCc4c([nH]c5ccccc45)[C@](C2)([C@H]13)C(=O)OC |r,TLB:1:2:19.18:6.5,21:18:2.3:6.5,THB:7:6:2.3:19.18,10:18:2.3:6.5| Show InChI InChI=1S/C21H26N2O2/c1-3-14-10-13-11-21(20(24)25-2)18-16(8-9-23(12-13)19(14)21)15-6-4-5-7-17(15)22-18/h4-7,13-14,19,22H,3,8-12H2,1-2H3/t13-,14-,19-,21+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem Lett 20: 6185-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.123
BindingDB Entry DOI: 10.7270/Q28053KV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
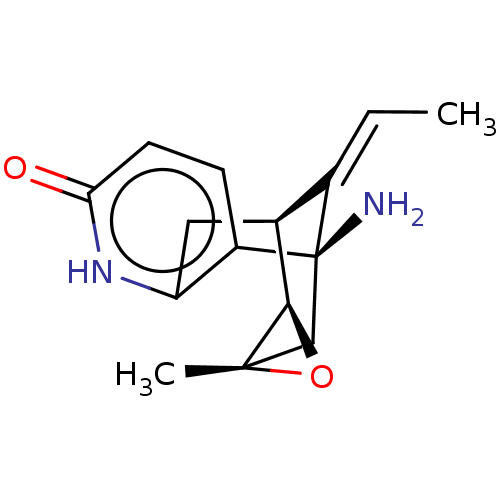
(Homo sapiens (Human)) | BDBM50027324
(CHEMBL3355594)Show SMILES [H][C@]12O[C@@]1(C)C[C@@]1(N)\C(=C/C)[C@]2([H])Cc2[nH]c(=O)ccc12 |r,TLB:9:8:13.20.14:5.3.1| Show InChI InChI=1S/C15H18N2O2/c1-3-9-8-6-11-10(4-5-12(18)17-11)15(9,16)7-14(2)13(8)19-14/h3-5,8,13H,6-7,16H2,1-2H3,(H,17,18)/b9-3-/t8-,13+,14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as inhibition of acetylcholine hydrolysis after 30 mins by Ellmann's method |
J Nat Prod 77: 2054-9 (2014)
Article DOI: 10.1021/np500412f
BindingDB Entry DOI: 10.7270/Q2BC4147 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
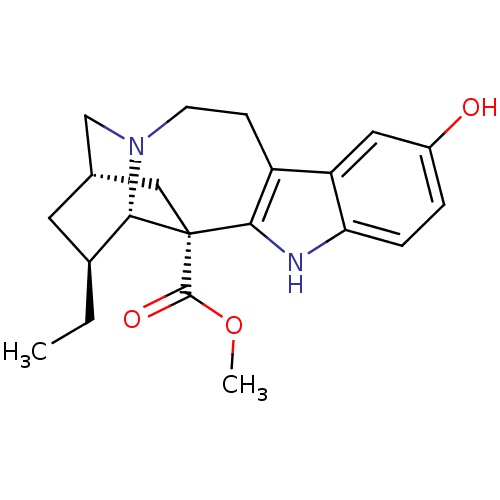
(Electrophorus electricus (Electric eel)) | BDBM50329103
(10-Hydroxycoronaridine | CHEMBL1271293)Show SMILES CC[C@H]1C[C@@H]2CN3CCc4c([nH]c5ccc(O)cc45)[C@](C2)([C@H]13)C(=O)OC |r,TLB:22:19:3.2:5.6,1:2:20.19:5.6,THB:7:6:3.2:20.19,10:19:3.2:5.6| Show InChI InChI=1S/C21H26N2O3/c1-3-13-8-12-10-21(20(25)26-2)18-15(6-7-23(11-12)19(13)21)16-9-14(24)4-5-17(16)22-18/h4-5,9,12-13,19,22,24H,3,6-8,10-11H2,1-2H3/t12-,13-,19-,21+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem Lett 20: 6185-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.123
BindingDB Entry DOI: 10.7270/Q28053KV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50329105

(19(S)-Heyneanine | CHEMBL463317 | voacristine)Show SMILES COC(=O)[C@@]12C[C@@H]3C[C@H]([C@H](C)O)[C@@H]1N(C3)CCc1c2[nH]c2ccc(OC)cc12 |r,TLB:8:12:18.17.16.15:5.6.14,9:8:4.5:13.14| Show InChI InChI=1S/C22H28N2O4/c1-12(25)16-8-13-10-22(21(26)28-3)19-15(6-7-24(11-13)20(16)22)17-9-14(27-2)4-5-18(17)23-19/h4-5,9,12-13,16,20,23,25H,6-8,10-11H2,1-3H3/t12-,13-,16+,20-,22+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem Lett 20: 6185-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.123
BindingDB Entry DOI: 10.7270/Q28053KV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
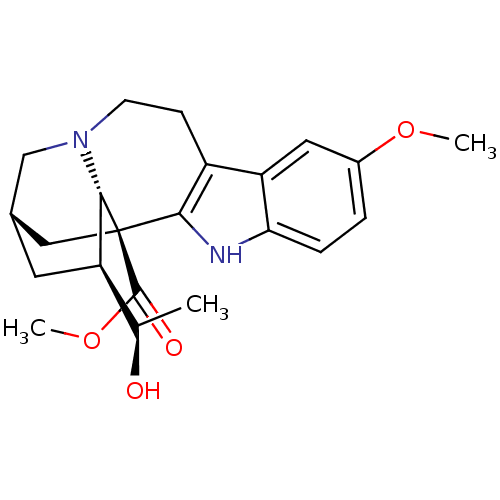
(Electrophorus electricus (Electric eel)) | BDBM50329106
(19(R)-Heyneanine | 19-epi-voacristine | CHEMBL5126...)Show SMILES COC(=O)[C@@]12C[C@@H]3C[C@H]([C@@H](C)O)[C@@H]1N(C3)CCc1c2[nH]c2ccc(OC)cc12 |r,TLB:8:12:18.17.16.15:5.6.14,9:8:4.5:13.14| Show InChI InChI=1S/C22H28N2O4/c1-12(25)16-8-13-10-22(21(26)28-3)19-15(6-7-24(11-13)20(16)22)17-9-14(27-2)4-5-18(17)23-19/h4-5,9,12-13,16,20,23,25H,6-8,10-11H2,1-3H3/t12-,13+,16-,20+,22-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem Lett 20: 6185-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.123
BindingDB Entry DOI: 10.7270/Q28053KV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50378856
(VOBASINE)Show SMILES COC(=O)[C@@H]1[C@@H]2Cc3c([nH]c4ccccc34)C(=O)C[C@@H]1C(CN2C)=CC |r,w:24.28| Show InChI InChI=1S/C21H24N2O3/c1-4-12-11-23(2)17-9-15-13-7-5-6-8-16(13)22-20(15)18(24)10-14(12)19(17)21(25)26-3/h4-8,14,17,19,22H,9-11H2,1-3H3/t14-,17+,19+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem Lett 20: 6185-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.123
BindingDB Entry DOI: 10.7270/Q28053KV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data