 Found 220 hits with Last Name = 'zhao' and Initial = 'yl'
Found 220 hits with Last Name = 'zhao' and Initial = 'yl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM25121

(4-{[(7R)-8-cyclopentyl-7-ethyl-5-methyl-6-oxo-5,6,...)Show SMILES CC[C@H]1N(C2CCCC2)c2nc(Nc3ccc(cc3OC)C(=O)NC3CCN(C)CC3)ncc2N(C)C1=O |r| Show InChI InChI=1S/C28H39N7O3/c1-5-22-27(37)34(3)23-17-29-28(32-25(23)35(22)20-8-6-7-9-20)31-21-11-10-18(16-24(21)38-4)26(36)30-19-12-14-33(2)15-13-19/h10-11,16-17,19-20,22H,5-9,12-15H2,1-4H3,(H,30,36)(H,29,31,32)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 (unknown origin) |
Bioorg Med Chem Lett 18: 4972-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.033
BindingDB Entry DOI: 10.7270/Q26973DV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Yes |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086454
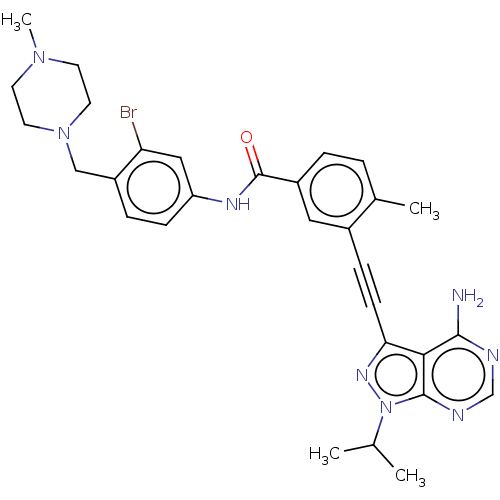
(CHEMBL3425518)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(Br)c2)c2c(N)ncnc12 Show InChI InChI=1S/C30H33BrN8O/c1-19(2)39-29-27(28(32)33-18-34-29)26(36-39)10-8-21-15-22(6-5-20(21)3)30(40)35-24-9-7-23(25(31)16-24)17-38-13-11-37(4)12-14-38/h5-7,9,15-16,18-19H,11-14,17H2,1-4H3,(H,35,40)(H2,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM418817

(N-methyl-4-({4-[({3- [methyl(methylsulfonyl)amino]...)Show SMILES CNC(=O)c1ccc(Nc2ncc(c(NCc3nccnc3N(C)S(C)(=O)=O)n2)C(F)(F)F)cc1 Show InChI InChI=1S/C20H21F3N8O3S/c1-24-18(32)12-4-6-13(7-5-12)29-19-28-10-14(20(21,22)23)16(30-19)27-11-15-17(26-9-8-25-15)31(2)35(3,33)34/h4-10H,11H2,1-3H3,(H,24,32)(H2,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAK (unknown origin) incubated for 40 mins by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113573
BindingDB Entry DOI: 10.7270/Q2125XFH |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50534666
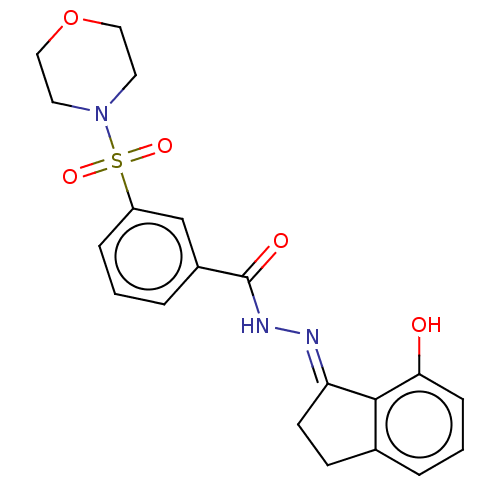
(CHEMBL4436953)Show SMILES Oc1cccc2CC\C(=N/NC(=O)c3cccc(c3)S(=O)(=O)N3CCOCC3)c12 Show InChI InChI=1S/C20H21N3O5S/c24-18-6-2-3-14-7-8-17(19(14)18)21-22-20(25)15-4-1-5-16(13-15)29(26,27)23-9-11-28-12-10-23/h1-6,13,24H,7-12H2,(H,22,25)/b21-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 (unknown origin) |
Bioorg Med Chem Lett 26: 4552-4557 (2016)
Article DOI: 10.1016/j.bmcl.2015.06.054
BindingDB Entry DOI: 10.7270/Q2ZS3117 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50534710

(CHEMBL4454677)Show SMILES Oc1ccc(Cl)c2CC\C(=N/NC(=O)c3cccc(c3)S(=O)(=O)N3CCOCC3)c12 Show InChI InChI=1S/C20H20ClN3O5S/c21-16-5-7-18(25)19-15(16)4-6-17(19)22-23-20(26)13-2-1-3-14(12-13)30(27,28)24-8-10-29-11-9-24/h1-3,5,7,12,25H,4,6,8-11H2,(H,23,26)/b22-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 (unknown origin) |
Bioorg Med Chem Lett 26: 4552-4557 (2016)
Article DOI: 10.1016/j.bmcl.2015.06.054
BindingDB Entry DOI: 10.7270/Q2ZS3117 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50571721
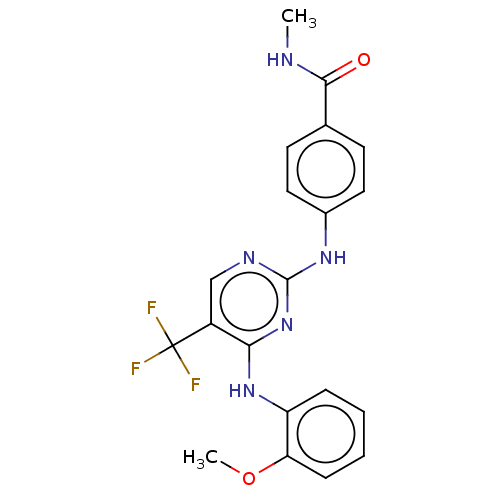
(CHEMBL4874046)Show SMILES CNC(=O)c1ccc(Nc2ncc(c(Nc3ccccc3OC)n2)C(F)(F)F)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAK (unknown origin) incubated for 40 mins by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113573
BindingDB Entry DOI: 10.7270/Q2125XFH |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086457
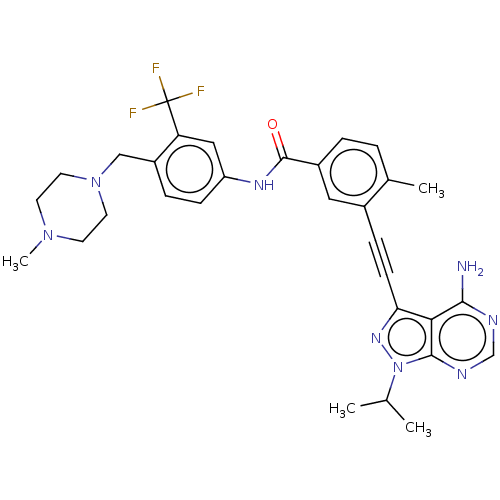
(CHEMBL3426217)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C31H33F3N8O/c1-19(2)42-29-27(28(35)36-18-37-29)26(39-42)10-8-21-15-22(6-5-20(21)3)30(43)38-24-9-7-23(25(16-24)31(32,33)34)17-41-13-11-40(4)12-14-41/h5-7,9,15-16,18-19H,11-14,17H2,1-4H3,(H,38,43)(H2,35,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086455
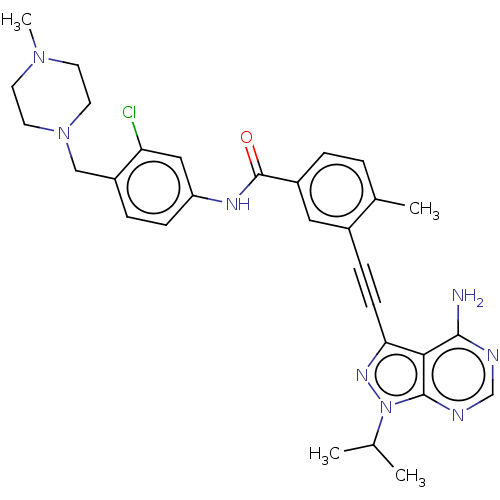
(CHEMBL3426219)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(Cl)c2)c2c(N)ncnc12 Show InChI InChI=1S/C30H33ClN8O/c1-19(2)39-29-27(28(32)33-18-34-29)26(36-39)10-8-21-15-22(6-5-20(21)3)30(40)35-24-9-7-23(25(31)16-24)17-38-13-11-37(4)12-14-38/h5-7,9,15-16,18-19H,11-14,17H2,1-4H3,(H,35,40)(H2,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086451
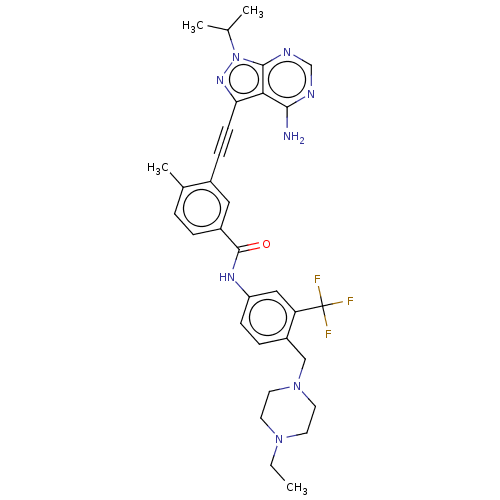
(CHEMBL3426222)Show SMILES CCN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn(C(C)C)c4ncnc(N)c34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C32H35F3N8O/c1-5-41-12-14-42(15-13-41)18-24-8-10-25(17-26(24)32(33,34)35)39-31(44)23-7-6-21(4)22(16-23)9-11-27-28-29(36)37-19-38-30(28)43(40-27)20(2)3/h6-8,10,16-17,19-20H,5,12-15,18H2,1-4H3,(H,39,44)(H2,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50534708
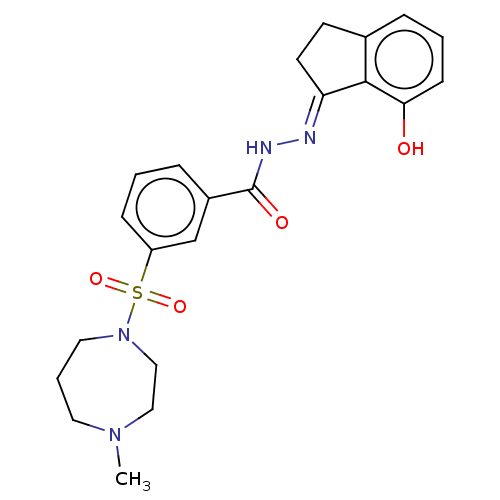
(CHEMBL4549707)Show SMILES CN1CCCN(CC1)S(=O)(=O)c1cccc(c1)C(=O)N\N=C1/CCc2cccc(O)c12 Show InChI InChI=1S/C22H26N4O4S/c1-25-11-4-12-26(14-13-25)31(29,30)18-7-2-6-17(15-18)22(28)24-23-19-10-9-16-5-3-8-20(27)21(16)19/h2-3,5-8,15,27H,4,9-14H2,1H3,(H,24,28)/b23-19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 (unknown origin) |
Bioorg Med Chem Lett 26: 4552-4557 (2016)
Article DOI: 10.1016/j.bmcl.2015.06.054
BindingDB Entry DOI: 10.7270/Q2ZS3117 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50571706
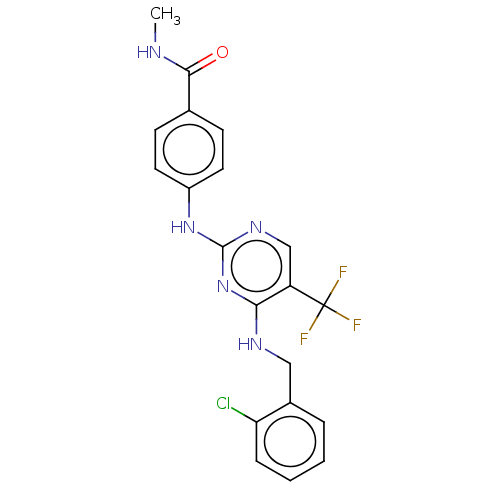
(CHEMBL4850588)Show SMILES CNC(=O)c1ccc(Nc2ncc(c(NCc3ccccc3Cl)n2)C(F)(F)F)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAK (unknown origin) incubated for 40 mins by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113573
BindingDB Entry DOI: 10.7270/Q2125XFH |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086453

(CHEMBL3426220 | US10266537, Compound 14)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2cccc(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C25H21F3N6O/c1-14(2)34-23-21(22(29)30-13-31-23)20(33-34)10-9-16-11-17(8-7-15(16)3)24(35)32-19-6-4-5-18(12-19)25(26,27)28/h4-8,11-14H,1-3H3,(H,32,35)(H2,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50571710
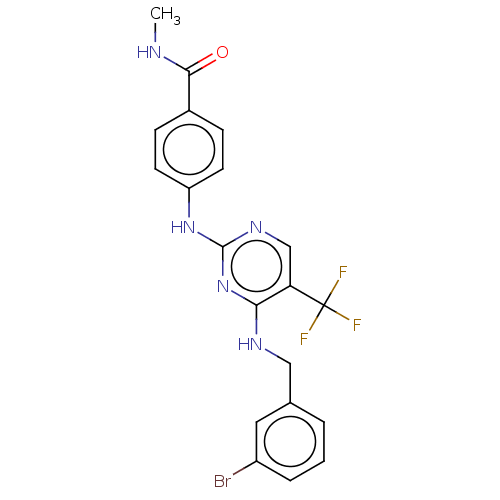
(CHEMBL4872224)Show SMILES CNC(=O)c1ccc(Nc2ncc(c(NCc3cccc(Br)c3)n2)C(F)(F)F)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAK (unknown origin) incubated for 40 mins by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113573
BindingDB Entry DOI: 10.7270/Q2125XFH |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086450
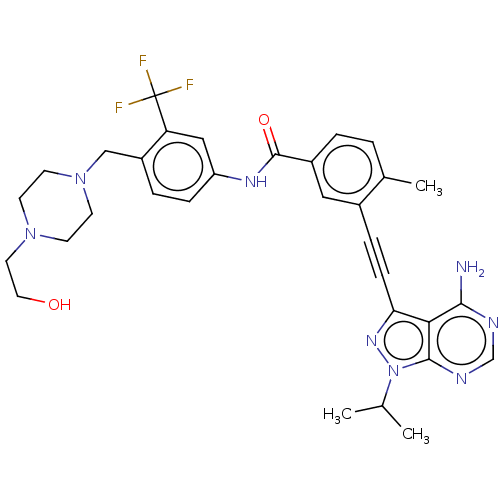
(CHEMBL3426223)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C32H35F3N8O2/c1-20(2)43-30-28(29(36)37-19-38-30)27(40-43)9-7-22-16-23(5-4-21(22)3)31(45)39-25-8-6-24(26(17-25)32(33,34)35)18-42-12-10-41(11-13-42)14-15-44/h4-6,8,16-17,19-20,44H,10-15,18H2,1-3H3,(H,39,45)(H2,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086456

(CHEMBL3426218 | US10266537, Compound 17)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)cc2)c2c(N)ncnc12 Show InChI InChI=1S/C30H34N8O/c1-20(2)38-29-27(28(31)32-19-33-29)26(35-38)12-9-23-17-24(8-5-21(23)3)30(39)34-25-10-6-22(7-11-25)18-37-15-13-36(4)14-16-37/h5-8,10-11,17,19-20H,13-16,18H2,1-4H3,(H,34,39)(H2,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50571708

(CHEMBL4873348)Show SMILES CNC(=O)c1ccc(Nc2ncc(c(NCc3cccc(F)c3)n2)C(F)(F)F)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAK (unknown origin) incubated for 40 mins by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113573
BindingDB Entry DOI: 10.7270/Q2125XFH |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50571711
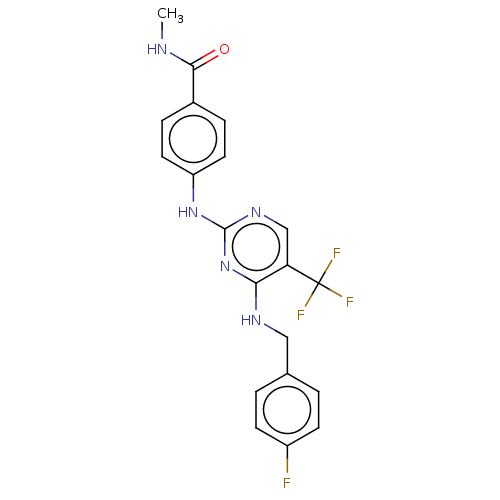
(CHEMBL4860092)Show SMILES CNC(=O)c1ccc(Nc2ncc(c(NCc3ccc(F)cc3)n2)C(F)(F)F)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAK (unknown origin) incubated for 40 mins by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113573
BindingDB Entry DOI: 10.7270/Q2125XFH |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50534673
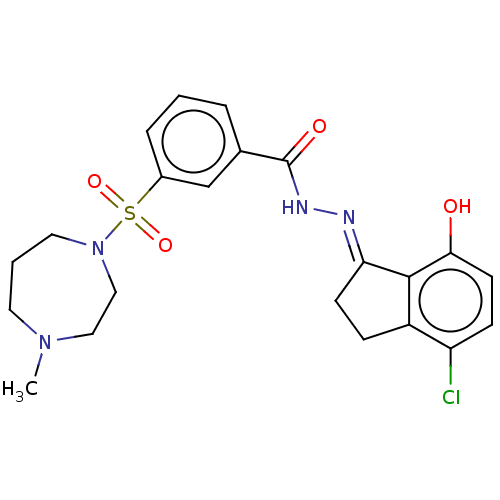
(CHEMBL4546158)Show SMILES CN1CCCN(CC1)S(=O)(=O)c1cccc(c1)C(=O)N\N=C1/CCc2c1c(O)ccc2Cl Show InChI InChI=1S/C22H25ClN4O4S/c1-26-10-3-11-27(13-12-26)32(30,31)16-5-2-4-15(14-16)22(29)25-24-19-8-6-17-18(23)7-9-20(28)21(17)19/h2,4-5,7,9,14,28H,3,6,8,10-13H2,1H3,(H,25,29)/b24-19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 (unknown origin) |
Bioorg Med Chem Lett 26: 4552-4557 (2016)
Article DOI: 10.1016/j.bmcl.2015.06.054
BindingDB Entry DOI: 10.7270/Q2ZS3117 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50534667
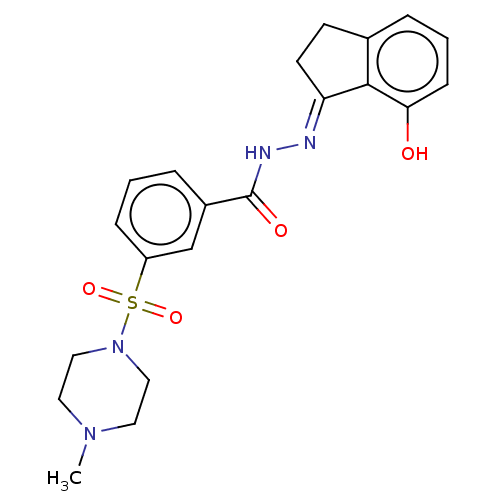
(CHEMBL4588399)Show SMILES CN1CCN(CC1)S(=O)(=O)c1cccc(c1)C(=O)N\N=C1/CCc2cccc(O)c12 Show InChI InChI=1S/C21H24N4O4S/c1-24-10-12-25(13-11-24)30(28,29)17-6-2-5-16(14-17)21(27)23-22-18-9-8-15-4-3-7-19(26)20(15)18/h2-7,14,26H,8-13H2,1H3,(H,23,27)/b22-18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 (unknown origin) |
Bioorg Med Chem Lett 26: 4552-4557 (2016)
Article DOI: 10.1016/j.bmcl.2015.06.054
BindingDB Entry DOI: 10.7270/Q2ZS3117 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086586

(CHEMBL3426234 | US10266537, Compound 29)Show SMILES CCn1nc(C#Cc2cccc(c2)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C29H29F3N8O/c1-3-40-27-25(26(33)34-18-35-27)24(37-40)10-7-19-5-4-6-20(15-19)28(41)36-22-9-8-21(23(16-22)29(30,31)32)17-39-13-11-38(2)12-14-39/h4-6,8-9,15-16,18H,3,11-14,17H2,1-2H3,(H,36,41)(H2,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Fyn |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50571714
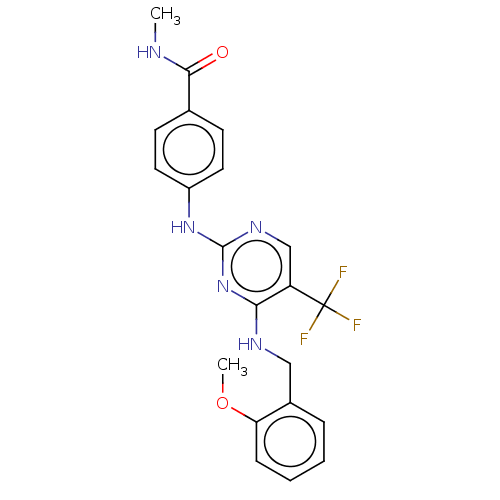
(CHEMBL4874518)Show SMILES CNC(=O)c1ccc(Nc2ncc(c(NCc3ccccc3OC)n2)C(F)(F)F)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAK (unknown origin) incubated for 40 mins by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113573
BindingDB Entry DOI: 10.7270/Q2125XFH |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50571709
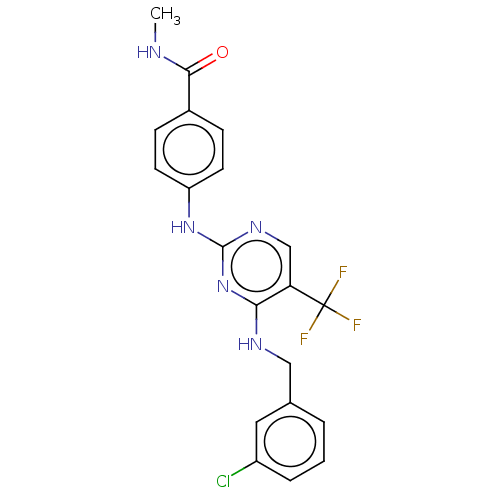
(CHEMBL4853818)Show SMILES CNC(=O)c1ccc(Nc2ncc(c(NCc3cccc(Cl)c3)n2)C(F)(F)F)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAK (unknown origin) incubated for 40 mins by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113573
BindingDB Entry DOI: 10.7270/Q2125XFH |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50571705
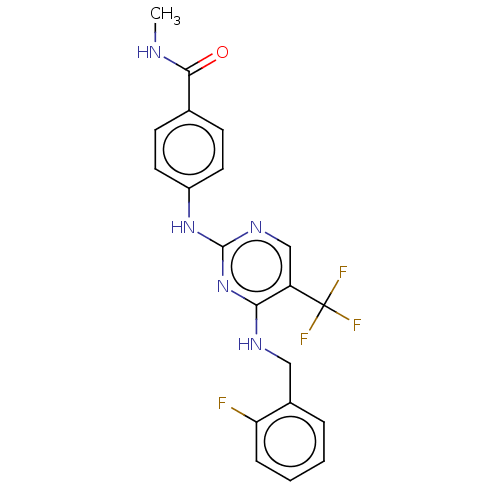
(CHEMBL4865567)Show SMILES CNC(=O)c1ccc(Nc2ncc(c(NCc3ccccc3F)n2)C(F)(F)F)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAK (unknown origin) incubated for 40 mins by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113573
BindingDB Entry DOI: 10.7270/Q2125XFH |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50571707
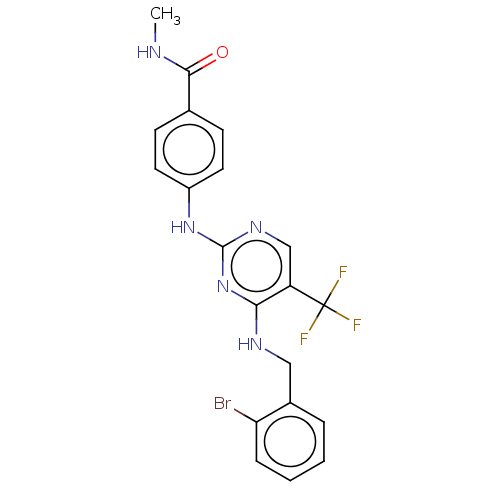
(CHEMBL4850002)Show SMILES CNC(=O)c1ccc(Nc2ncc(c(NCc3ccccc3Br)n2)C(F)(F)F)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAK (unknown origin) incubated for 40 mins by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113573
BindingDB Entry DOI: 10.7270/Q2125XFH |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086445

(CHEMBL3426229 | US10266537, Compound 21)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn([C@H]4CCOC4)c4ncnc(N)c34)cc2C(F)(F)F)CC1 |r| Show InChI InChI=1S/C32H33F3N8O2/c1-20-3-4-22(15-21(20)6-8-27-28-29(36)37-19-38-30(28)43(40-27)25-9-14-45-18-25)31(44)39-24-7-5-23(26(16-24)32(33,34)35)17-42-12-10-41(2)11-13-42/h3-5,7,15-16,19,25H,9-14,17-18H2,1-2H3,(H,39,44)(H2,36,37,38)/t25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086444

(CHEMBL3426230 | US10266537, Compound 20)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn([C@@H]4CCOC4)c4ncnc(N)c34)cc2C(F)(F)F)CC1 |r| Show InChI InChI=1S/C32H33F3N8O2/c1-20-3-4-22(15-21(20)6-8-27-28-29(36)37-19-38-30(28)43(40-27)25-9-14-45-18-25)31(44)39-24-7-5-23(26(16-24)32(33,34)35)17-42-12-10-41(2)11-13-42/h3-5,7,15-16,19,25H,9-14,17-18H2,1-2H3,(H,39,44)(H2,36,37,38)/t25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086449

(CHEMBL3426224 | US10266537, Compound 8)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn(C)c4ncnc(N)c34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H29F3N8O/c1-18-4-5-20(14-19(18)7-9-24-25-26(33)34-17-35-27(25)39(3)37-24)28(41)36-22-8-6-21(23(15-22)29(30,31)32)16-40-12-10-38(2)11-13-40/h4-6,8,14-15,17H,10-13,16H2,1-3H3,(H,36,41)(H2,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086446
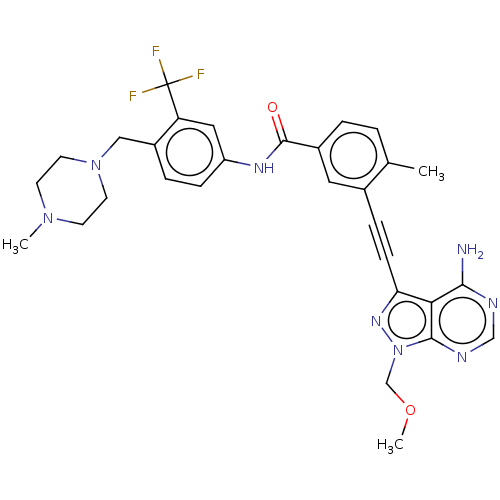
(CHEMBL3426228 | US10266537, Compound 19)Show SMILES COCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O2/c1-19-4-5-21(14-20(19)7-9-25-26-27(34)35-17-36-28(26)41(38-25)18-43-3)29(42)37-23-8-6-22(24(15-23)30(31,32)33)16-40-12-10-39(2)11-13-40/h4-6,8,14-15,17H,10-13,16,18H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086448

(CHEMBL3426226 | US10266537, Compound 6)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn(C4CCC4)c4ncnc(N)c34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C32H33F3N8O/c1-20-6-7-22(16-21(20)9-11-27-28-29(36)37-19-38-30(28)43(40-27)25-4-3-5-25)31(44)39-24-10-8-23(26(17-24)32(33,34)35)18-42-14-12-41(2)13-15-42/h6-8,10,16-17,19,25H,3-5,12-15,18H2,1-2H3,(H,39,44)(H2,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50534661
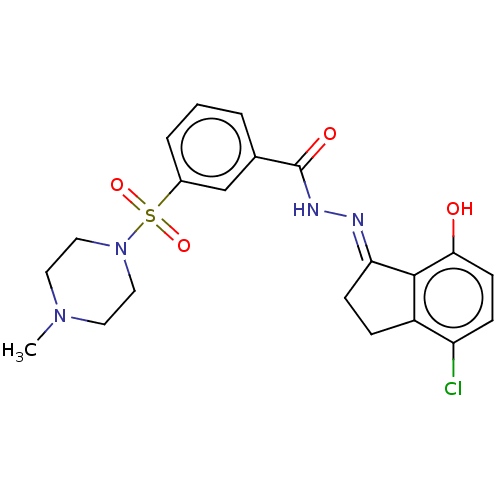
(CHEMBL4540594)Show SMILES CN1CCN(CC1)S(=O)(=O)c1cccc(c1)C(=O)N\N=C1/CCc2c1c(O)ccc2Cl Show InChI InChI=1S/C21H23ClN4O4S/c1-25-9-11-26(12-10-25)31(29,30)15-4-2-3-14(13-15)21(28)24-23-18-7-5-16-17(22)6-8-19(27)20(16)18/h2-4,6,8,13,27H,5,7,9-12H2,1H3,(H,24,28)/b23-18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 (unknown origin) |
Bioorg Med Chem Lett 26: 4552-4557 (2016)
Article DOI: 10.1016/j.bmcl.2015.06.054
BindingDB Entry DOI: 10.7270/Q2ZS3117 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086585
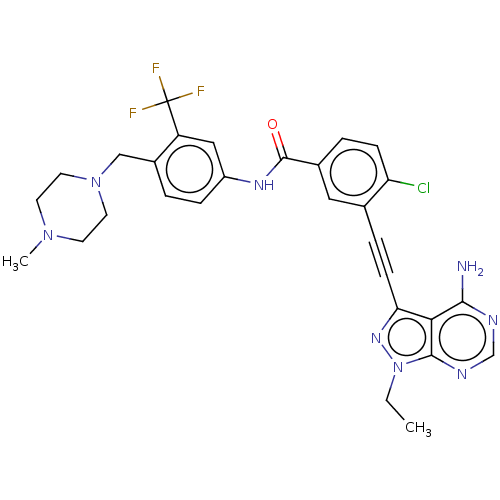
(CHEMBL3426235 | US10266537, Compound 26)Show SMILES CCn1nc(C#Cc2cc(ccc2Cl)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C29H28ClF3N8O/c1-3-41-27-25(26(34)35-17-36-27)24(38-41)9-6-18-14-19(5-8-23(18)30)28(42)37-21-7-4-20(22(15-21)29(31,32)33)16-40-12-10-39(2)11-13-40/h4-5,7-8,14-15,17H,3,10-13,16H2,1-2H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50571718
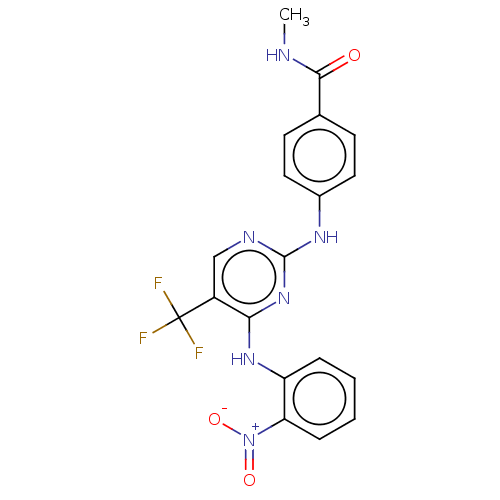
(CHEMBL4849851)Show SMILES CNC(=O)c1ccc(Nc2ncc(c(Nc3ccccc3[N+]([O-])=O)n2)C(F)(F)F)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAK (unknown origin) incubated for 40 mins by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113573
BindingDB Entry DOI: 10.7270/Q2125XFH |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086443
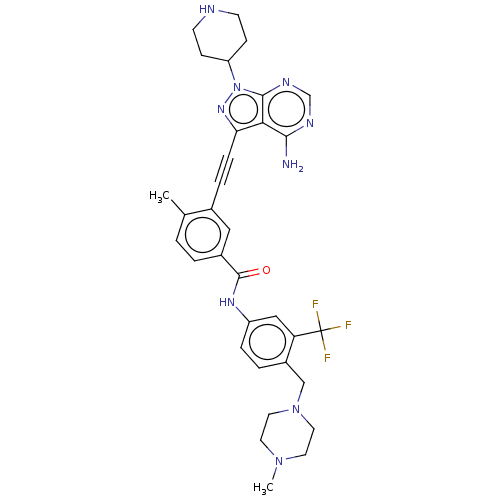
(CHEMBL3426232)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn(C4CCNCC4)c4ncnc(N)c34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C33H36F3N9O/c1-21-3-4-23(32(46)41-25-7-5-24(27(18-25)33(34,35)36)19-44-15-13-43(2)14-16-44)17-22(21)6-8-28-29-30(37)39-20-40-31(29)45(42-28)26-9-11-38-12-10-26/h3-5,7,17-18,20,26,38H,9-16,19H2,1-2H3,(H,41,46)(H2,37,39,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086442
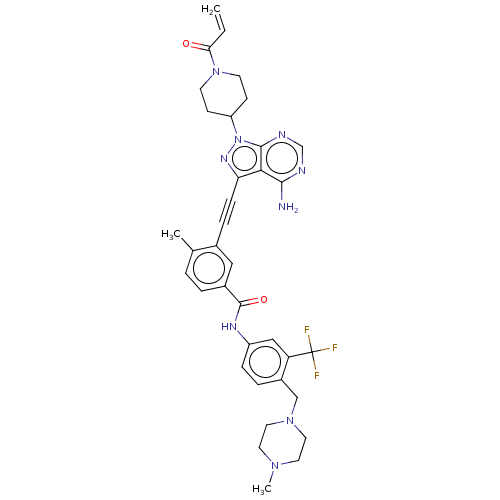
(CHEMBL3426233 | US10266537, Compound 121)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn(C4CCN(CC4)C(=O)C=C)c4ncnc(N)c34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C36H38F3N9O2/c1-4-31(49)47-13-11-28(12-14-47)48-34-32(33(40)41-22-42-34)30(44-48)10-8-24-19-25(6-5-23(24)2)35(50)43-27-9-7-26(29(20-27)36(37,38)39)21-46-17-15-45(3)16-18-46/h4-7,9,19-20,22,28H,1,11-18,21H2,2-3H3,(H,43,50)(H2,40,41,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human B-RAF V600E mutant |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human EphA2 |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50571727
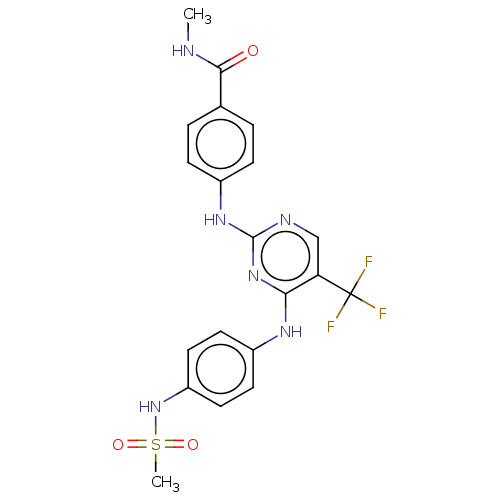
(CHEMBL4869055)Show SMILES CNC(=O)c1ccc(Nc2ncc(c(Nc3ccc(NS(C)(=O)=O)cc3)n2)C(F)(F)F)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAK (unknown origin) incubated for 40 mins by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113573
BindingDB Entry DOI: 10.7270/Q2125XFH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50571721

(CHEMBL4874046)Show SMILES CNC(=O)c1ccc(Nc2ncc(c(Nc3ccccc3OC)n2)C(F)(F)F)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JAK2 (unknown origin) measured after 40 mins by HTRF |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113573
BindingDB Entry DOI: 10.7270/Q2125XFH |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50571721

(CHEMBL4874046)Show SMILES CNC(=O)c1ccc(Nc2ncc(c(Nc3ccccc3OC)n2)C(F)(F)F)cc1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TRKA (unknown origin) measured after 40 mins by HTRF |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113573
BindingDB Entry DOI: 10.7270/Q2125XFH |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086447
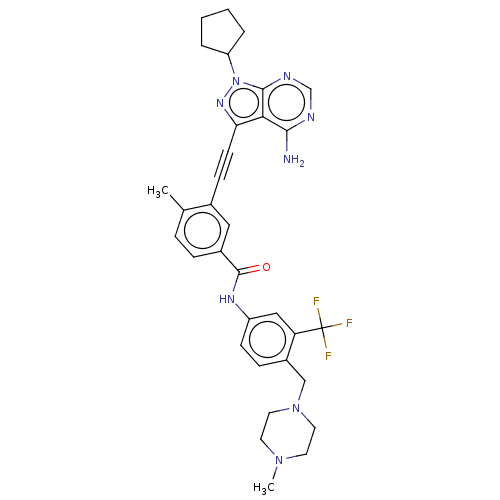
(CHEMBL3426227 | US10266537, Compound 7)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn(C4CCCC4)c4ncnc(N)c34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C33H35F3N8O/c1-21-7-8-23(17-22(21)10-12-28-29-30(37)38-20-39-31(29)44(41-28)26-5-3-4-6-26)32(45)40-25-11-9-24(27(18-25)33(34,35)36)19-43-15-13-42(2)14-16-43/h7-9,11,17-18,20,26H,3-6,13-16,19H2,1-2H3,(H,40,45)(H2,37,38,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Blk |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50249522

(2-chloro-N-(4-chloro-3-(pyridin-2-yl)phenyl)-4-(me...)Show SMILES CS(=O)(=O)c1ccc(C(=O)Nc2ccc(Cl)c(c2)-c2ccccn2)c(Cl)c1 Show InChI InChI=1S/C19H14Cl2N2O3S/c1-27(25,26)13-6-7-14(17(21)11-13)19(24)23-12-5-8-16(20)15(10-12)18-4-2-3-9-22-18/h2-11H,1H3,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of smoothened (unknown origin)-mediated Shh signaling |
Bioorg Med Chem Lett 24: 1426-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.006
BindingDB Entry DOI: 10.7270/Q27D2WMH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50571721

(CHEMBL4874046)Show SMILES CNC(=O)c1ccc(Nc2ncc(c(Nc3ccccc3OC)n2)C(F)(F)F)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JAK3 (unknown origin) measured after 40 mins by HTRF |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113573
BindingDB Entry DOI: 10.7270/Q2125XFH |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086452

(CHEMBL3426221)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCOCC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H30F3N7O2/c1-18(2)40-28-26(27(34)35-17-36-28)25(38-40)9-7-20-14-21(5-4-19(20)3)29(41)37-23-8-6-22(24(15-23)30(31,32)33)16-39-10-12-42-13-11-39/h4-6,8,14-15,17-18H,10-13,16H2,1-3H3,(H,37,41)(H2,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50571726
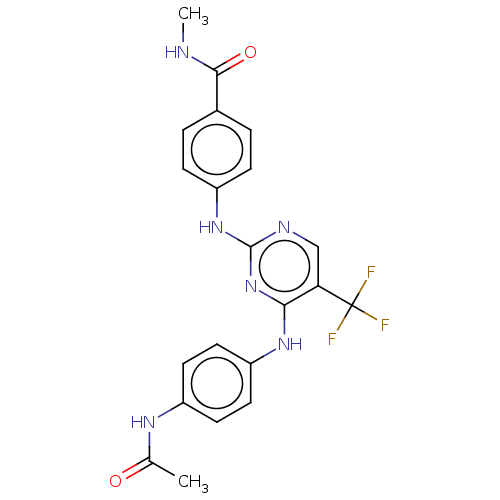
(CHEMBL4877387)Show SMILES CNC(=O)c1ccc(Nc2ncc(c(Nc3ccc(NC(C)=O)cc3)n2)C(F)(F)F)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAK (unknown origin) incubated for 40 mins by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113573
BindingDB Entry DOI: 10.7270/Q2125XFH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data