 Found 43 hits with Last Name = 'zhou' and Initial = 'bn'
Found 43 hits with Last Name = 'zhou' and Initial = 'bn' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of human platelet COX1 by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 expressed in insect Sf21 cells by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM32020
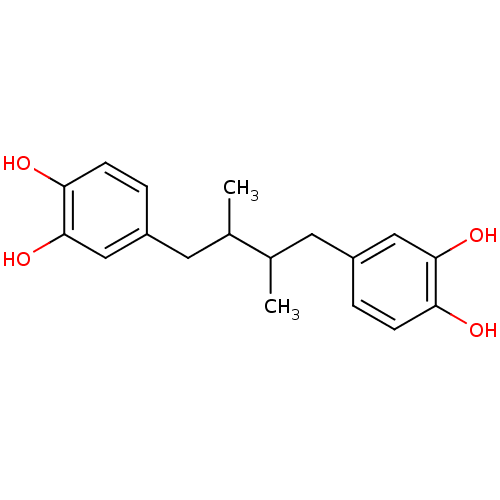
(4-[4-(3,4-dihydroxyphenyl)-2,3-dimethyl-butyl]pyro...)Show InChI InChI=1S/C18H22O4/c1-11(7-13-3-5-15(19)17(21)9-13)12(2)8-14-4-6-16(20)18(22)10-14/h3-6,9-12,19-22H,7-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of 5LOX in human PBMC by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Oryctolagus cuniculus (rabbit)) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of 15LOX in rabbit reticulocytes by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Oryctolagus cuniculus (rabbit)) | BDBM50208822
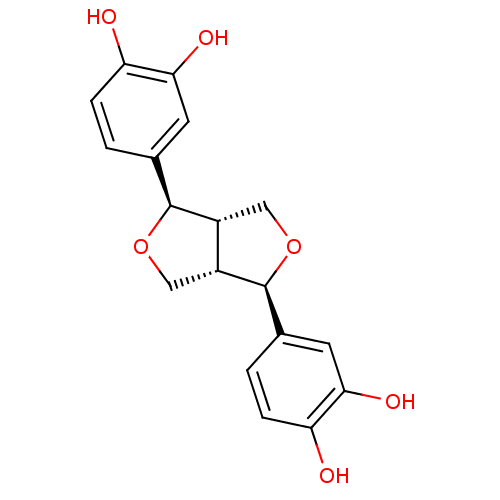
((-)-3,3'-bisdemethylpinoresinol | CHEMBL227187)Show SMILES Oc1ccc(cc1O)[C@@H]1OC[C@@H]2[C@H]1CO[C@H]2c1ccc(O)c(O)c1 Show InChI InChI=1S/C18H18O6/c19-13-3-1-9(5-15(13)21)17-11-7-24-18(12(11)8-23-17)10-2-4-14(20)16(22)6-10/h1-6,11-12,17-22H,7-8H2/t11-,12-,17+,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of 15LOX in rabbit reticulocytes by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Oryctolagus cuniculus (rabbit)) | BDBM50208825
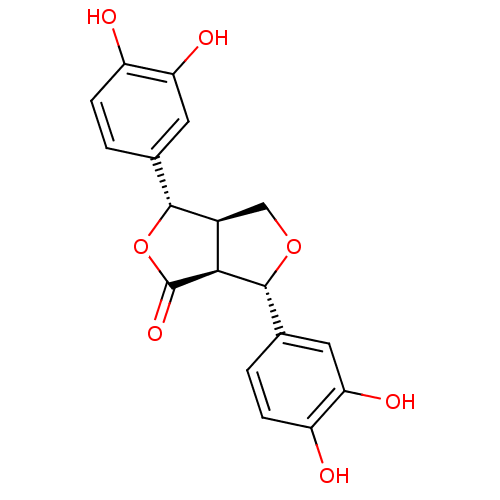
((+)-3,4,3',4'-tetrahydroxy-9,7'alpha-epoxylignano-...)Show SMILES Oc1ccc(cc1O)[C@H]1OC(=O)[C@H]2[C@@H]1CO[C@@H]2c1ccc(O)c(O)c1 Show InChI InChI=1S/C18H16O7/c19-11-3-1-8(5-13(11)21)16-10-7-24-17(15(10)18(23)25-16)9-2-4-12(20)14(22)6-9/h1-6,10,15-17,19-22H,7H2/t10-,15-,16+,17+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of 15LOX in rabbit reticulocytes by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50259819

(CHEMBL481635 | Calceolarioside | Calceolarioside A)Show SMILES OC[C@H]1O[C@@H](OCCc2ccc(O)c(O)c2)[C@H](O)[C@@H](O)[C@@H]1OC(=O)\C=C\c1ccc(O)c(O)c1 |r| Show InChI InChI=1S/C23H26O11/c24-11-18-22(34-19(29)6-3-12-1-4-14(25)16(27)9-12)20(30)21(31)23(33-18)32-8-7-13-2-5-15(26)17(28)10-13/h1-6,9-10,18,20-28,30-31H,7-8,11H2/b6-3+/t18-,20-,21-,22-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PKCalpha |
J Nat Prod 61: 1410-2 (1999)
Article DOI: 10.1021/np980147s
BindingDB Entry DOI: 10.7270/Q2348M80 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Oryctolagus cuniculus (rabbit)) | BDBM50208824
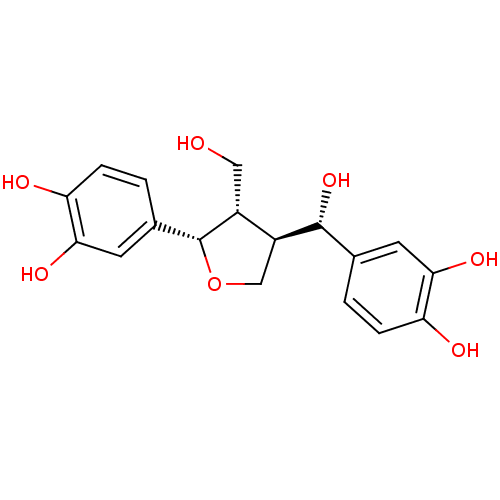
((+)-3,3'-bisdemethyltanegool | CHEMBL436990)Show SMILES OC[C@@H]1[C@H](CO[C@@H]1c1ccc(O)c(O)c1)[C@H](O)c1ccc(O)c(O)c1 Show InChI InChI=1S/C18H20O7/c19-7-11-12(17(24)9-1-3-13(20)15(22)5-9)8-25-18(11)10-2-4-14(21)16(23)6-10/h1-6,11-12,17-24H,7-8H2/t11-,12+,17-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of 15LOX in rabbit reticulocytes by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of 5LOX in human PBMC by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Oryctolagus cuniculus (rabbit)) | BDBM50208823

(6,11-dihydrothiochromeno[4,3-b]indole | CHEMBL1809...)Show InChI InChI=1S/C15H11NS/c1-3-7-13-10(5-1)12-9-17-14-8-4-2-6-11(14)15(12)16-13/h1-8,16H,9H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of 15LOX in rabbit reticulocytes by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50269517

(CHEMBL504363 | Forsythiaside | forsythoside A)Show SMILES C[C@@H]1O[C@@H](OC[C@H]2O[C@@H](OCCc3ccc(O)c(O)c3)[C@H](O)[C@@H](O)[C@@H]2OC(=O)\C=C\c2ccc(O)c(O)c2)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C29H36O15/c1-13-22(35)23(36)25(38)29(42-13)41-12-20-27(44-21(34)7-4-14-2-5-16(30)18(32)10-14)24(37)26(39)28(43-20)40-9-8-15-3-6-17(31)19(33)11-15/h2-7,10-11,13,20,22-33,35-39H,8-9,12H2,1H3/b7-4+/t13-,20+,22-,23+,24+,25+,26+,27+,28+,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PKCalpha |
J Nat Prod 61: 1410-2 (1999)
Article DOI: 10.1021/np980147s
BindingDB Entry DOI: 10.7270/Q2348M80 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Oryctolagus cuniculus (rabbit)) | BDBM7462

(3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...)Show InChI InChI=1S/C15H10O6/c16-8-3-1-7(2-4-8)15-14(20)13(19)12-10(18)5-9(17)6-11(12)21-15/h1-6,16-18,20H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of 15LOX in rabbit reticulocytes by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM7462

(3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...)Show InChI InChI=1S/C15H10O6/c16-8-3-1-7(2-4-8)15-14(20)13(19)12-10(18)5-9(17)6-11(12)21-15/h1-6,16-18,20H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of 5LOX in human PBMC by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Oryctolagus cuniculus (rabbit)) | BDBM50292291
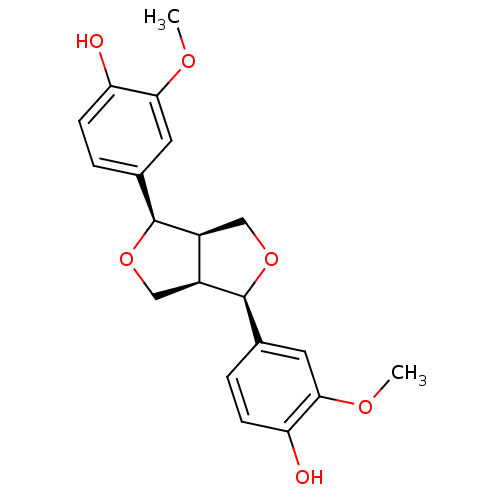
((-)-pinoresinol | CHEMBL460862)Show SMILES COc1cc(ccc1O)[C@@H]1OC[C@H]2[C@@H]1CO[C@H]2c1ccc(O)c(OC)c1 |r| Show InChI InChI=1S/C20H22O6/c1-23-17-7-11(3-5-15(17)21)19-13-9-26-20(14(13)10-25-19)12-4-6-16(22)18(8-12)24-2/h3-8,13-14,19-22H,9-10H2,1-2H3/t13-,14-,19-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of 15LOX in rabbit reticulocytes by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50269516
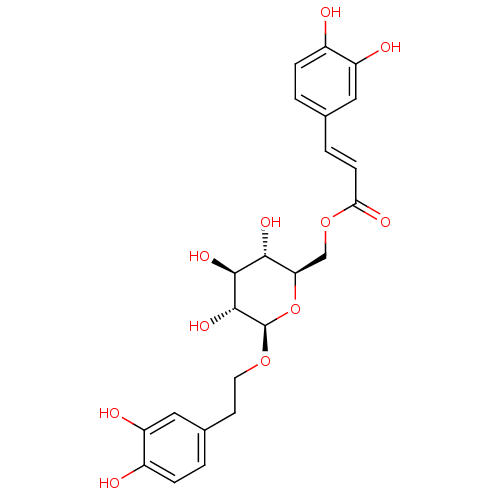
(CHEMBL518414 | Calceolarioside B)Show SMILES O[C@@H]1[C@@H](COC(=O)\C=C\c2ccc(O)c(O)c2)O[C@@H](OCCc2ccc(O)c(O)c2)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C23H26O11/c24-14-4-1-12(9-16(14)26)3-6-19(28)33-11-18-20(29)21(30)22(31)23(34-18)32-8-7-13-2-5-15(25)17(27)10-13/h1-6,9-10,18,20-27,29-31H,7-8,11H2/b6-3+/t18-,20-,21+,22-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PKCalpha |
J Nat Prod 61: 1410-2 (1999)
Article DOI: 10.1021/np980147s
BindingDB Entry DOI: 10.7270/Q2348M80 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Saccharomyces cerevisiae S288c) | BDBM50090044

((Z)-2',3'-dihydroxy-3,4,4',5-tetramethoxystilbene ...)Show InChI InChI=1S/C18H20O6/c1-21-13-8-7-12(16(19)17(13)20)6-5-11-9-14(22-2)18(24-4)15(10-11)23-3/h5-10,19-20H,1-4H3/b6-5- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase 1 in Saccharomyces cerevisiae RS321N in galactose medium by microtiter plate assay |
J Nat Prod 63: 457-60 (2000)
Article DOI: 10.1021/np9904410
BindingDB Entry DOI: 10.7270/Q2930WZR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50208825

((+)-3,4,3',4'-tetrahydroxy-9,7'alpha-epoxylignano-...)Show SMILES Oc1ccc(cc1O)[C@H]1OC(=O)[C@H]2[C@@H]1CO[C@@H]2c1ccc(O)c(O)c1 Show InChI InChI=1S/C18H16O7/c19-11-3-1-8(5-13(11)21)16-10-7-24-17(15(10)18(23)25-16)9-2-4-12(20)14(22)6-9/h1-6,10,15-17,19-22H,7H2/t10-,15-,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of 5LOX in human PBMC by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Saccharomyces cerevisiae S288c) | BDBM50090044

((Z)-2',3'-dihydroxy-3,4,4',5-tetramethoxystilbene ...)Show InChI InChI=1S/C18H20O6/c1-21-13-8-7-12(16(19)17(13)20)6-5-11-9-14(22-2)18(24-4)15(10-11)23-3/h5-10,19-20H,1-4H3/b6-5- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase 1 in Saccharomyces cerevisiae RS321N in glucose medium by microtiter plate assay |
J Nat Prod 63: 457-60 (2000)
Article DOI: 10.1021/np9904410
BindingDB Entry DOI: 10.7270/Q2930WZR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50208822

((-)-3,3'-bisdemethylpinoresinol | CHEMBL227187)Show SMILES Oc1ccc(cc1O)[C@@H]1OC[C@@H]2[C@H]1CO[C@H]2c1ccc(O)c(O)c1 Show InChI InChI=1S/C18H18O6/c19-13-3-1-9(5-15(13)21)17-11-7-24-18(12(11)8-23-17)10-2-4-14(20)16(22)6-10/h1-6,11-12,17-22H,7-8H2/t11-,12-,17+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of 5LOX in human PBMC by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50208824

((+)-3,3'-bisdemethyltanegool | CHEMBL436990)Show SMILES OC[C@@H]1[C@H](CO[C@@H]1c1ccc(O)c(O)c1)[C@H](O)c1ccc(O)c(O)c1 Show InChI InChI=1S/C18H20O7/c19-7-11-12(17(24)9-1-3-13(20)15(22)5-9)8-25-18(11)10-2-4-14(21)16(23)6-10/h1-6,11-12,17-24H,7-8H2/t11-,12+,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of 5LOX in human PBMC by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50377909

(ACETOSIDE)Show SMILES C[C@H]1O[C@H](O[C@@H]2[C@@H](O)[C@H](OCCc3ccc(O)c(O)c3)O[C@H](CO)[C@H]2OC(=O)\C=C\c2ccc(O)c(O)c2)[C@@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C29H36O15/c1-13-22(36)23(37)24(38)29(41-13)44-27-25(39)28(40-9-8-15-3-6-17(32)19(34)11-15)42-20(12-30)26(27)43-21(35)7-4-14-2-5-16(31)18(33)10-14/h2-7,10-11,13,20,22-34,36-39H,8-9,12H2,1H3/b7-4+/t13-,20-,22-,23+,24+,25-,26-,27-,28-,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PKCalpha |
J Nat Prod 61: 1410-2 (1999)
Article DOI: 10.1021/np980147s
BindingDB Entry DOI: 10.7270/Q2348M80 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50292291

((-)-pinoresinol | CHEMBL460862)Show SMILES COc1cc(ccc1O)[C@@H]1OC[C@H]2[C@@H]1CO[C@H]2c1ccc(O)c(OC)c1 |r| Show InChI InChI=1S/C20H22O6/c1-23-17-7-11(3-5-15(17)21)19-13-9-26-20(14(13)10-25-19)12-4-6-16(22)18(8-12)24-2/h3-8,13-14,19-22H,9-10H2,1-2H3/t13-,14-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of 5LOX in human PBMC by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50269518

(CHEMBL452413 | Plantainoside D)Show SMILES OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](COC(=O)\C=C\c3ccc(O)c(O)c3)O[C@@H](OCCc3ccc(O)c(O)c3)[C@@H]2O)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C29H36O16/c30-11-19-22(36)24(38)25(39)29(43-19)45-27-23(37)20(12-42-21(35)6-3-13-1-4-15(31)17(33)9-13)44-28(26(27)40)41-8-7-14-2-5-16(32)18(34)10-14/h1-6,9-10,19-20,22-34,36-40H,7-8,11-12H2/b6-3+/t19-,20-,22-,23-,24+,25-,26-,27+,28-,29+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PKCalpha |
J Nat Prod 61: 1410-2 (1999)
Article DOI: 10.1021/np980147s
BindingDB Entry DOI: 10.7270/Q2348M80 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Oryctolagus cuniculus (rabbit)) | BDBM50206008
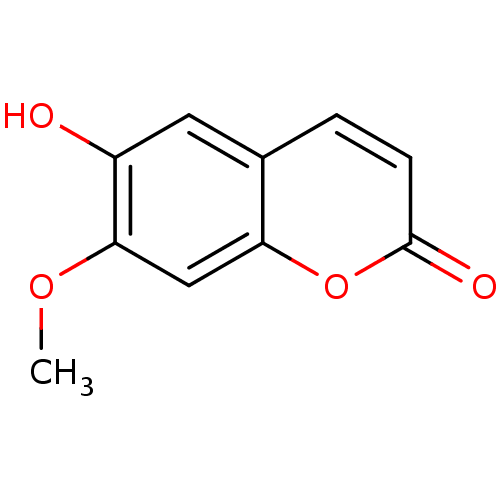
(6-hydroxy-7-methoxy-2H-chromen-2-one | CHEMBL39032...)Show InChI InChI=1S/C10H8O4/c1-13-9-5-8-6(4-7(9)11)2-3-10(12)14-8/h2-5,11H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of 15LOX in rabbit reticulocytes by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Oryctolagus cuniculus (rabbit)) | BDBM50156693
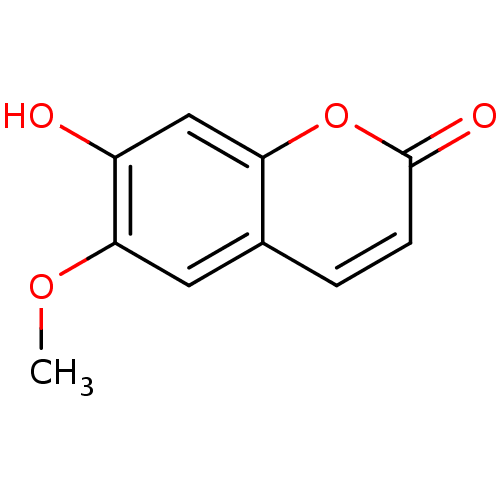
(6-Methoxy-7-hydroxycoumarin | 6-Methylesculetin | ...)Show InChI InChI=1S/C10H8O4/c1-13-9-4-6-2-3-10(12)14-8(6)5-7(9)11/h2-5,11H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of 15LOX in rabbit reticulocytes by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50250350
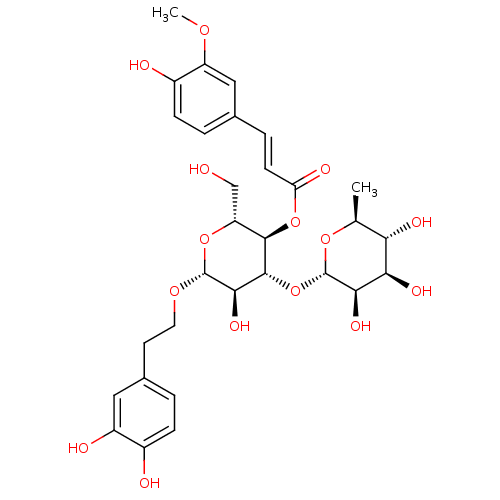
(CHEMBL450121 | Leucosceptoside A)Show SMILES COc1cc(\C=C\C(=O)O[C@@H]2[C@@H](CO)O[C@@H](OCCc3ccc(O)c(O)c3)[C@H](O)[C@H]2O[C@@H]2O[C@@H](C)[C@H](O)[C@@H](O)[C@H]2O)ccc1O |r| Show InChI InChI=1S/C30H38O15/c1-14-23(36)24(37)25(38)30(42-14)45-28-26(39)29(41-10-9-16-3-6-17(32)19(34)11-16)43-21(13-31)27(28)44-22(35)8-5-15-4-7-18(33)20(12-15)40-2/h3-8,11-12,14,21,23-34,36-39H,9-10,13H2,1-2H3/b8-5+/t14-,21+,23-,24+,25+,26+,27+,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PKCalpha |
J Nat Prod 61: 1410-2 (1999)
Article DOI: 10.1021/np980147s
BindingDB Entry DOI: 10.7270/Q2348M80 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Saccharomyces cerevisiae S288c) | BDBM50005480

((-)-combretastatin | (Z)-3'-hydroxy-3,4,4',5-tetra...)Show InChI InChI=1S/C18H20O5/c1-20-15-8-7-12(9-14(15)19)5-6-13-10-16(21-2)18(23-4)17(11-13)22-3/h5-11,19H,1-4H3/b6-5- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase 1 in Saccharomyces cerevisiae RS321N in galactose medium by microtiter plate assay |
J Nat Prod 63: 457-60 (2000)
Article DOI: 10.1021/np9904410
BindingDB Entry DOI: 10.7270/Q2930WZR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Saccharomyces cerevisiae S288c) | BDBM50005480

((-)-combretastatin | (Z)-3'-hydroxy-3,4,4',5-tetra...)Show InChI InChI=1S/C18H20O5/c1-20-15-8-7-12(9-14(15)19)5-6-13-10-16(21-2)18(23-4)17(11-13)22-3/h5-11,19H,1-4H3/b6-5- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase 1 in Saccharomyces cerevisiae RS321N in glucose medium by microtiter plate assay |
J Nat Prod 63: 457-60 (2000)
Article DOI: 10.1021/np9904410
BindingDB Entry DOI: 10.7270/Q2930WZR |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50241873

(CHEMBL499145 | Poliumoside)Show SMILES C[C@@H]1O[C@@H](OC[C@H]2O[C@@H](OCCc3ccc(O)c(O)c3)[C@H](O)[C@@H](O[C@@H]3O[C@@H](C)[C@H](O)[C@@H](O)[C@H]3O)[C@@H]2OC(=O)\C=C\c2ccc(O)c(O)c2)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C35H46O19/c1-14-24(41)26(43)28(45)33(50-14)49-13-22-31(53-23(40)8-5-16-3-6-18(36)20(38)11-16)32(54-35-29(46)27(44)25(42)15(2)51-35)30(47)34(52-22)48-10-9-17-4-7-19(37)21(39)12-17/h3-8,11-12,14-15,22,24-39,41-47H,9-10,13H2,1-2H3/b8-5+/t14-,15-,22+,24-,25-,26+,27+,28+,29+,30+,31+,32+,33+,34+,35-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PKCalpha |
J Nat Prod 61: 1410-2 (1999)
Article DOI: 10.1021/np980147s
BindingDB Entry DOI: 10.7270/Q2348M80 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 expressed in insect Sf21 cells by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50208824

((+)-3,3'-bisdemethyltanegool | CHEMBL436990)Show SMILES OC[C@@H]1[C@H](CO[C@@H]1c1ccc(O)c(O)c1)[C@H](O)c1ccc(O)c(O)c1 Show InChI InChI=1S/C18H20O7/c19-7-11-12(17(24)9-1-3-13(20)15(22)5-9)8-25-18(11)10-2-4-14(21)16(23)6-10/h1-6,11-12,17-24H,7-8H2/t11-,12+,17-,18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 expressed in insect Sf21 cells by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50208825

((+)-3,4,3',4'-tetrahydroxy-9,7'alpha-epoxylignano-...)Show SMILES Oc1ccc(cc1O)[C@H]1OC(=O)[C@H]2[C@@H]1CO[C@@H]2c1ccc(O)c(O)c1 Show InChI InChI=1S/C18H16O7/c19-11-3-1-8(5-13(11)21)16-10-7-24-17(15(10)18(23)25-16)9-2-4-12(20)14(22)6-9/h1-6,10,15-17,19-22H,7H2/t10-,15-,16+,17+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 expressed in insect Sf21 cells by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM7462

(3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...)Show InChI InChI=1S/C15H10O6/c16-8-3-1-7(2-4-8)15-14(20)13(19)12-10(18)5-9(17)6-11(12)21-15/h1-6,16-18,20H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of human platelet COX1 by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50156693

(6-Methoxy-7-hydroxycoumarin | 6-Methylesculetin | ...)Show InChI InChI=1S/C10H8O4/c1-13-9-4-6-2-3-10(12)14-8(6)5-7(9)11/h2-5,11H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of 5LOX in human PBMC by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM7462

(3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...)Show InChI InChI=1S/C15H10O6/c16-8-3-1-7(2-4-8)15-14(20)13(19)12-10(18)5-9(17)6-11(12)21-15/h1-6,16-18,20H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 expressed in insect Sf21 cells by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50208822

((-)-3,3'-bisdemethylpinoresinol | CHEMBL227187)Show SMILES Oc1ccc(cc1O)[C@@H]1OC[C@@H]2[C@H]1CO[C@H]2c1ccc(O)c(O)c1 Show InChI InChI=1S/C18H18O6/c19-13-3-1-9(5-15(13)21)17-11-7-24-18(12(11)8-23-17)10-2-4-14(20)16(22)6-10/h1-6,11-12,17-22H,7-8H2/t11-,12-,17+,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 expressed in insect Sf21 cells by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50292291

((-)-pinoresinol | CHEMBL460862)Show SMILES COc1cc(ccc1O)[C@@H]1OC[C@H]2[C@@H]1CO[C@H]2c1ccc(O)c(OC)c1 |r| Show InChI InChI=1S/C20H22O6/c1-23-17-7-11(3-5-15(17)21)19-13-9-26-20(14(13)10-25-19)12-4-6-16(22)18(8-12)24-2/h3-8,13-14,19-22H,9-10H2,1-2H3/t13-,14-,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 expressed in insect Sf21 cells by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50156693

(6-Methoxy-7-hydroxycoumarin | 6-Methylesculetin | ...)Show InChI InChI=1S/C10H8O4/c1-13-9-4-6-2-3-10(12)14-8(6)5-7(9)11/h2-5,11H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 expressed in insect Sf21 cells by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50206008

(6-hydroxy-7-methoxy-2H-chromen-2-one | CHEMBL39032...)Show InChI InChI=1S/C10H8O4/c1-13-9-5-8-6(4-7(9)11)2-3-10(12)14-8/h2-5,11H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 expressed in insect Sf21 cells by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50206008

(6-hydroxy-7-methoxy-2H-chromen-2-one | CHEMBL39032...)Show InChI InChI=1S/C10H8O4/c1-13-9-5-8-6(4-7(9)11)2-3-10(12)14-8/h2-5,11H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tahitian Noni International
Curated by ChEMBL
| Assay Description
Inhibition of 5LOX in human PBMC by EIA assay |
J Nat Prod 70: 859-62 (2007)
Article DOI: 10.1021/np0605539
BindingDB Entry DOI: 10.7270/Q2J38S79 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50269515

(2-(3-hydroxy-4-methoxy-phenyl)-ethyl-O-(alpha-L-rh...)Show SMILES COc1ccc(CCO[C@@H]2O[C@H](CO[C@@H]3O[C@@H](C)[C@H](O)[C@@H](O)[C@H]3O)[C@@H](OC(=O)\C=C\c3ccc(O)c(OC)c3)[C@H](O[C@@H]3O[C@@H](C)[C@H](O)[C@@H](O)[C@H]3O)[C@H]2O)cc1O |r| Show InChI InChI=1S/C37H50O19/c1-16-26(41)28(43)30(45)35(52-16)51-15-24-33(55-25(40)10-7-18-5-8-20(38)23(14-18)49-4)34(56-37-31(46)29(44)27(42)17(2)53-37)32(47)36(54-24)50-12-11-19-6-9-22(48-3)21(39)13-19/h5-10,13-14,16-17,24,26-39,41-47H,11-12,15H2,1-4H3/b10-7+/t16-,17-,24+,26-,27-,28+,29+,30+,31+,32+,33+,34+,35+,36+,37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PKCalpha |
J Nat Prod 61: 1410-2 (1999)
Article DOI: 10.1021/np980147s
BindingDB Entry DOI: 10.7270/Q2348M80 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Saccharomyces cerevisiae S288c) | BDBM50478907

(CHEMBL497532)Show SMILES COc1ccc(CCc2cc(OC)c(OC)c(OC)c2)c(O[C@H]2O[C@@H](CO)[C@H](O)[C@@H](O)[C@@H]2O)c1O |r| Show InChI InChI=1S/C24H32O11/c1-30-14-8-7-13(6-5-12-9-15(31-2)23(33-4)16(10-12)32-3)22(19(14)27)35-24-21(29)20(28)18(26)17(11-25)34-24/h7-10,17-18,20-21,24-29H,5-6,11H2,1-4H3/t17-,18-,20+,21-,24+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase 1 in Saccharomyces cerevisiae RS321N in galactose medium by microtiter plate assay |
J Nat Prod 63: 457-60 (2000)
Article DOI: 10.1021/np9904410
BindingDB Entry DOI: 10.7270/Q2930WZR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Saccharomyces cerevisiae S288c) | BDBM50478907

(CHEMBL497532)Show SMILES COc1ccc(CCc2cc(OC)c(OC)c(OC)c2)c(O[C@H]2O[C@@H](CO)[C@H](O)[C@@H](O)[C@@H]2O)c1O |r| Show InChI InChI=1S/C24H32O11/c1-30-14-8-7-13(6-5-12-9-15(31-2)23(33-4)16(10-12)32-3)22(19(14)27)35-24-21(29)20(28)18(26)17(11-25)34-24/h7-10,17-18,20-21,24-29H,5-6,11H2,1-4H3/t17-,18-,20+,21-,24+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase 1 in Saccharomyces cerevisiae RS321N in glucose medium by microtiter plate assay |
J Nat Prod 63: 457-60 (2000)
Article DOI: 10.1021/np9904410
BindingDB Entry DOI: 10.7270/Q2930WZR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data