 Found 14 hits in this display
Found 14 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM20192
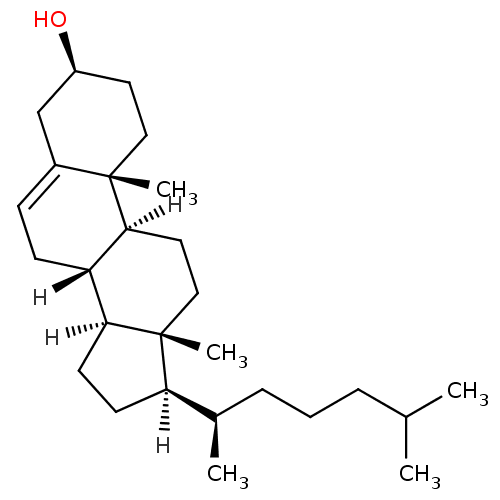
((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C |t:9| Show InChI InChI=1S/C27H46O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h9,18-19,21-25,28H,6-8,10-17H2,1-5H3/t19-,21+,22+,23-,24+,25+,26+,27-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: increase in Daunorubicn intracellular accumulation (Daunorubicin: ? uM) in NIH-G185 cells |
Biochem Biophys Res Commun 276: 909-16 (2000)
Article DOI: 10.1006/bbrc.2000.3554
BindingDB Entry DOI: 10.7270/Q2862HR0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM20192

((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C |t:9| Show InChI InChI=1S/C27H46O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h9,18-19,21-25,28H,6-8,10-17H2,1-5H3/t19-,21+,22+,23-,24+,25+,26+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >4.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant rat androgen receptor expressed in Escherichia coli using [3H]methyltrienolone (R 1881) |
J Med Chem 48: 5666-74 (2005)
Article DOI: 10.1021/jm050403f
BindingDB Entry DOI: 10.7270/Q2TM7CBZ |
More data for this
Ligand-Target Pair | |
Lanosterol 14-alpha demethylase
(Homo sapiens (Human)) | BDBM20192

((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C |t:9| Show InChI InChI=1S/C27H46O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h9,18-19,21-25,28H,6-8,10-17H2,1-5H3/t19-,21+,22+,23-,24+,25+,26+,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
ACT LLC
Curated by ChEMBL
| Assay Description
Inhibition of human CYP51 expressed in Topp 3 cells by lanosterol demethylase assay |
Drug Metab Dispos 35: 493-500 (2007)
Article DOI: 10.1124/dmd.106.013888
BindingDB Entry DOI: 10.7270/Q2DF6S2H |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM20192

((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C |t:9| Show InChI InChI=1S/C27H46O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h9,18-19,21-25,28H,6-8,10-17H2,1-5H3/t19-,21+,22+,23-,24+,25+,26+,27-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human DNA polymerase alpha incubated with 0.05 units |
J Med Chem 47: 4971-4 (2004)
Article DOI: 10.1021/jm030553v
BindingDB Entry DOI: 10.7270/Q2RN37BH |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Rattus norvegicus) | BDBM20192

((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C |t:9| Show InChI InChI=1S/C27H46O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h9,18-19,21-25,28H,6-8,10-17H2,1-5H3/t19-,21+,22+,23-,24+,25+,26+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat DNA polymerase beta incubated with 0.05 units |
J Med Chem 47: 4971-4 (2004)
Article DOI: 10.1021/jm030553v
BindingDB Entry DOI: 10.7270/Q2RN37BH |
More data for this
Ligand-Target Pair | |
Oxysterol-binding protein 2
(Homo sapiens (Human)) | BDBM20192

((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C |t:9| Show InChI InChI=1S/C27H46O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h9,18-19,21-25,28H,6-8,10-17H2,1-5H3/t19-,21+,22+,23-,24+,25+,26+,27-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 68 | n/a | n/a | n/a | 7.4 | 20 |
Dalhousie University
| Assay Description
Recombinant OSBP, ORP4L, or ORP4S (8 pmol) was incubated in 75 μl of binding buffer (10 mM HEPES (pH 7.4), 150 mM KCl, 2% (w/v) polyvinyl alcohol)... |
J Biol Chem 289: 15705-17 (2014)
Article DOI: 10.1074/jbc.M114.571216
BindingDB Entry DOI: 10.7270/Q2N29VTQ |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM20192

((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C |t:9| Show InChI InChI=1S/C27H46O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h9,18-19,21-25,28H,6-8,10-17H2,1-5H3/t19-,21+,22+,23-,24+,25+,26+,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 418 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Orthosteric agonist activity at recombinant human N-terminal His6-tagged RORgammat ligand binding domain (265 to 518 residues) expressed in Escherich... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00029
BindingDB Entry DOI: 10.7270/Q2GX4G7Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM20192

((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C |t:9| Show InChI InChI=1S/C27H46O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h9,18-19,21-25,28H,6-8,10-17H2,1-5H3/t19-,21+,22+,23-,24+,25+,26+,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Agonist activity at 6xHis tagged human RORgammat LBD (262 to 507 residues) expressed in Escherichia coli BL21 (DE3) assessed as biotinylated SRC1-2 p... |
J Med Chem 61: 5794-5804 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01314
BindingDB Entry DOI: 10.7270/Q2M32Z85 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
TspO-like protein
(Fremyella diplosiphon (Cyanobacterium)) | BDBM20192

((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C |t:9| Show InChI InChI=1S/C27H46O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h9,18-19,21-25,28H,6-8,10-17H2,1-5H3/t19-,21+,22+,23-,24+,25+,26+,27-/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.02E+5 | n/a | n/a | n/a | n/a | n/a |
Michigan State University
| Assay Description
Tryptophan fluorescence measurements were performed with purified FdTSPO1 protein and ligand essentially as described by Li et al.; 2.5 μM prote... |
Biochemistry 56: 73-84 (2017)
Article DOI: 10.1021/acs.biochem.6b01019
BindingDB Entry DOI: 10.7270/Q2F47N0J |
More data for this
Ligand-Target Pair | |
TspO-like protein
(Fremyella diplosiphon (Cyanobacterium)) | BDBM20192

((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C |t:9| Show InChI InChI=1S/C27H46O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h9,18-19,21-25,28H,6-8,10-17H2,1-5H3/t19-,21+,22+,23-,24+,25+,26+,27-/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.75E+5 | n/a | n/a | n/a | n/a | n/a |
Michigan State University
| Assay Description
Tryptophan fluorescence measurements were performed with purified FdTSPO1 protein and ligand essentially as described by Li et al.; 2.5 μM prote... |
Biochemistry 56: 73-84 (2017)
Article DOI: 10.1021/acs.biochem.6b01019
BindingDB Entry DOI: 10.7270/Q2F47N0J |
More data for this
Ligand-Target Pair | |
Steroid C26-monooxygenase
(Mycobacterium tuberculosis) | BDBM20192

((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C |t:9| Show InChI InChI=1S/C27H46O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h9,18-19,21-25,28H,6-8,10-17H2,1-5H3/t19-,21+,22+,23-,24+,25+,26+,27-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a |
Manchester Interdisciplinary Biocentre
| Assay Description
Substrate and ligand binding assay using UV- visible absorbance analysis of CYP142 was done on a Cary UV-50 UV-visible scanning spectrophotometer (Va... |
J Biol Chem 285: 38270-82 (2010)
Article DOI: 10.1074/jbc.M110.164293
BindingDB Entry DOI: 10.7270/Q2251GR0 |
More data for this
Ligand-Target Pair | |
Steroid C26-monooxygenase
(Mycobacterium tuberculosis) | BDBM20192

((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C |t:9| Show InChI InChI=1S/C27H46O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h9,18-19,21-25,28H,6-8,10-17H2,1-5H3/t19-,21+,22+,23-,24+,25+,26+,27-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a |
Manchester Interdisciplinary Biocentre
| Assay Description
Substrate and ligand binding assay using UV- visible absorbance analysis of CYP142 was done on a Cary UV-50 UV-visible scanning spectrophotometer (Va... |
J Biol Chem 285: 38270-82 (2010)
Article DOI: 10.1074/jbc.M110.164293
BindingDB Entry DOI: 10.7270/Q2251GR0 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM20192

((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C |t:9| Show InChI InChI=1S/C27H46O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h9,18-19,21-25,28H,6-8,10-17H2,1-5H3/t19-,21+,22+,23-,24+,25+,26+,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Dartmouth College
| Assay Description
The LXR LiSA measures the ligand-dependent recruitment of a 25 amino acid fragment of the steroid receptor coactivator 1 (SRC1) to the ligand-binding... |
J Med Chem 44: 886-97 (2001)
Article DOI: 10.1021/jm0004749
BindingDB Entry DOI: 10.7270/Q2Z899P5 |
More data for this
Ligand-Target Pair | |
Oxysterol-binding protein 2 [1-181,275-916]
(Homo sapiens (Human)) | BDBM20192

((1S,2R,5S,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C |t:9| Show InChI InChI=1S/C27H46O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h9,18-19,21-25,28H,6-8,10-17H2,1-5H3/t19-,21+,22+,23-,24+,25+,26+,27-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 60 | n/a | n/a | n/a | 7.4 | 20 |
Dalhousie University
| Assay Description
Recombinant OSBP, ORP4L, or ORP4S (8 pmol) was incubated in 75 μl of binding buffer (10 mM HEPES (pH 7.4), 150 mM KCl, 2% (w/v) polyvinyl alcohol)... |
J Biol Chem 289: 15705-17 (2014)
Article DOI: 10.1074/jbc.M114.571216
BindingDB Entry DOI: 10.7270/Q2N29VTQ |
More data for this
Ligand-Target Pair | |