 Found 17 hits in this display
Found 17 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Corticosteroid-binding globulin
(Homo sapiens) | BDBM91713
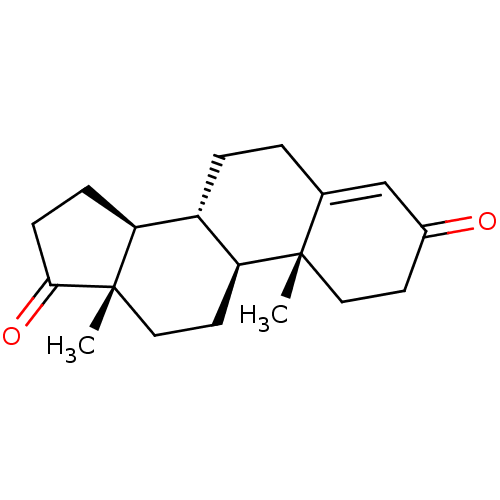
(Androst-4-en-3,17-dione, 2 | Androst-4-ene-3,17-di...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@]34C)[C@@H]1CCC2=O |t:8| Show InChI InChI=1S/C19H26O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-16H,3-10H2,1-2H3/t14-,15-,16-,18-,19-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Zoki Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human CBG receptor (corticosteroid-binding globulins) |
J Med Chem 47: 2732-42 (2004)
Article DOI: 10.1021/jm030364c
BindingDB Entry DOI: 10.7270/Q2WM1H5Q |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member
(Danio rerio (Zebrafish)) | BDBM91713

(Androst-4-en-3,17-dione, 2 | Androst-4-ene-3,17-di...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@]34C)[C@@H]1CCC2=O |t:8| Show InChI InChI=1S/C19H26O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-16H,3-10H2,1-2H3/t14-,15-,16-,18-,19-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rudjer Boskovic Institute
| Assay Description
In the inhibition experiments, the cells were preincubated for 20 s with test compounds, followed by a 5-min incubation with [3H]E3S (5 nM) or 30-min... |
J Biol Chem 288: 33894-911 (2013)
Article DOI: 10.1074/jbc.M113.518506
BindingDB Entry DOI: 10.7270/Q29Z93RK |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM91713

(Androst-4-en-3,17-dione, 2 | Androst-4-ene-3,17-di...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@]34C)[C@@H]1CCC2=O |t:8| Show InChI InChI=1S/C19H26O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-16H,3-10H2,1-2H3/t14-,15-,16-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro antagonist activity against rat prostatic androgen receptor (AR) |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM91713

(Androst-4-en-3,17-dione, 2 | Androst-4-ene-3,17-di...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@]34C)[C@@H]1CCC2=O |t:8| Show InChI InChI=1S/C19H26O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-16H,3-10H2,1-2H3/t14-,15-,16-,18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Steroid 5-alpha-reductase type 2 in transfected SW-13 cells using [3H]- delta4-Androstenedione as substrate |
J Med Chem 38: 1456-61 (1995)
BindingDB Entry DOI: 10.7270/Q2W096K6 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Rattus norvegicus) | BDBM91713

(Androst-4-en-3,17-dione, 2 | Androst-4-ene-3,17-di...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@]34C)[C@@H]1CCC2=O |t:8| Show InChI InChI=1S/C19H26O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-16H,3-10H2,1-2H3/t14-,15-,16-,18-,19-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 169 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec-Research Center (CHUL)
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 in rat testes microsomes using [14C]-4-androstene-3,17-dione as substrate after 2 hrs |
Bioorg Med Chem 23: 5433-51 (2015)
Article DOI: 10.1016/j.bmc.2015.07.049
BindingDB Entry DOI: 10.7270/Q20C4XHT |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM91713

(Androst-4-en-3,17-dione, 2 | Androst-4-ene-3,17-di...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@]34C)[C@@H]1CCC2=O |t:8| Show InChI InChI=1S/C19H26O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-16H,3-10H2,1-2H3/t14-,15-,16-,18-,19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 489 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire de Qu£bec and Universit£ Laval
Curated by ChEMBL
| Assay Description
Ability to inhibit the Type-3 17-beta- hydroxysteroid dehydrogenase activity transfected in human embryonic kidney (HEK)-293 cells experiment 3 |
J Med Chem 45: 640-53 (2002)
BindingDB Entry DOI: 10.7270/Q2K93881 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM91713

(Androst-4-en-3,17-dione, 2 | Androst-4-ene-3,17-di...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@]34C)[C@@H]1CCC2=O |t:8| Show InChI InChI=1S/C19H26O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-16H,3-10H2,1-2H3/t14-,15-,16-,18-,19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire de Qu£bec and Universit£ Laval
Curated by ChEMBL
| Assay Description
Ability to inhibit the Type-3 17-beta- hydroxysteroid dehydrogenase activity transfected in human embryonic kidney (HEK)-293 cells experiment 2 |
J Med Chem 45: 640-53 (2002)
BindingDB Entry DOI: 10.7270/Q2K93881 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM91713

(Androst-4-en-3,17-dione, 2 | Androst-4-ene-3,17-di...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@]34C)[C@@H]1CCC2=O |t:8| Show InChI InChI=1S/C19H26O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-16H,3-10H2,1-2H3/t14-,15-,16-,18-,19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 758 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUQ-Pavillon CHUL and Universit£ Laval
Curated by ChEMBL
| Assay Description
Inhibition of type-3 17 beta-hydroxysteroid dehydrogenase expressed in HEK293 cells at 37 degree C pH7.4 |
J Med Chem 48: 5257-68 (2005)
Article DOI: 10.1021/jm058179h
BindingDB Entry DOI: 10.7270/Q21C1XNS |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM91713

(Androst-4-en-3,17-dione, 2 | Androst-4-ene-3,17-di...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@]34C)[C@@H]1CCC2=O |t:8| Show InChI InChI=1S/C19H26O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-16H,3-10H2,1-2H3/t14-,15-,16-,18-,19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 758 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Center (CHUL) and Laval University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against type-3 17-beta-HSD expressed in HEK293 cells |
Bioorg Med Chem Lett 10: 2533-6 (2001)
BindingDB Entry DOI: 10.7270/Q2GH9JGF |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM91713

(Androst-4-en-3,17-dione, 2 | Androst-4-ene-3,17-di...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@]34C)[C@@H]1CCC2=O |t:8| Show InChI InChI=1S/C19H26O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-16H,3-10H2,1-2H3/t14-,15-,16-,18-,19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 758 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire de Qu£bec and Universit£ Laval
Curated by ChEMBL
| Assay Description
Ability to inhibit the Type-3 17-beta- hydroxysteroid dehydrogenase activity transfected in human embryonic kidney (HEK)-293 cells experiment 1 |
J Med Chem 45: 640-53 (2002)
BindingDB Entry DOI: 10.7270/Q2K93881 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM91713

(Androst-4-en-3,17-dione, 2 | Androst-4-ene-3,17-di...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@]34C)[C@@H]1CCC2=O |t:8| Show InChI InChI=1S/C19H26O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-16H,3-10H2,1-2H3/t14-,15-,16-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant rat androgen receptor expressed in Escherichia coli using [3H]methyltrienolone (R 1881) |
J Med Chem 48: 5666-74 (2005)
Article DOI: 10.1021/jm050403f
BindingDB Entry DOI: 10.7270/Q2TM7CBZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM91713

(Androst-4-en-3,17-dione, 2 | Androst-4-ene-3,17-di...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@]34C)[C@@H]1CCC2=O |t:8| Show InChI InChI=1S/C19H26O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-16H,3-10H2,1-2H3/t14-,15-,16-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jordan
| Assay Description
AChE and BChE inhibiting activities were measured in vitro by a modified spectrophotometric method previously developed by Ellman et. al. |
J Enzyme Inhib Med Chem 24: 553-8 (2009)
Article DOI: 10.1080/14756360802236393
BindingDB Entry DOI: 10.7270/Q20K274X |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM91713

(Androst-4-en-3,17-dione, 2 | Androst-4-ene-3,17-di...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@]34C)[C@@H]1CCC2=O |t:8| Show InChI InChI=1S/C19H26O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-16H,3-10H2,1-2H3/t14-,15-,16-,18-,19-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUL Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of human tSteroid 5-alpha-reductase type I in transfected 293 cells using [3H]- delta4-Androstenedione as substrate. |
J Med Chem 38: 1456-61 (1995)
BindingDB Entry DOI: 10.7270/Q2W096K6 |
More data for this
Ligand-Target Pair | |
Alpha-synuclein
(Homo sapiens (Human)) | BDBM91713

(Androst-4-en-3,17-dione, 2 | Androst-4-ene-3,17-di...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@]34C)[C@@H]1CCC2=O |t:8| Show InChI InChI=1S/C19H26O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-16H,3-10H2,1-2H3/t14-,15-,16-,18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of alpha-synuclein aggregation (unknown origin) incubated for 8 days by thioflavin S based fluorescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2019.01.045
BindingDB Entry DOI: 10.7270/Q2H998V9 |
More data for this
Ligand-Target Pair | |
Beta-glucuronidase
(Escherichia coli (Enterobacteria)) | BDBM91713

(Androst-4-en-3,17-dione, 2 | Androst-4-ene-3,17-di...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@]34C)[C@@H]1CCC2=O |t:8| Show InChI InChI=1S/C19H26O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-16H,3-10H2,1-2H3/t14-,15-,16-,18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.30E+5 | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Karachi
| Assay Description
β-Glucuronidase inhibitory activity was evaluated by a biochemical assay, based on the measurement of the absorbance of p-nitrophenol at 405 nm,... |
J Enzyme Inhib Med Chem 27: 348-55 (2012)
Article DOI: 10.3109/14756366.2011.590804
BindingDB Entry DOI: 10.7270/Q2N29VV5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM91713

(Androst-4-en-3,17-dione, 2 | Androst-4-ene-3,17-di...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@]34C)[C@@H]1CCC2=O |t:8| Show InChI InChI=1S/C19H26O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-16H,3-10H2,1-2H3/t14-,15-,16-,18-,19-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >6.98E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Sex hormone-binding globulin
(Homo sapiens (Human)) | BDBM91713

(Androst-4-en-3,17-dione, 2 | Androst-4-ene-3,17-di...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@]34C)[C@@H]1CCC2=O |t:8| Show InChI InChI=1S/C19H26O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-16H,3-10H2,1-2H3/t14-,15-,16-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a |
University of British Columbia
Curated by ChEMBL
| Assay Description
Displacement of [3H]5alpha dihydrotestosterone from human sex hormone binding globulin |
J Med Chem 51: 2047-56 (2008)
Article DOI: 10.1021/jm7011485
BindingDB Entry DOI: 10.7270/Q2RX9DC2 |
More data for this
Ligand-Target Pair | |