 Found 22 hits in this display
Found 22 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM21653
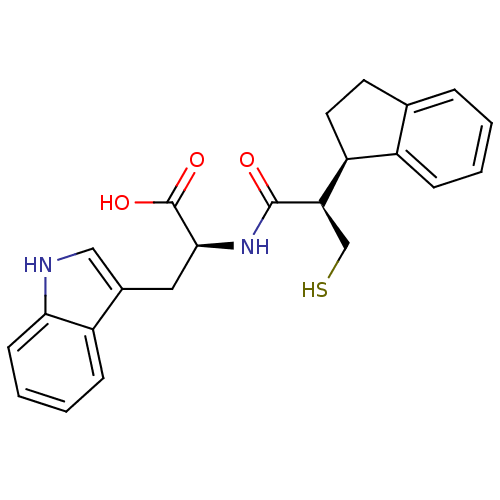
((2S)-2-[(2R)-2-[(1R)-2,3-dihydro-1H-inden-1-yl]-3-...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CS)[C@H]1CCc2ccccc12 |r| Show InChI InChI=1S/C23H24N2O3S/c26-22(19(13-29)18-10-9-14-5-1-2-6-16(14)18)25-21(23(27)28)11-15-12-24-20-8-4-3-7-17(15)20/h1-8,12,18-19,21,24,29H,9-11,13H2,(H,25,26)(H,27,28)/t18-,19+,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of Neutral endopeptidase. |
Bioorg Med Chem Lett 12: 2001-5 (2002)
BindingDB Entry DOI: 10.7270/Q27080S4 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM21653

((2S)-2-[(2R)-2-[(1R)-2,3-dihydro-1H-inden-1-yl]-3-...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CS)[C@H]1CCc2ccccc12 |r| Show InChI InChI=1S/C23H24N2O3S/c26-22(19(13-29)18-10-9-14-5-1-2-6-16(14)18)25-21(23(27)28)11-15-12-24-20-8-4-3-7-17(15)20/h1-8,12,18-19,21,24,29H,9-11,13H2,(H,25,26)(H,27,28)/t18-,19+,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | -54.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS
| Assay Description
NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... |
J Med Chem 45: 1477-86 (2002)
Article DOI: 10.1021/jm0005454
BindingDB Entry DOI: 10.7270/Q2T72FQ1 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50115842
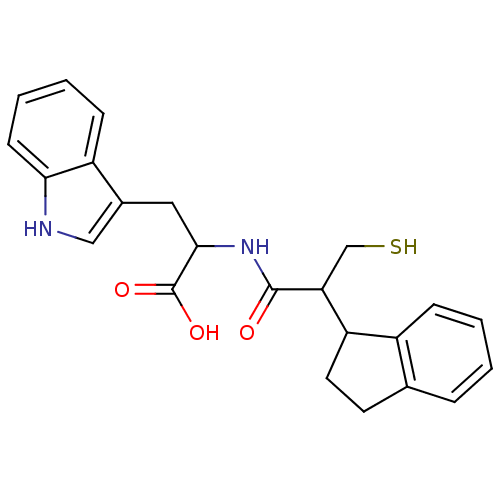
(2-(2-Indan-1-yl-3-mercapto-propionylamino)-3-(1H-i...)Show SMILES OC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)C(CS)C1CCc2ccccc12 Show InChI InChI=1S/C23H24N2O3S/c26-22(19(13-29)18-10-9-14-5-1-2-6-16(14)18)25-21(23(27)28)11-15-12-24-20-8-4-3-7-17(15)20/h1-8,12,18-19,21,24,29H,9-11,13H2,(H,25,26)(H,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of Neutral endopeptidase. |
Bioorg Med Chem Lett 12: 2001-5 (2002)
BindingDB Entry DOI: 10.7270/Q27080S4 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM21639
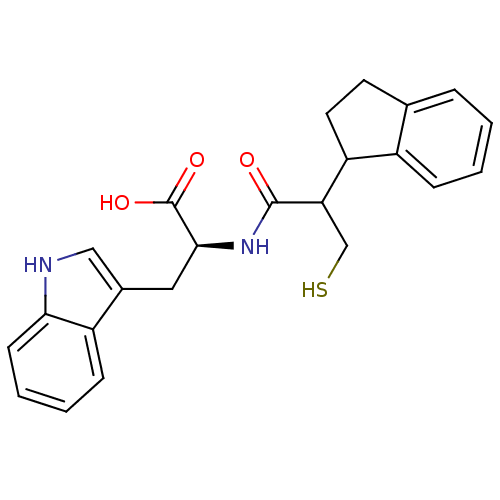
((2S)-2-[2-(2,3-dihydro-1H-inden-1-yl)-3-sulfanylpr...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CS)C1CCc2ccccc12 |r| Show InChI InChI=1S/C23H24N2O3S/c26-22(19(13-29)18-10-9-14-5-1-2-6-16(14)18)25-21(23(27)28)11-15-12-24-20-8-4-3-7-17(15)20/h1-8,12,18-19,21,24,29H,9-11,13H2,(H,25,26)(H,27,28)/t18?,19?,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS
| Assay Description
NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... |
J Med Chem 45: 1477-86 (2002)
Article DOI: 10.1021/jm0005454
BindingDB Entry DOI: 10.7270/Q2T72FQ1 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM21654
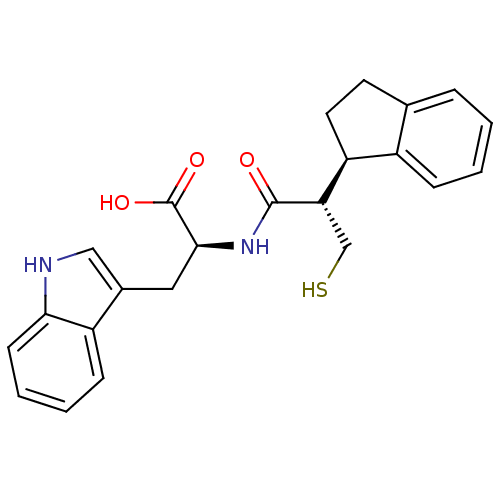
((2S)-2-[(2S)-2-[(1R)-2,3-dihydro-1H-inden-1-yl]-3-...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CS)[C@H]1CCc2ccccc12 |r| Show InChI InChI=1S/C23H24N2O3S/c26-22(19(13-29)18-10-9-14-5-1-2-6-16(14)18)25-21(23(27)28)11-15-12-24-20-8-4-3-7-17(15)20/h1-8,12,18-19,21,24,29H,9-11,13H2,(H,25,26)(H,27,28)/t18-,19-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | -51.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS
| Assay Description
NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... |
J Med Chem 45: 1477-86 (2002)
Article DOI: 10.1021/jm0005454
BindingDB Entry DOI: 10.7270/Q2T72FQ1 |
More data for this
Ligand-Target Pair | |
EEF1AKMT4-ECE2 readthrough transcript protein
(Homo sapiens (Human)) | BDBM92503
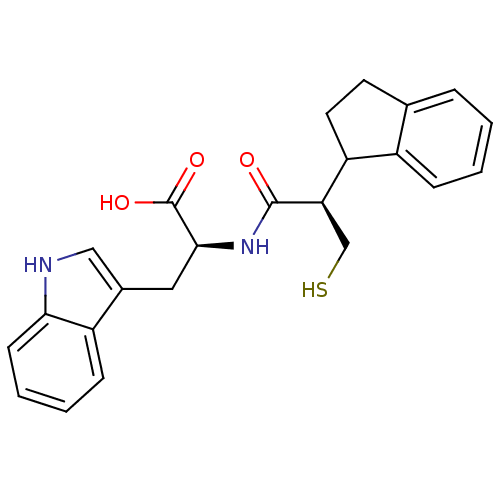
((2S)-2-[(2R)-2-(2,3-dihydro-1H-inden-1-yl)-3-sulfa...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CS)C1CCc2ccccc12 |r| Show InChI InChI=1S/C23H24N2O3S/c26-22(19(13-29)18-10-9-14-5-1-2-6-16(14)18)25-21(23(27)28)11-15-12-24-20-8-4-3-7-17(15)20/h1-8,12,18-19,21,24,29H,9-11,13H2,(H,25,26)(H,27,28)/t18?,19-,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.70 | n/a | 15.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaleads
| Assay Description
Inhibitory assay against ECE-2. |
J Biol Chem 285: 34390-400 (2010)
Article DOI: 10.1074/jbc.M110.120576
BindingDB Entry DOI: 10.7270/Q29P307K |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM21654

((2S)-2-[(2S)-2-[(1R)-2,3-dihydro-1H-inden-1-yl]-3-...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CS)[C@H]1CCc2ccccc12 |r| Show InChI InChI=1S/C23H24N2O3S/c26-22(19(13-29)18-10-9-14-5-1-2-6-16(14)18)25-21(23(27)28)11-15-12-24-20-8-4-3-7-17(15)20/h1-8,12,18-19,21,24,29H,9-11,13H2,(H,25,26)(H,27,28)/t18-,19-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
| Assay Description
Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... |
J Med Chem 45: 1477-86 (2002)
Article DOI: 10.1021/jm0005454
BindingDB Entry DOI: 10.7270/Q2T72FQ1 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM21652
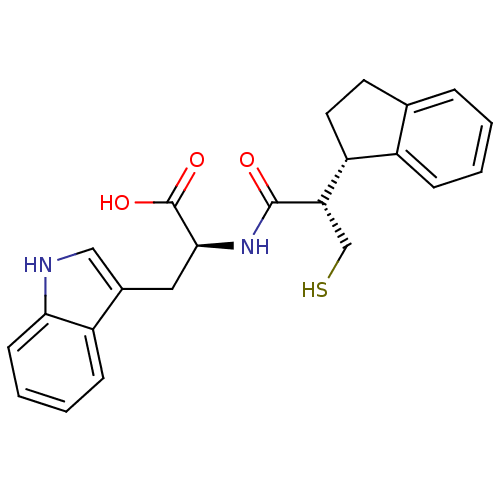
((2S)-2-[(2S)-2-[(1S)-2,3-dihydro-1H-inden-1-yl]-3-...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CS)[C@@H]1CCc2ccccc12 |r| Show InChI InChI=1S/C23H24N2O3S/c26-22(19(13-29)18-10-9-14-5-1-2-6-16(14)18)25-21(23(27)28)11-15-12-24-20-8-4-3-7-17(15)20/h1-8,12,18-19,21,24,29H,9-11,13H2,(H,25,26)(H,27,28)/t18-,19+,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS
| Assay Description
NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... |
J Med Chem 45: 1477-86 (2002)
Article DOI: 10.1021/jm0005454
BindingDB Entry DOI: 10.7270/Q2T72FQ1 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50115842

(2-(2-Indan-1-yl-3-mercapto-propionylamino)-3-(1H-i...)Show SMILES OC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)C(CS)C1CCc2ccccc12 Show InChI InChI=1S/C23H24N2O3S/c26-22(19(13-29)18-10-9-14-5-1-2-6-16(14)18)25-21(23(27)28)11-15-12-24-20-8-4-3-7-17(15)20/h1-8,12,18-19,21,24,29H,9-11,13H2,(H,25,26)(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme. |
Bioorg Med Chem Lett 12: 2001-5 (2002)
BindingDB Entry DOI: 10.7270/Q27080S4 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM21639

((2S)-2-[2-(2,3-dihydro-1H-inden-1-yl)-3-sulfanylpr...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CS)C1CCc2ccccc12 |r| Show InChI InChI=1S/C23H24N2O3S/c26-22(19(13-29)18-10-9-14-5-1-2-6-16(14)18)25-21(23(27)28)11-15-12-24-20-8-4-3-7-17(15)20/h1-8,12,18-19,21,24,29H,9-11,13H2,(H,25,26)(H,27,28)/t18?,19?,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
| Assay Description
Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... |
J Med Chem 45: 1477-86 (2002)
Article DOI: 10.1021/jm0005454
BindingDB Entry DOI: 10.7270/Q2T72FQ1 |
More data for this
Ligand-Target Pair | |
Endothelin-converting enzyme 1
(Homo sapiens (Human)) | BDBM21653

((2S)-2-[(2R)-2-[(1R)-2,3-dihydro-1H-inden-1-yl]-3-...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CS)[C@H]1CCc2ccccc12 |r| Show InChI InChI=1S/C23H24N2O3S/c26-22(19(13-29)18-10-9-14-5-1-2-6-16(14)18)25-21(23(27)28)11-15-12-24-20-8-4-3-7-17(15)20/h1-8,12,18-19,21,24,29H,9-11,13H2,(H,25,26)(H,27,28)/t18-,19+,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of endothelin converting enzyme. |
Bioorg Med Chem Lett 12: 2001-5 (2002)
BindingDB Entry DOI: 10.7270/Q27080S4 |
More data for this
Ligand-Target Pair | |
Endothelin-converting enzyme 1
(Homo sapiens (Human)) | BDBM21653

((2S)-2-[(2R)-2-[(1R)-2,3-dihydro-1H-inden-1-yl]-3-...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CS)[C@H]1CCc2ccccc12 |r| Show InChI InChI=1S/C23H24N2O3S/c26-22(19(13-29)18-10-9-14-5-1-2-6-16(14)18)25-21(23(27)28)11-15-12-24-20-8-4-3-7-17(15)20/h1-8,12,18-19,21,24,29H,9-11,13H2,(H,25,26)(H,27,28)/t18-,19+,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
| Assay Description
ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... |
J Med Chem 45: 1477-86 (2002)
Article DOI: 10.1021/jm0005454
BindingDB Entry DOI: 10.7270/Q2T72FQ1 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM21655
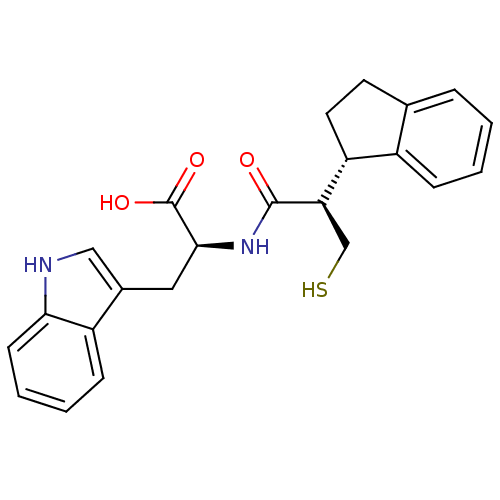
((2S)-2-[(2R)-2-[(1S)-2,3-dihydro-1H-inden-1-yl]-3-...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CS)[C@@H]1CCc2ccccc12 |r| Show InChI InChI=1S/C23H24N2O3S/c26-22(19(13-29)18-10-9-14-5-1-2-6-16(14)18)25-21(23(27)28)11-15-12-24-20-8-4-3-7-17(15)20/h1-8,12,18-19,21,24,29H,9-11,13H2,(H,25,26)(H,27,28)/t18-,19-,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | -44.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS
| Assay Description
NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... |
J Med Chem 45: 1477-86 (2002)
Article DOI: 10.1021/jm0005454
BindingDB Entry DOI: 10.7270/Q2T72FQ1 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM21652

((2S)-2-[(2S)-2-[(1S)-2,3-dihydro-1H-inden-1-yl]-3-...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CS)[C@@H]1CCc2ccccc12 |r| Show InChI InChI=1S/C23H24N2O3S/c26-22(19(13-29)18-10-9-14-5-1-2-6-16(14)18)25-21(23(27)28)11-15-12-24-20-8-4-3-7-17(15)20/h1-8,12,18-19,21,24,29H,9-11,13H2,(H,25,26)(H,27,28)/t18-,19+,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
| Assay Description
Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... |
J Med Chem 45: 1477-86 (2002)
Article DOI: 10.1021/jm0005454
BindingDB Entry DOI: 10.7270/Q2T72FQ1 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM21653

((2S)-2-[(2R)-2-[(1R)-2,3-dihydro-1H-inden-1-yl]-3-...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CS)[C@H]1CCc2ccccc12 |r| Show InChI InChI=1S/C23H24N2O3S/c26-22(19(13-29)18-10-9-14-5-1-2-6-16(14)18)25-21(23(27)28)11-15-12-24-20-8-4-3-7-17(15)20/h1-8,12,18-19,21,24,29H,9-11,13H2,(H,25,26)(H,27,28)/t18-,19+,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
| Assay Description
Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... |
J Med Chem 45: 1477-86 (2002)
Article DOI: 10.1021/jm0005454
BindingDB Entry DOI: 10.7270/Q2T72FQ1 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM21653

((2S)-2-[(2R)-2-[(1R)-2,3-dihydro-1H-inden-1-yl]-3-...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CS)[C@H]1CCc2ccccc12 |r| Show InChI InChI=1S/C23H24N2O3S/c26-22(19(13-29)18-10-9-14-5-1-2-6-16(14)18)25-21(23(27)28)11-15-12-24-20-8-4-3-7-17(15)20/h1-8,12,18-19,21,24,29H,9-11,13H2,(H,25,26)(H,27,28)/t18-,19+,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme. |
Bioorg Med Chem Lett 12: 2001-5 (2002)
BindingDB Entry DOI: 10.7270/Q27080S4 |
More data for this
Ligand-Target Pair | |
Endothelin-converting enzyme 1
(Homo sapiens (Human)) | BDBM21639

((2S)-2-[2-(2,3-dihydro-1H-inden-1-yl)-3-sulfanylpr...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C(CS)C1CCc2ccccc12 |r| Show InChI InChI=1S/C23H24N2O3S/c26-22(19(13-29)18-10-9-14-5-1-2-6-16(14)18)25-21(23(27)28)11-15-12-24-20-8-4-3-7-17(15)20/h1-8,12,18-19,21,24,29H,9-11,13H2,(H,25,26)(H,27,28)/t18?,19?,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
| Assay Description
ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... |
J Med Chem 45: 1477-86 (2002)
Article DOI: 10.1021/jm0005454
BindingDB Entry DOI: 10.7270/Q2T72FQ1 |
More data for this
Ligand-Target Pair | |
Endothelin-converting enzyme 1
(Homo sapiens (Human)) | BDBM50115842

(2-(2-Indan-1-yl-3-mercapto-propionylamino)-3-(1H-i...)Show SMILES OC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)C(CS)C1CCc2ccccc12 Show InChI InChI=1S/C23H24N2O3S/c26-22(19(13-29)18-10-9-14-5-1-2-6-16(14)18)25-21(23(27)28)11-15-12-24-20-8-4-3-7-17(15)20/h1-8,12,18-19,21,24,29H,9-11,13H2,(H,25,26)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of endothelin converting enzyme. |
Bioorg Med Chem Lett 12: 2001-5 (2002)
BindingDB Entry DOI: 10.7270/Q27080S4 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM21655

((2S)-2-[(2R)-2-[(1S)-2,3-dihydro-1H-inden-1-yl]-3-...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CS)[C@@H]1CCc2ccccc12 |r| Show InChI InChI=1S/C23H24N2O3S/c26-22(19(13-29)18-10-9-14-5-1-2-6-16(14)18)25-21(23(27)28)11-15-12-24-20-8-4-3-7-17(15)20/h1-8,12,18-19,21,24,29H,9-11,13H2,(H,25,26)(H,27,28)/t18-,19-,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
| Assay Description
Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... |
J Med Chem 45: 1477-86 (2002)
Article DOI: 10.1021/jm0005454
BindingDB Entry DOI: 10.7270/Q2T72FQ1 |
More data for this
Ligand-Target Pair | |
Endothelin-converting enzyme 1
(Homo sapiens (Human)) | BDBM21654

((2S)-2-[(2S)-2-[(1R)-2,3-dihydro-1H-inden-1-yl]-3-...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CS)[C@H]1CCc2ccccc12 |r| Show InChI InChI=1S/C23H24N2O3S/c26-22(19(13-29)18-10-9-14-5-1-2-6-16(14)18)25-21(23(27)28)11-15-12-24-20-8-4-3-7-17(15)20/h1-8,12,18-19,21,24,29H,9-11,13H2,(H,25,26)(H,27,28)/t18-,19-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
| Assay Description
ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... |
J Med Chem 45: 1477-86 (2002)
Article DOI: 10.1021/jm0005454
BindingDB Entry DOI: 10.7270/Q2T72FQ1 |
More data for this
Ligand-Target Pair | |
Endothelin-converting enzyme 1
(Homo sapiens (Human)) | BDBM21655

((2S)-2-[(2R)-2-[(1S)-2,3-dihydro-1H-inden-1-yl]-3-...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CS)[C@@H]1CCc2ccccc12 |r| Show InChI InChI=1S/C23H24N2O3S/c26-22(19(13-29)18-10-9-14-5-1-2-6-16(14)18)25-21(23(27)28)11-15-12-24-20-8-4-3-7-17(15)20/h1-8,12,18-19,21,24,29H,9-11,13H2,(H,25,26)(H,27,28)/t18-,19-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
| Assay Description
ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... |
J Med Chem 45: 1477-86 (2002)
Article DOI: 10.1021/jm0005454
BindingDB Entry DOI: 10.7270/Q2T72FQ1 |
More data for this
Ligand-Target Pair | |
Endothelin-converting enzyme 1
(Homo sapiens (Human)) | BDBM21652

((2S)-2-[(2S)-2-[(1S)-2,3-dihydro-1H-inden-1-yl]-3-...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](CS)[C@@H]1CCc2ccccc12 |r| Show InChI InChI=1S/C23H24N2O3S/c26-22(19(13-29)18-10-9-14-5-1-2-6-16(14)18)25-21(23(27)28)11-15-12-24-20-8-4-3-7-17(15)20/h1-8,12,18-19,21,24,29H,9-11,13H2,(H,25,26)(H,27,28)/t18-,19+,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
| Assay Description
ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... |
J Med Chem 45: 1477-86 (2002)
Article DOI: 10.1021/jm0005454
BindingDB Entry DOI: 10.7270/Q2T72FQ1 |
More data for this
Ligand-Target Pair | |