

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM36656 (Cyclobutylmethyl cyclic urea) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | 6.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Enzyme inhibition assay using cyclic ureas to inhibit GAG polyprotein. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM36657 (2-Naphthylmethyl cyclic urea) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 6.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Enzyme inhibition assay using cyclic ureas to inhibit GAG polyprotein. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 6.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Enzyme inhibition assay using cyclic ureas to inhibit GAG polyprotein. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 13.1 | n/a | n/a | n/a | n/a | 6.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Enzyme inhibition assay using cyclic ureas to inhibit GAG polyprotein. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 6.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Enzyme inhibition assay using cyclic ureas to inhibit GAG polyprotein. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM36655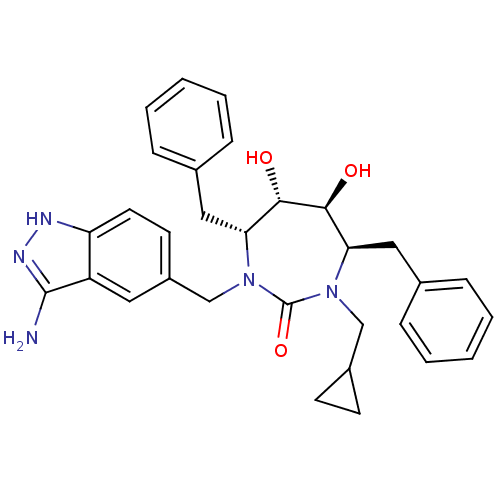 (Cyclopropylmethyl cyclic urea) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 6.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Enzyme inhibition assay using cyclic ureas to inhibit GAG polyprotein. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50124714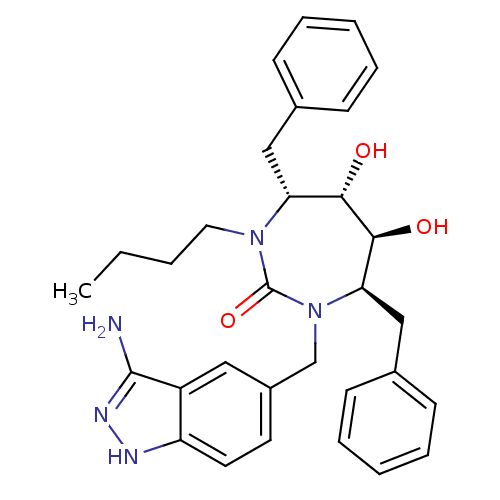 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-4,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 39.5 | n/a | n/a | n/a | n/a | 6.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Enzyme inhibition assay using cyclic ureas to inhibit GAG polyprotein. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM36658 (n-Pentyl cyclic urea) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | 6.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Enzyme inhibition assay using cyclic ureas to inhibit GAG polyprotein. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM36646 (DMP850) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | 6.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Enzyme inhibition assay using cyclic ureas to inhibit GAG polyprotein. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM36659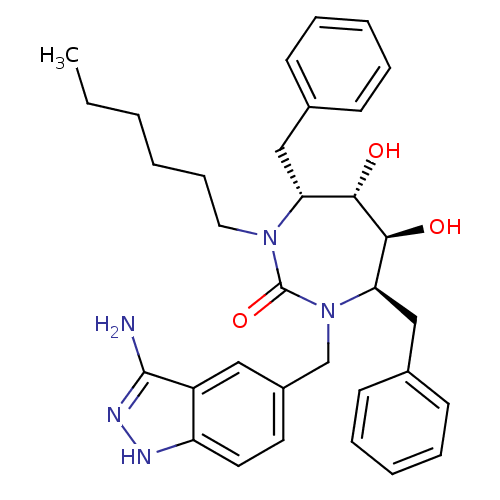 (n-Hexyl cyclic urea) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 6.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Enzyme inhibition assay using cyclic ureas to inhibit GAG polyprotein. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Avian erythroblastosis virus (strain ES4)) | BDBM50273882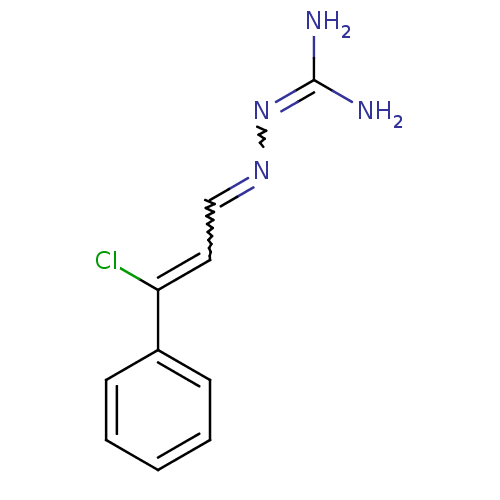 (2-[3-chloro-3-phenylprop-2-enylidene]hydrazinecarb...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor alpha (unknown origin) expressed in human HEC1 cells assessed as inhibition of 1 nM estradiol-induced transc... | Bioorg Med Chem 16: 10075-84 (2008) Article DOI: 10.1016/j.bmc.2008.10.007 BindingDB Entry DOI: 10.7270/Q28915Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Avian erythroblastosis virus (strain ES4)) | BDBM50273849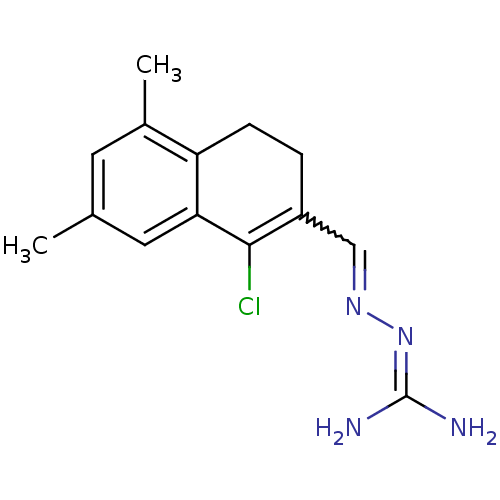 (1-Chloro-5,7-dimethyl-3,4-dihydronaphthalene-2-car...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor alpha (unknown origin) expressed in human HEC1 cells assessed as inhibition of 1 nM estradiol-induced transc... | Bioorg Med Chem 16: 10075-84 (2008) Article DOI: 10.1016/j.bmc.2008.10.007 BindingDB Entry DOI: 10.7270/Q28915Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Avian erythroblastosis virus (strain ES4)) | BDBM50273851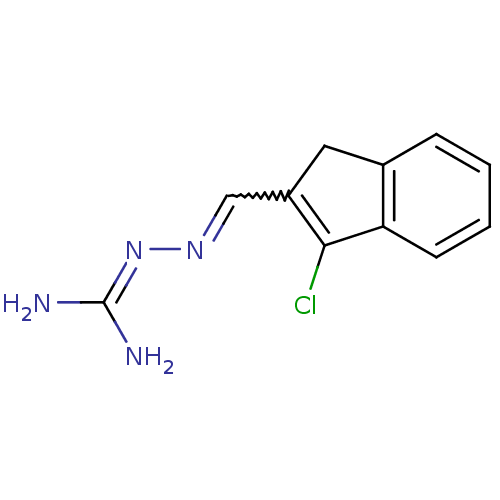 (2-[(3-chloro-1H-inden-2-yl)methylene]hydrazinecarb...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor alpha (unknown origin) expressed in human HEC1 cells assessed as inhibition of 1 nM estradiol-induced transc... | Bioorg Med Chem 16: 10075-84 (2008) Article DOI: 10.1016/j.bmc.2008.10.007 BindingDB Entry DOI: 10.7270/Q28915Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Avian erythroblastosis virus (strain ES4)) | BDBM50253469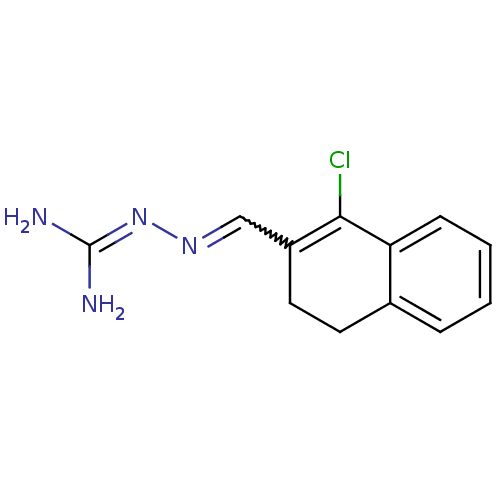 (1-Chloro-3,4-dihydronaphthalene-2-carboxaldehyde g...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor alpha (unknown origin) expressed in human HEC1 cells assessed as inhibition of 1 nM estradiol-induced transc... | Bioorg Med Chem 16: 10075-84 (2008) Article DOI: 10.1016/j.bmc.2008.10.007 BindingDB Entry DOI: 10.7270/Q28915Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Avian erythroblastosis virus (strain ES4)) | BDBM50273883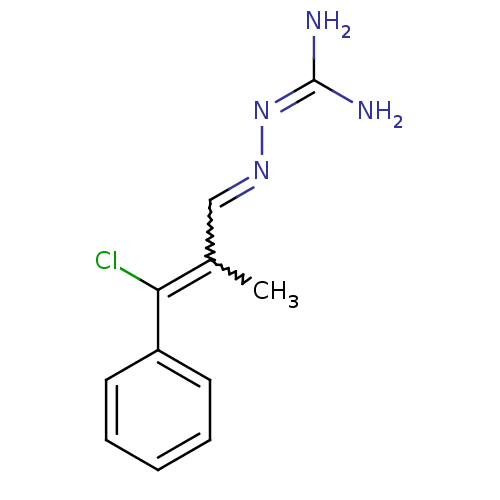 (CHEMBL460174 | beta-Chloro-alpha-methylcinnamaldeh...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor alpha (unknown origin) expressed in human HEC1 cells assessed as inhibition of 1 nM estradiol-induced transc... | Bioorg Med Chem 16: 10075-84 (2008) Article DOI: 10.1016/j.bmc.2008.10.007 BindingDB Entry DOI: 10.7270/Q28915Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Avian erythroblastosis virus (strain ES4)) | BDBM50273850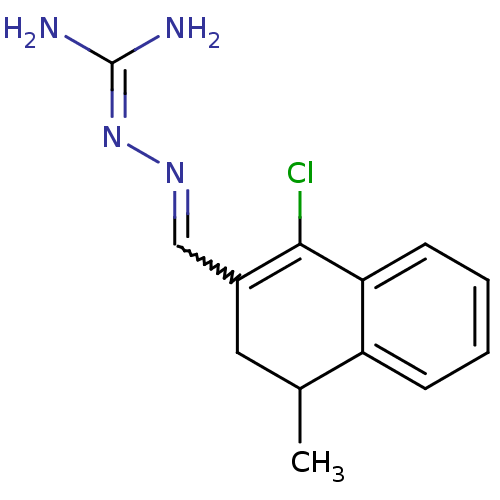 (1-Chloro-3-dihydro-4-methylnaphthalene-2-carboxald...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor alpha (unknown origin) expressed in human HEC1 cells assessed as inhibition of 1 nM estradiol-induced transc... | Bioorg Med Chem 16: 10075-84 (2008) Article DOI: 10.1016/j.bmc.2008.10.007 BindingDB Entry DOI: 10.7270/Q28915Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Avian erythroblastosis virus (strain ES4)) | BDBM50273848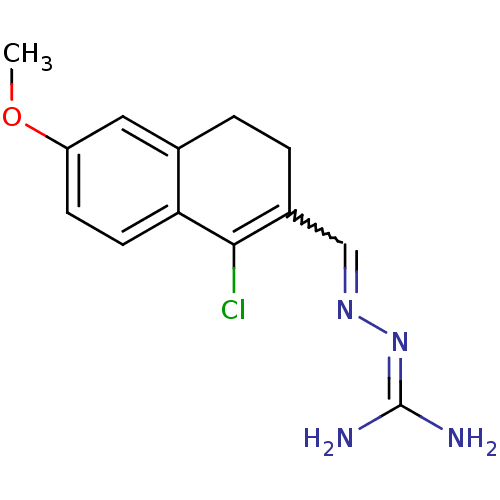 (1-Chloro-6-methoxy-3,4-dihydronaphthalene-2-carbox...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor alpha (unknown origin) expressed in human HEC1 cells assessed as inhibition of 1 nM estradiol-induced transc... | Bioorg Med Chem 16: 10075-84 (2008) Article DOI: 10.1016/j.bmc.2008.10.007 BindingDB Entry DOI: 10.7270/Q28915Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Avian erythroblastosis virus (strain ES4)) | BDBM50273854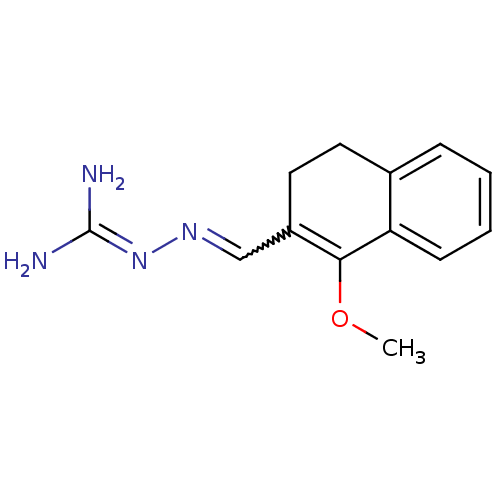 (1-Methoxy-3,4-dihydronaphthalene-2-carboxaldehyde ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor alpha (unknown origin) expressed in human HEC1 cells assessed as inhibition of 1 nM estradiol-induced transc... | Bioorg Med Chem 16: 10075-84 (2008) Article DOI: 10.1016/j.bmc.2008.10.007 BindingDB Entry DOI: 10.7270/Q28915Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Avian erythroblastosis virus (strain ES4)) | BDBM50273852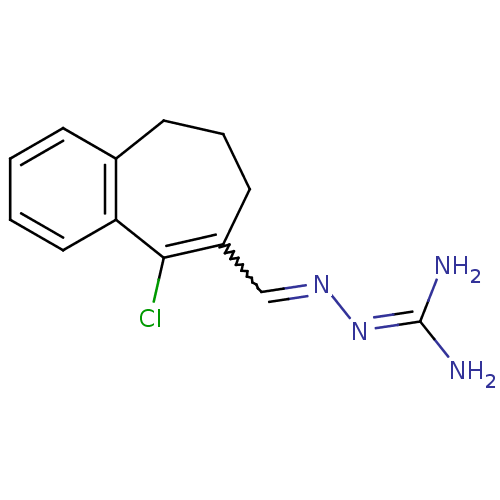 (9-Chloro-6,7-dihydro-5-benzocycloheptene-8-carboxa...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor alpha (unknown origin) expressed in human HEC1 cells assessed as inhibition of 1 nM estradiol-induced transc... | Bioorg Med Chem 16: 10075-84 (2008) Article DOI: 10.1016/j.bmc.2008.10.007 BindingDB Entry DOI: 10.7270/Q28915Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Avian erythroblastosis virus (strain ES4)) | BDBM50273880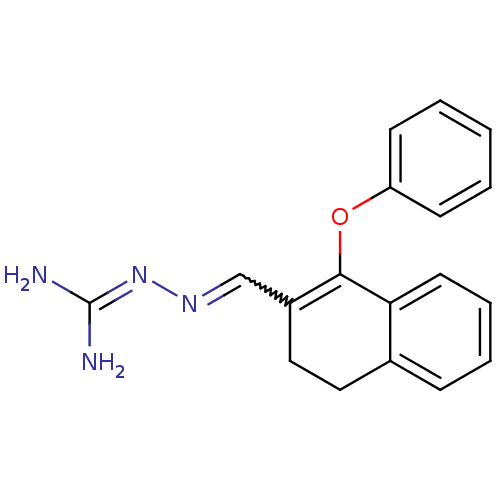 (1-Phenoxy-3,4-dihydronaphthalene-2-carboxaldehyde ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor alpha (unknown origin) expressed in human HEC1 cells assessed as inhibition of 1 nM estradiol-induced transc... | Bioorg Med Chem 16: 10075-84 (2008) Article DOI: 10.1016/j.bmc.2008.10.007 BindingDB Entry DOI: 10.7270/Q28915Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Avian erythroblastosis virus (strain ES4)) | BDBM50273847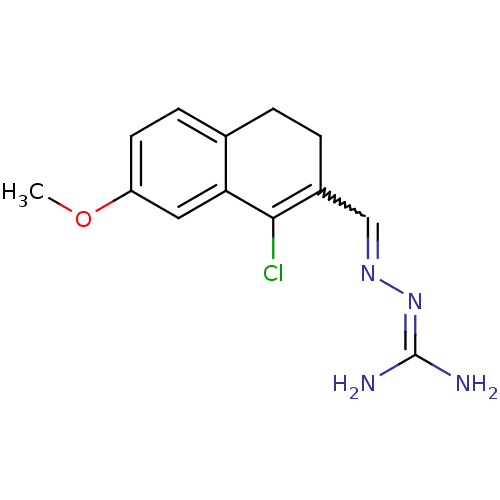 (1-Chloro-7-methoxy-3,4-dihydronaphthalene-2-carbox...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor alpha (unknown origin) expressed in human HEC1 cells assessed as inhibition of 1 nM estradiol-induced transc... | Bioorg Med Chem 16: 10075-84 (2008) Article DOI: 10.1016/j.bmc.2008.10.007 BindingDB Entry DOI: 10.7270/Q28915Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Avian erythroblastosis virus (strain ES4)) | BDBM50240439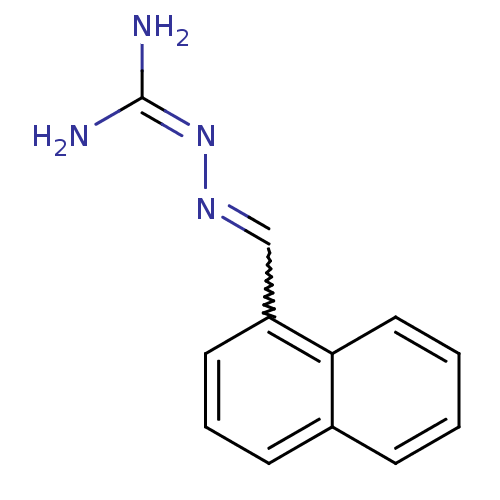 (2-(1-Naphthalenylmethylene) hydrazinecarboximidami...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor alpha (unknown origin) expressed in human HEC1 cells assessed as inhibition of 1 nM estradiol-induced transc... | Bioorg Med Chem 16: 10075-84 (2008) Article DOI: 10.1016/j.bmc.2008.10.007 BindingDB Entry DOI: 10.7270/Q28915Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Avian erythroblastosis virus (strain ES4)) | BDBM50240438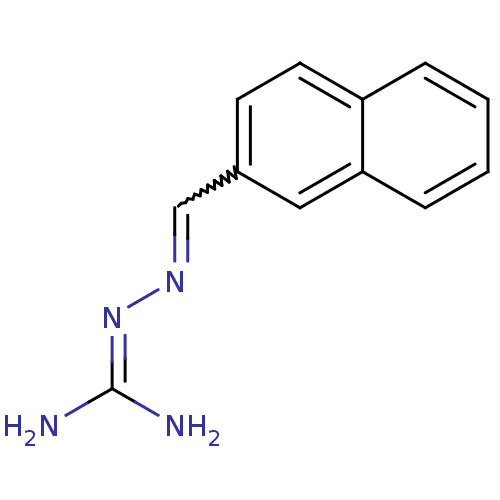 (2-(2-Naphthalenylmethylene)hydrazinecarboximidamid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor alpha (unknown origin) expressed in human HEC1 cells assessed as inhibition of 1 nM estradiol-induced transc... | Bioorg Med Chem 16: 10075-84 (2008) Article DOI: 10.1016/j.bmc.2008.10.007 BindingDB Entry DOI: 10.7270/Q28915Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Avian erythroblastosis virus (strain ES4)) | BDBM50273853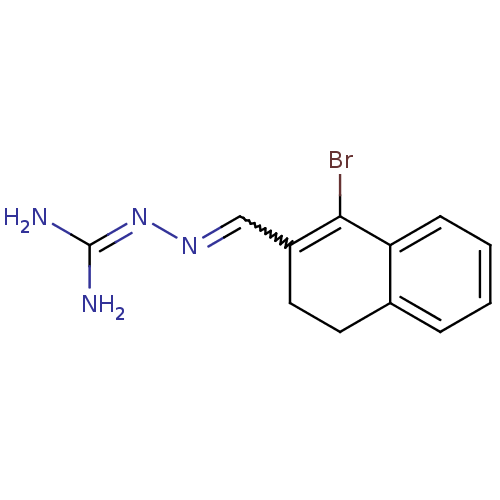 (1-Bromo-3,4-dihydronaphthalene-2-carboxaldehyde gu...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor alpha (unknown origin) expressed in human HEC1 cells assessed as inhibition of 1 nM estradiol-induced transc... | Bioorg Med Chem 16: 10075-84 (2008) Article DOI: 10.1016/j.bmc.2008.10.007 BindingDB Entry DOI: 10.7270/Q28915Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Avian erythroblastosis virus (strain ES4)) | BDBM50273881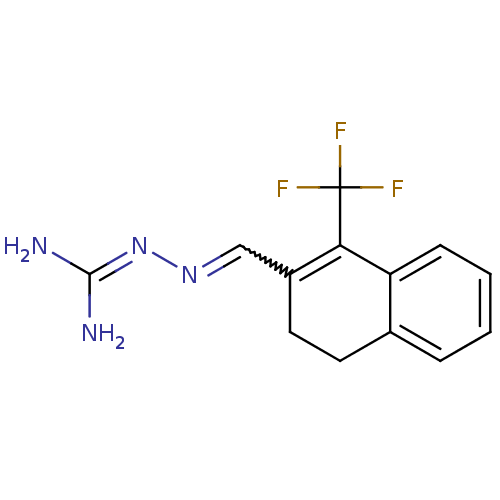 (1-(1,1,1,-Trifluoromethyl)-3,4-dihydronaphthalene-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor alpha (unknown origin) expressed in human HEC1 cells assessed as inhibition of 1 nM estradiol-induced transc... | Bioorg Med Chem 16: 10075-84 (2008) Article DOI: 10.1016/j.bmc.2008.10.007 BindingDB Entry DOI: 10.7270/Q28915Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Human immunodeficiency virus 1) | BDBM50450015 (Bms-955176 | GSK-3532795) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to wild type HIV1 gag polyprotein containing viral like particle | J Med Chem 61: 7289-7313 (2018) Article DOI: 10.1021/acs.jmedchem.8b00854 BindingDB Entry DOI: 10.7270/Q2377C8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag polyprotein (Human immunodeficiency virus 1) | BDBM50450016 (CHEMBL4163247) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to wild type HIV1 gag polyprotein containing viral like particle | J Med Chem 61: 7289-7313 (2018) Article DOI: 10.1021/acs.jmedchem.8b00854 BindingDB Entry DOI: 10.7270/Q2377C8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||