

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM85043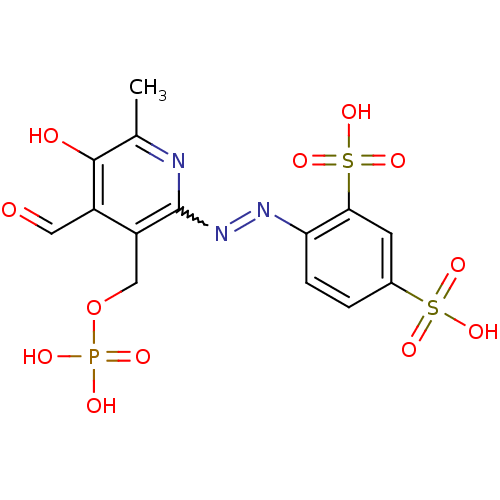 (CAS_149017-66-3 | CHEMBL69234 | NSC_6093163 | PPAD...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 27.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Institute for Experimental Medicine Curated by PDSP Ki Database | Mol Pharmacol 51: 109-18 (1997) Article DOI: 10.1124/mol.51.1.109 BindingDB Entry DOI: 10.7270/Q218351B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50300129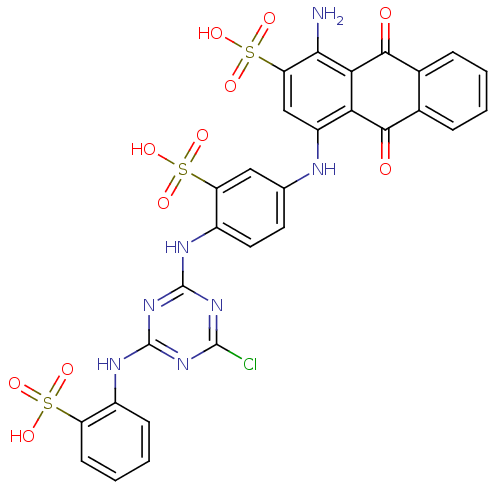 (CHEMBL572528 | CIBACRON BLUE | Cibacron Blue 3Ga) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 39.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Institute for Experimental Medicine Curated by PDSP Ki Database | Mol Pharmacol 51: 109-18 (1997) Article DOI: 10.1124/mol.51.1.109 BindingDB Entry DOI: 10.7270/Q218351B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (RAT) | BDBM50029031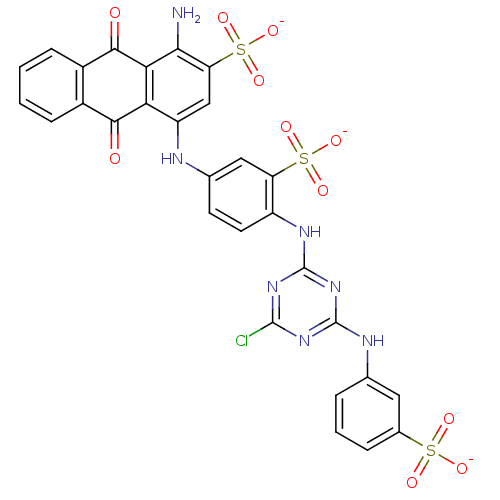 (1-Amino-4-{4-[4-chloro-6-(3-sulfo-phenylamino)-[1,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 46.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Institute for Experimental Medicine Curated by PDSP Ki Database | Mol Pharmacol 51: 109-18 (1997) Article DOI: 10.1124/mol.51.1.109 BindingDB Entry DOI: 10.7270/Q218351B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50370141 (TNP-ATP) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Competitive antagonist activity at human P2X4 receptor expressed in 1321N1 cell membrane assessed as inhibition of [35S]ATPgammaS binding by scintill... | J Med Chem 55: 9576-88 (2012) Article DOI: 10.1021/jm300845v BindingDB Entry DOI: 10.7270/Q2XK8GPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM85044 (Bromphenol blue | Brophenol Blue | CAS_115-39-9 | ...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 78.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Institute for Experimental Medicine Curated by PDSP Ki Database | Mol Pharmacol 51: 109-18 (1997) Article DOI: 10.1124/mol.51.1.109 BindingDB Entry DOI: 10.7270/Q218351B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (RAT) | BDBM50300129 (CHEMBL572528 | CIBACRON BLUE | Cibacron Blue 3Ga) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Institute for Experimental Medicine Curated by PDSP Ki Database | Mol Pharmacol 51: 109-18 (1997) Article DOI: 10.1124/mol.51.1.109 BindingDB Entry DOI: 10.7270/Q218351B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50000029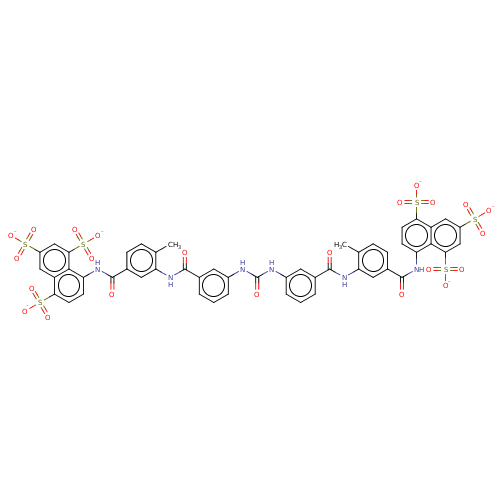 (4-Methyl-8-{4-methyl-3-[3-(3-{3-[2-methyl-5-(4,6,8...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | 178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Institute for Experimental Medicine Curated by PDSP Ki Database | Mol Pharmacol 51: 109-18 (1997) Article DOI: 10.1124/mol.51.1.109 BindingDB Entry DOI: 10.7270/Q218351B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (RAT) | BDBM85044 (Bromphenol blue | Brophenol Blue | CAS_115-39-9 | ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Institute for Experimental Medicine Curated by PDSP Ki Database | Mol Pharmacol 51: 109-18 (1997) Article DOI: 10.1124/mol.51.1.109 BindingDB Entry DOI: 10.7270/Q218351B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (RAT) | BDBM50000029 (4-Methyl-8-{4-methyl-3-[3-(3-{3-[2-methyl-5-(4,6,8...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Institute for Experimental Medicine Curated by PDSP Ki Database | Mol Pharmacol 51: 109-18 (1997) Article DOI: 10.1124/mol.51.1.109 BindingDB Entry DOI: 10.7270/Q218351B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (RAT) | BDBM85043 (CAS_149017-66-3 | CHEMBL69234 | NSC_6093163 | PPAD...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Institute for Experimental Medicine Curated by PDSP Ki Database | Mol Pharmacol 51: 109-18 (1997) Article DOI: 10.1124/mol.51.1.109 BindingDB Entry DOI: 10.7270/Q218351B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50000029 (4-Methyl-8-{4-methyl-3-[3-(3-{3-[2-methyl-5-(4,6,8...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biocentre Niederursel Curated by PDSP Ki Database | Naunyn Schmiedebergs Arch Pharmacol 362: 340-50 (2000) Article DOI: 10.1007/s002100000312 BindingDB Entry DOI: 10.7270/Q2CF9NPR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM85730 (CAS_5311313 | NF023 | NSC_5311313) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biocentre Niederursel Curated by PDSP Ki Database | Naunyn Schmiedebergs Arch Pharmacol 362: 340-50 (2000) Article DOI: 10.1007/s002100000312 BindingDB Entry DOI: 10.7270/Q2CF9NPR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM85682 (CAS_5311315 | NF279 | NSC_5311315) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biocentre Niederursel Curated by PDSP Ki Database | Naunyn Schmiedebergs Arch Pharmacol 362: 340-50 (2000) Article DOI: 10.1007/s002100000312 BindingDB Entry DOI: 10.7270/Q2CF9NPR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506156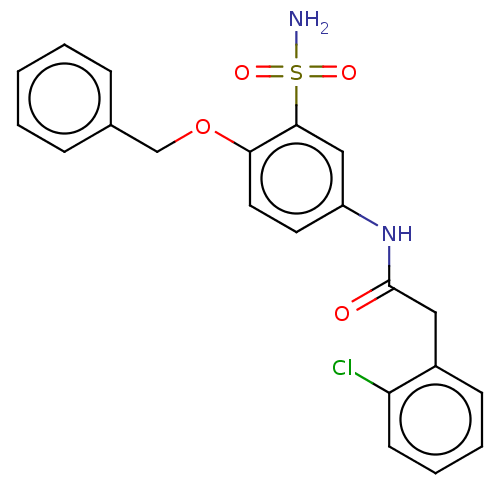 (CHEMBL4471140) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506203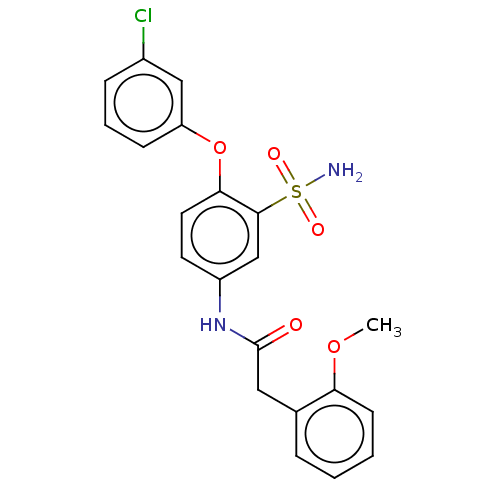 (CHEMBL4452312) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506173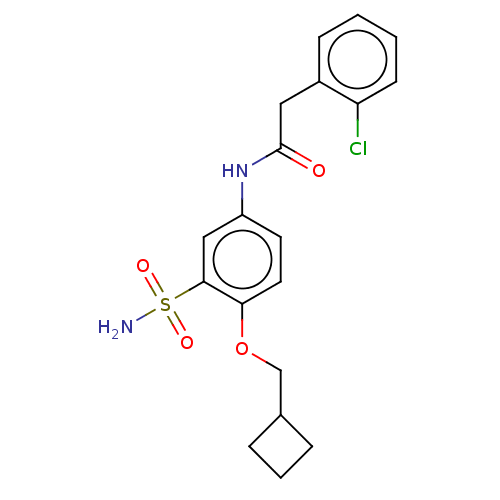 (CHEMBL4589444) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506179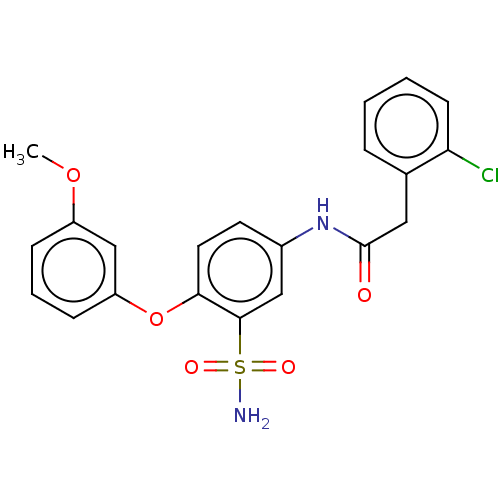 (CHEMBL4579583) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506184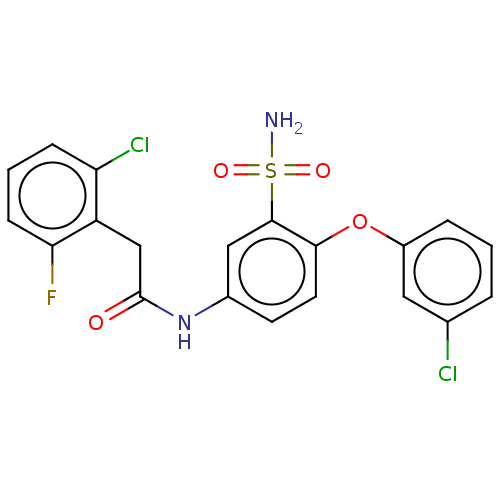 (CHEMBL4447043) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506180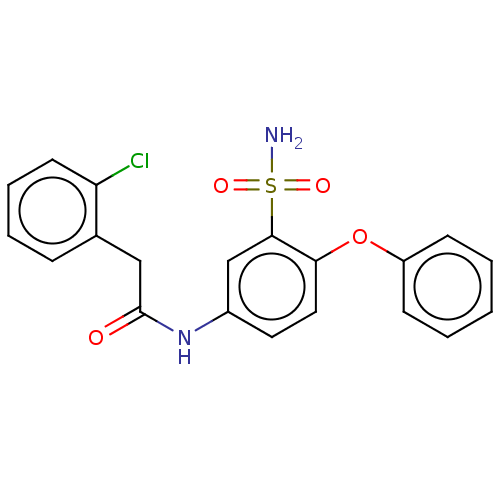 (CHEMBL4556573) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506187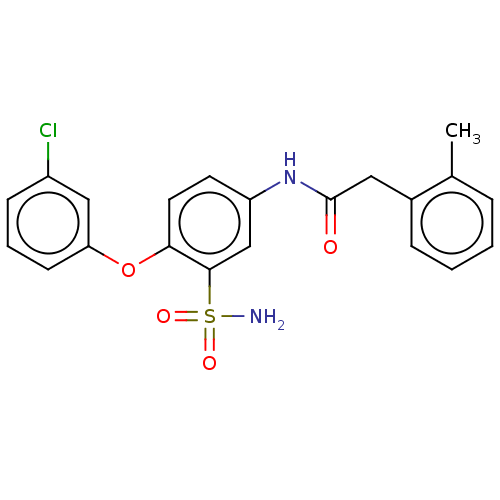 (CHEMBL4522504) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506178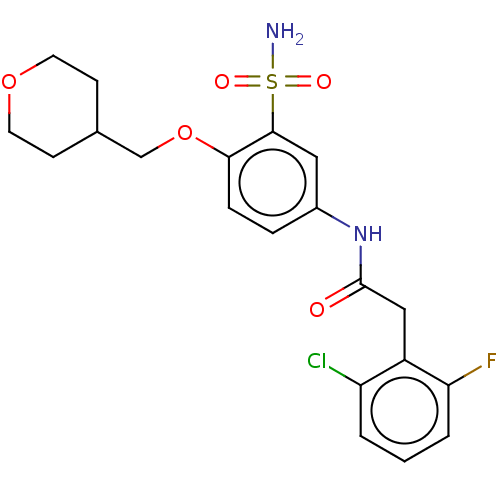 (CHEMBL4555657) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506201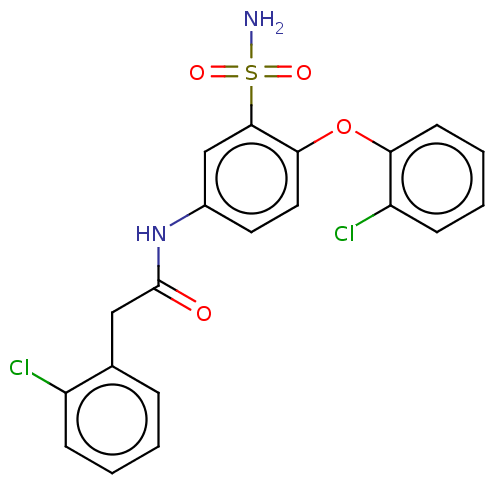 (CHEMBL4516176) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506183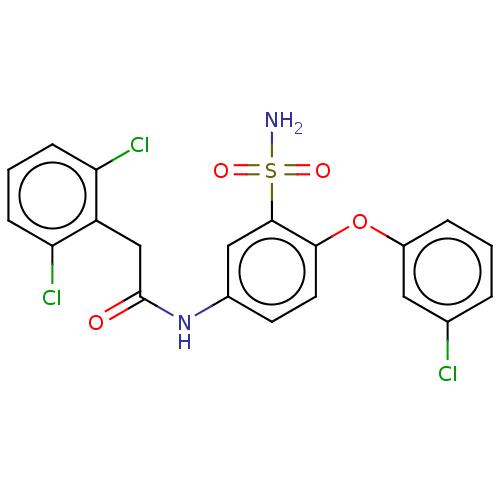 (CHEMBL4521017) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506172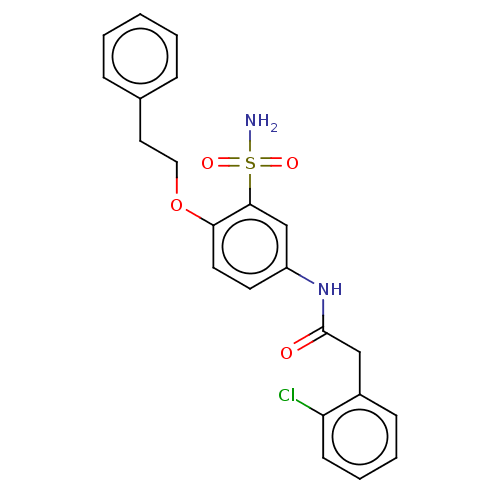 (CHEMBL4563994) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506185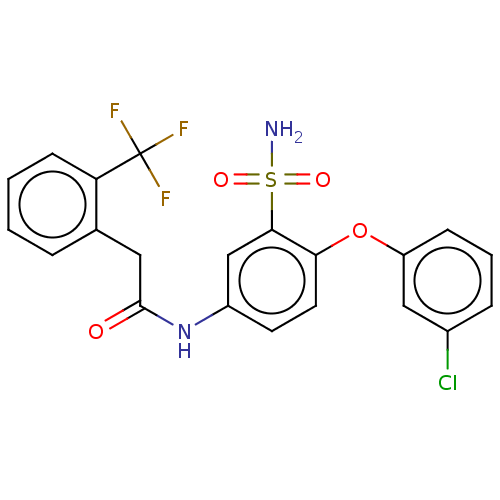 (CHEMBL4438327) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM366072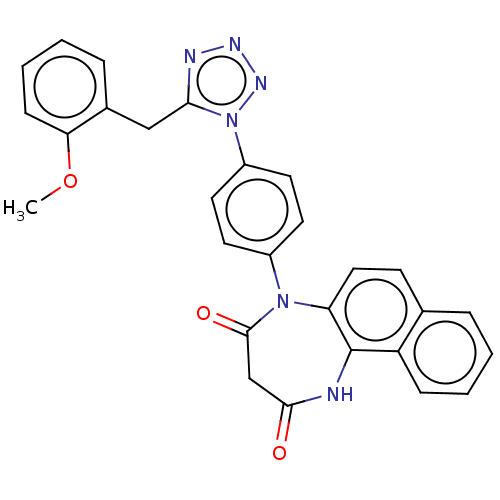 (5-[4-[5-(2-Methoxybenzyl)-1H-tetrazol-1-yl]phenyl]...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description The 1321N1 cells stably expressing human P2X4 receptor were seeded on a 96-well plate, cultured under the conditions of 37° C. and 5% CO2 for 24 hour... | J Med Chem 51: 68-76 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506191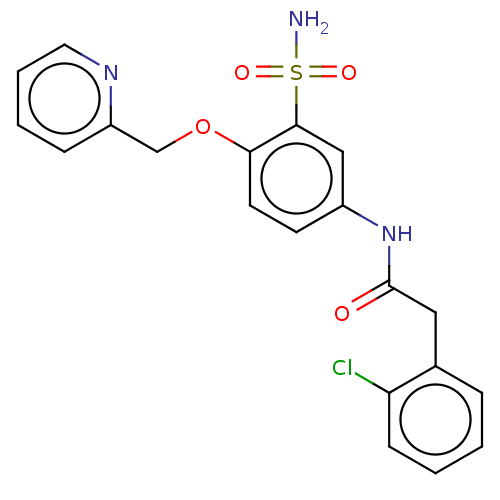 (CHEMBL4439570) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506169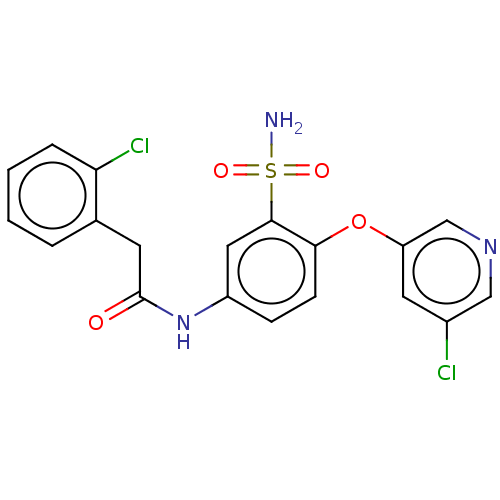 (CHEMBL4535761) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506162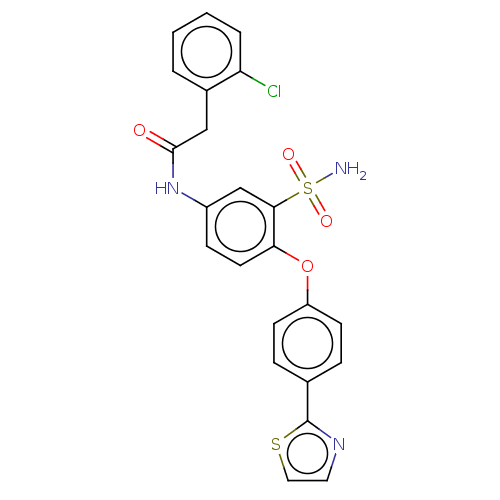 (CHEMBL4463320) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506157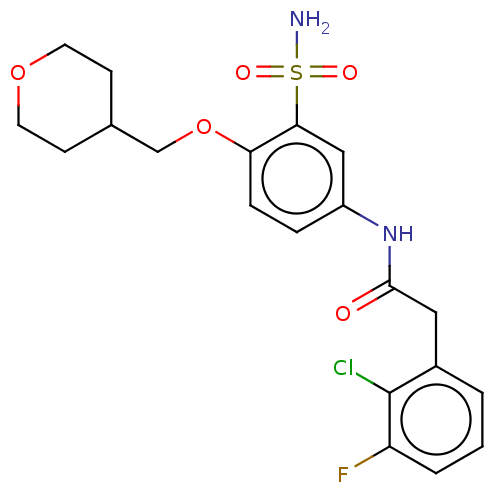 (CHEMBL4517831) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM366073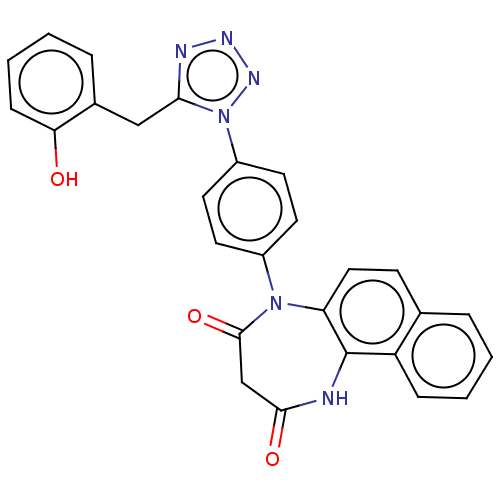 (5-[4-[5-(2-Hydroxybenzyl)-1H-tetrazol-1-yl]phenyl]...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description The 1321N1 cells stably expressing human P2X4 receptor were seeded on a 96-well plate, cultured under the conditions of 37° C. and 5% CO2 for 24 hour... | J Med Chem 51: 68-76 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM366069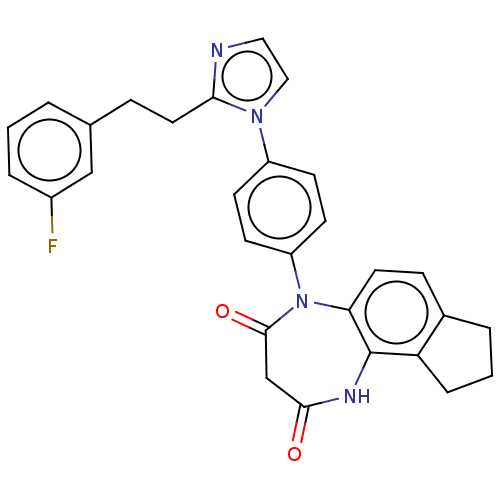 (US9873683, Example 79) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description The 1321N1 cells stably expressing human P2X4 receptor were seeded on a 96-well plate, cultured under the conditions of 37° C. and 5% CO2 for 24 hour... | J Med Chem 51: 68-76 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506198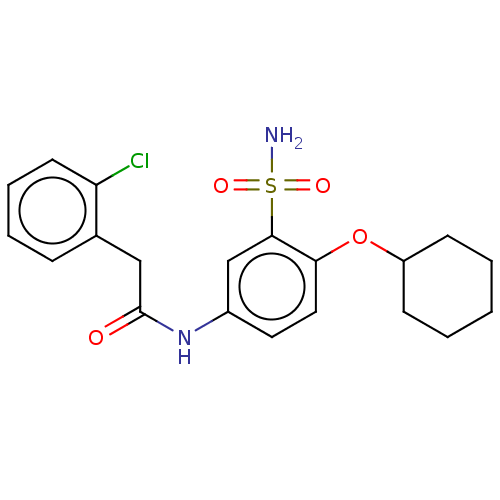 (CHEMBL4539658) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50598326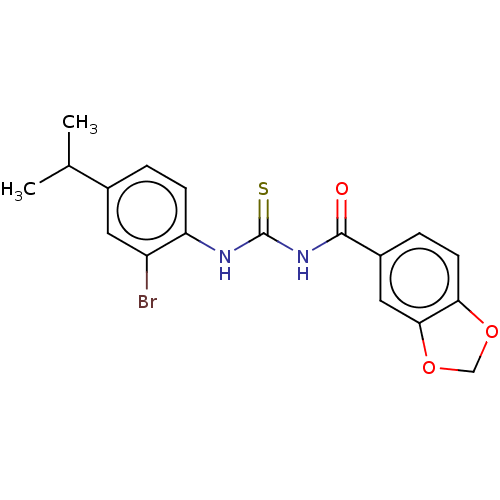 (CHEMBL5206892) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114491 BindingDB Entry DOI: 10.7270/Q2BG2T1P | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50596624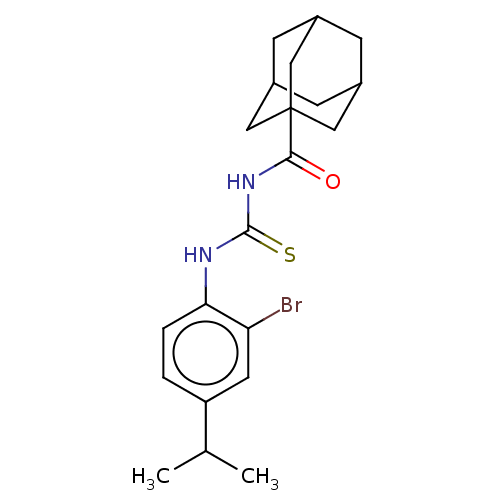 (CHEMBL5204261) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114162 BindingDB Entry DOI: 10.7270/Q2S46X0K | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50540434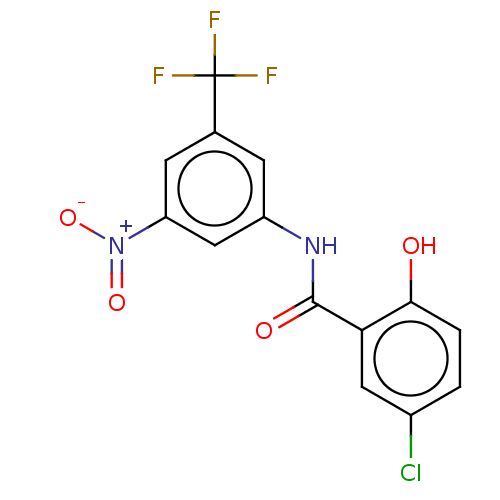 (CHEMBL4640175) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor expressed in human 1321N1 cells assessed as inhibition of ATP-induced cytosolic calcium influx preincubate... | J Med Chem 63: 6164-6178 (2020) Article DOI: 10.1021/acs.jmedchem.0c00435 BindingDB Entry DOI: 10.7270/Q24F1V93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM366075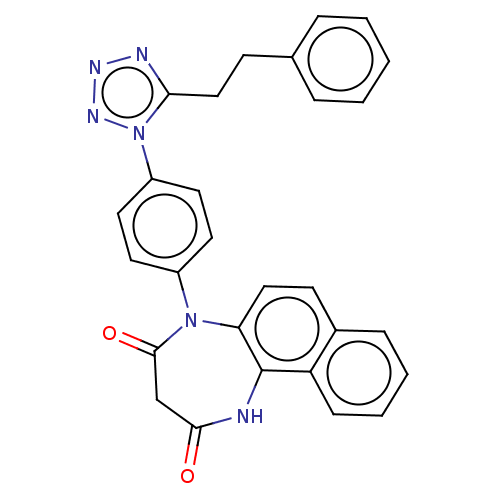 (5-[4-(5-Phenethyl-1H-tetrazol-1-yl)phenyl]-1H-naph...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description The 1321N1 cells stably expressing human P2X4 receptor were seeded on a 96-well plate, cultured under the conditions of 37° C. and 5% CO2 for 24 hour... | J Med Chem 51: 68-76 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506166 (CHEMBL4474747) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50430156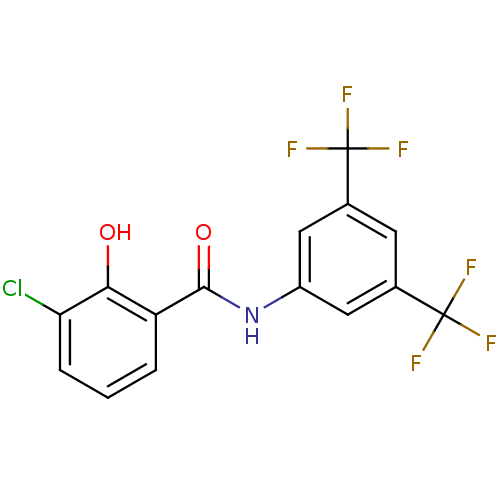 (CHEMBL2338695) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor expressed in human 1321N1 cells assessed as inhibition of ATP-induced cytosolic calcium influx preincubate... | J Med Chem 63: 6164-6178 (2020) Article DOI: 10.1021/acs.jmedchem.0c00435 BindingDB Entry DOI: 10.7270/Q24F1V93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM365870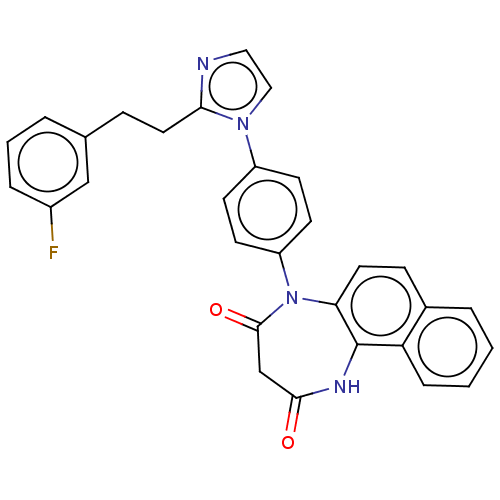 (US9873683, Example 31) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description The 1321N1 cells stably expressing human P2X4 receptor were seeded on a 96-well plate, cultured under the conditions of 37° C. and 5% CO2 for 24 hour... | J Med Chem 51: 68-76 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506204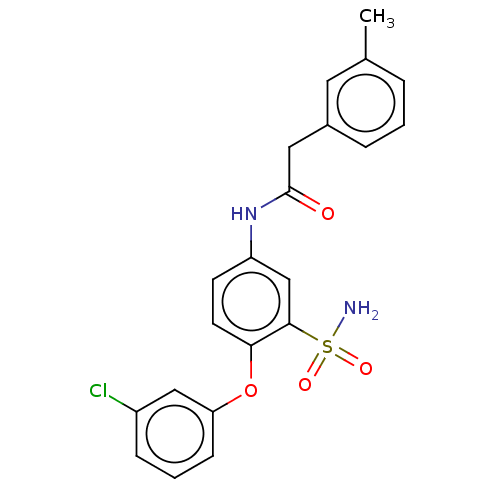 (CHEMBL4443234) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM366062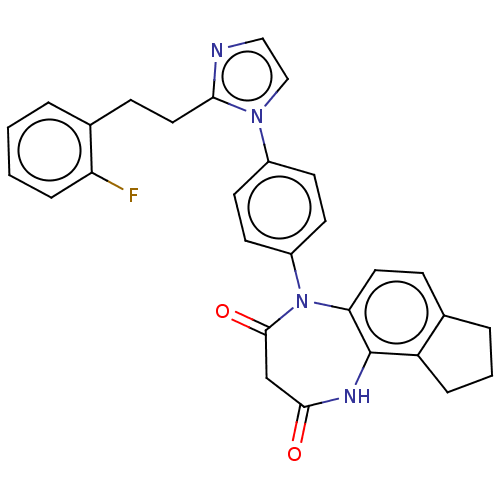 (US9873683, Example 67) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description The 1321N1 cells stably expressing human P2X4 receptor were seeded on a 96-well plate, cultured under the conditions of 37° C. and 5% CO2 for 24 hour... | J Med Chem 51: 68-76 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM366049 (US9873683, Example 43) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description The 1321N1 cells stably expressing human P2X4 receptor were seeded on a 96-well plate, cultured under the conditions of 37° C. and 5% CO2 for 24 hour... | J Med Chem 51: 68-76 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM393615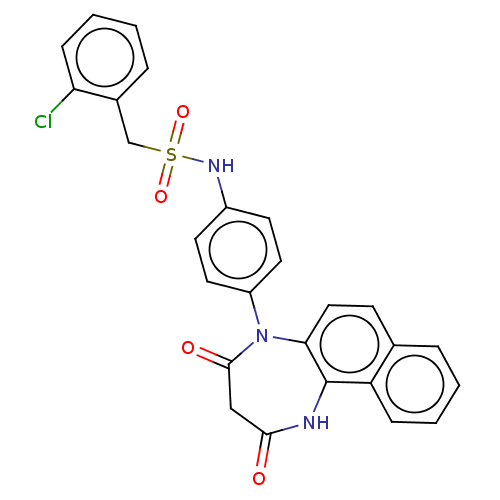 (US09969700, 173) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor expressed in HEK cells assessed as reduction in ATP induced calcium influx | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50598322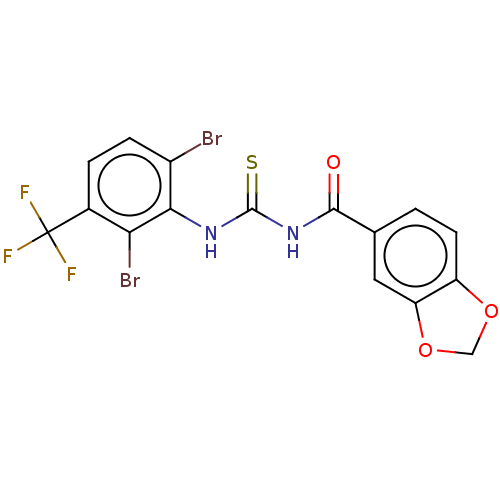 (CHEMBL5200114) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114491 BindingDB Entry DOI: 10.7270/Q2BG2T1P | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM393615 (US09969700, 173) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita del Piemonte Orientale | Assay Description The P2X4 receptor antagonist activity of the compounds of the present invention was measured as follows. The 1321N1 cells stably expressing human P2X... | J Med Chem 52: 3001-9 (2009) BindingDB Entry DOI: 10.7270/Q2542QX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM393615 (US09969700, 173) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in human 1321N1 cells assessed as inhibition of ATP-induced calcium influx incubated for 15 min... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506195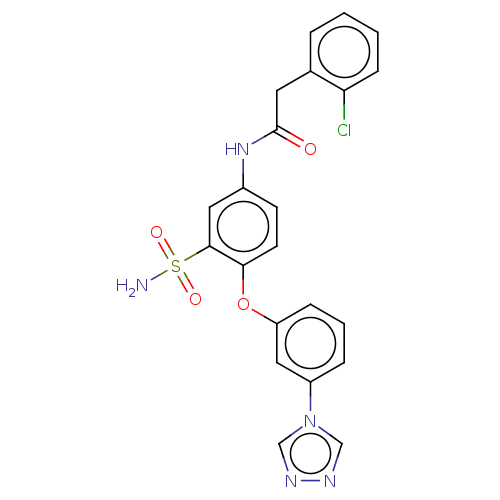 (CHEMBL4438981) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM366057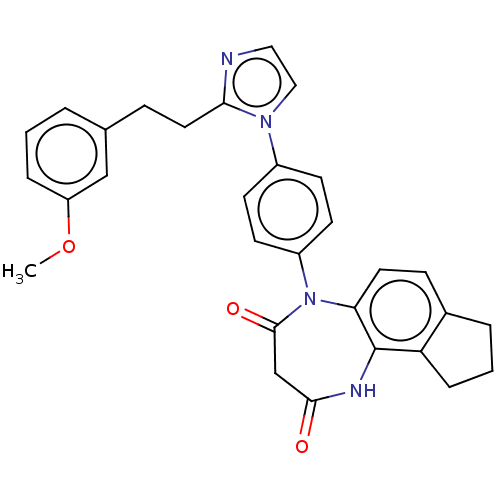 (US9873683, Example 61) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description The 1321N1 cells stably expressing human P2X4 receptor were seeded on a 96-well plate, cultured under the conditions of 37° C. and 5% CO2 for 24 hour... | J Med Chem 51: 68-76 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM50506163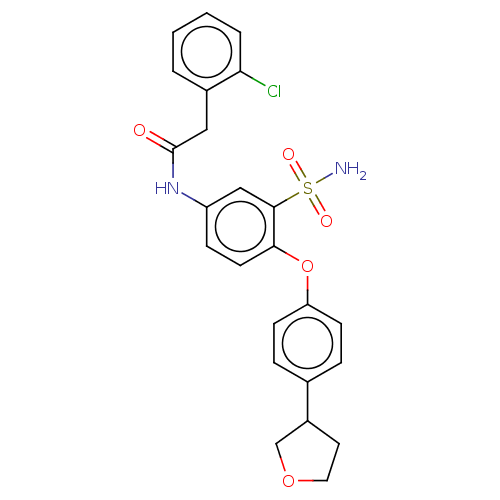 (CHEMBL4561582) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human P2X4 receptor tranfected in HEK293 cells assessed as inhibition of Bz-ATP-induced calcium influx incubated for 30 mins a... | J Med Chem 62: 11194-11217 (2019) Article DOI: 10.1021/acs.jmedchem.9b01304 BindingDB Entry DOI: 10.7270/Q2M048RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 475 total ) | Next | Last >> |