

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM85043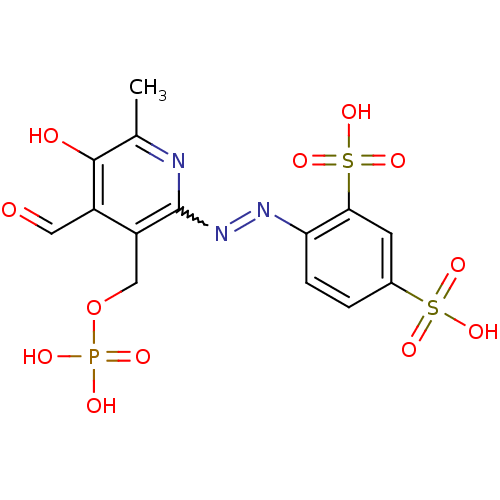 (CAS_149017-66-3 | CHEMBL69234 | NSC_6093163 | PPAD...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 27.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Institute for Experimental Medicine Curated by PDSP Ki Database | Mol Pharmacol 51: 109-18 (1997) Article DOI: 10.1124/mol.51.1.109 BindingDB Entry DOI: 10.7270/Q218351B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (RAT) | BDBM85043 (CAS_149017-66-3 | CHEMBL69234 | NSC_6093163 | PPAD...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Institute for Experimental Medicine Curated by PDSP Ki Database | Mol Pharmacol 51: 109-18 (1997) Article DOI: 10.1124/mol.51.1.109 BindingDB Entry DOI: 10.7270/Q218351B | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (RAT) | BDBM85043 (CAS_149017-66-3 | CHEMBL69234 | NSC_6093163 | PPAD...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at rat P2X4 receptor (mutant type) | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM85043 (CAS_149017-66-3 | CHEMBL69234 | NSC_6093163 | PPAD...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 2.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at human P2X4 receptor (mutant type) | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (RAT) | BDBM85043 (CAS_149017-66-3 | CHEMBL69234 | NSC_6093163 | PPAD...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at rat P2X4 receptor (wild type) | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (Homo sapiens (Human)) | BDBM85043 (CAS_149017-66-3 | CHEMBL69234 | NSC_6093163 | PPAD...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against recombinant human P2X purinoceptor 4 (P2X4) | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (RAT) | BDBM85043 (CAS_149017-66-3 | CHEMBL69234 | NSC_6093163 | PPAD...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against recombinant rat P2X purinoceptor 4 (P2X4) at 3 uM,expressed in Xenopus oocytes | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||