

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM81515 (VEA-499) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM50565392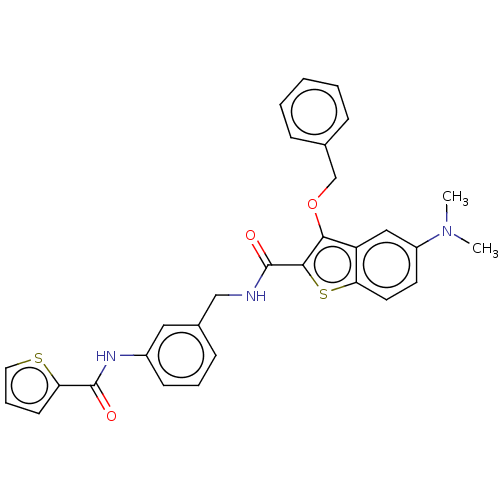 (CHEMBL4791433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human SNEP2 (365 to 589 residues) expressed in Escherichia coli (DE3) cells using RanGAP-SUMO2 substrate preincubated for 1... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112553 BindingDB Entry DOI: 10.7270/Q2H135RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM50565398 (CHEMBL4776158) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human SNEP2 (365 to 589 residues) expressed in Escherichia coli (DE3) cells using RanGAP-SUMO2 substrate preincubated for 1... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112553 BindingDB Entry DOI: 10.7270/Q2H135RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM81516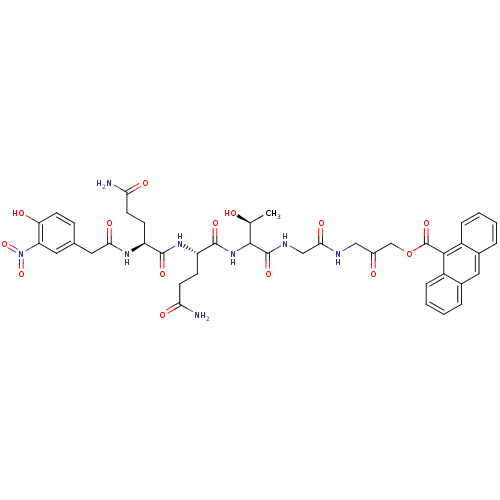 (VEA-500) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM103826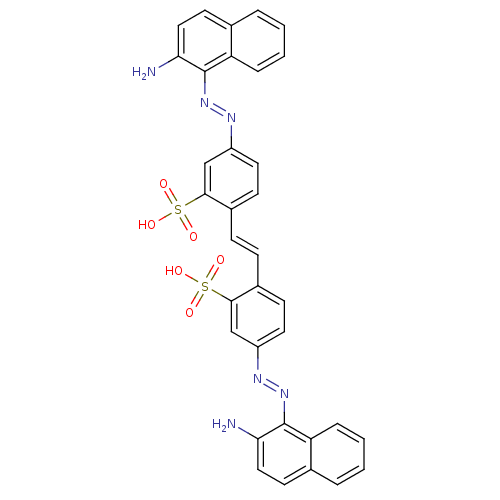 (SPI-01 | US11041859, Code SPI-01 | US9791447, Comp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Beckman Research Institute of the City of Hope | Assay Description Assays were performedby incubating SENPs with various concentrations of the inhibitor (0−60 μM) at RT for 10 min in assay buffer (50 mM Tr... | ACS Chem Biol 8: 1435-41 (2013) Article DOI: 10.1021/cb400177q BindingDB Entry DOI: 10.7270/Q2KH0KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM50565346 (CHEMBL4781794) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged SENP2 (364 to 589 residues) expressed in Escherichia coli (DE3) cells using DUB-Glo as substrate preincubated for 10 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112553 BindingDB Entry DOI: 10.7270/Q2H135RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM50565391 (CHEMBL4792569) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human SNEP2 (365 to 589 residues) expressed in Escherichia coli (DE3) cells using RanGAP-SUMO2 substrate preincubated for 1... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112553 BindingDB Entry DOI: 10.7270/Q2H135RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM50565393 (CHEMBL4780770) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human SNEP2 (365 to 589 residues) expressed in Escherichia coli (DE3) cells using RanGAP-SUMO2 substrate preincubated for 1... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112553 BindingDB Entry DOI: 10.7270/Q2H135RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2/Small ubiquitin-related modifier 1 (Homo sapiens (Human)) | BDBM103826 (SPI-01 | US11041859, Code SPI-01 | US9791447, Comp...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitors confer the non-competitive inhibitory mechanism, as shown by nuclear magnetic resonance (NMR) and quantitative enzyme kinetic analysis... | Citation and Details BindingDB Entry DOI: 10.7270/Q29W0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM103826 (SPI-01 | US11041859, Code SPI-01 | US9791447, Comp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Beckman Research Institute of the City of Hope | Assay Description Assays were performedby incubating SENPs with various concentrations of the inhibitor (0−60 μM) at RT for 10 min in assay buffer (50 mM Tr... | ACS Chem Biol 8: 1435-41 (2013) Article DOI: 10.1021/cb400177q BindingDB Entry DOI: 10.7270/Q2KH0KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM103829 (SPI-04 | US11041859, Code SPI-04 | US9791447, Comp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Beckman Research Institute of the City of Hope | Assay Description Assays were performedby incubating SENPs with various concentrations of the inhibitor (0−60 μM) at RT for 10 min in assay buffer (50 mM Tr... | ACS Chem Biol 8: 1435-41 (2013) Article DOI: 10.1021/cb400177q BindingDB Entry DOI: 10.7270/Q2KH0KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2/Small ubiquitin-related modifier 1 (Homo sapiens (Human)) | BDBM103829 (SPI-04 | US11041859, Code SPI-04 | US9791447, Comp...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitors confer the non-competitive inhibitory mechanism, as shown by nuclear magnetic resonance (NMR) and quantitative enzyme kinetic analysis... | Citation and Details BindingDB Entry DOI: 10.7270/Q29W0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM50565390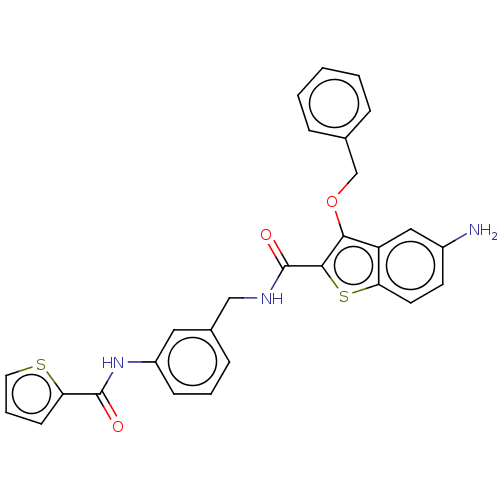 (CHEMBL4780586) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human SNEP2 (365 to 589 residues) expressed in Escherichia coli (DE3) cells using RanGAP-SUMO2 substrate preincubated for 1... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112553 BindingDB Entry DOI: 10.7270/Q2H135RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM103827 (SPI-02 | US11041859, Code SPI-02 | US9791447, Comp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
City of Hope US Patent | Assay Description The binding of SPI-01 to the enzyme-substrate complex was investigated. CSP analysis was carried out on the 40 kDa complex of 15N-labeled full length... | US Patent US9791447 (2017) BindingDB Entry DOI: 10.7270/Q2J67K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM103827 (SPI-02 | US11041859, Code SPI-02 | US9791447, Comp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitors confer the non-competitive inhibitory mechanism, as shown by nuclear magnetic resonance (NMR) and quantitative enzyme kinetic analysis... | Citation and Details BindingDB Entry DOI: 10.7270/Q29W0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM103827 (SPI-02 | US11041859, Code SPI-02 | US9791447, Comp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114227 BindingDB Entry DOI: 10.7270/Q2T43Z3H | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM103827 (SPI-02 | US11041859, Code SPI-02 | US9791447, Comp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM103829 (SPI-04 | US11041859, Code SPI-04 | US9791447, Comp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.15E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Beckman Research Institute of the City of Hope | Assay Description Assays were performedby incubating SENPs with various concentrations of the inhibitor (0−60 μM) at RT for 10 min in assay buffer (50 mM Tr... | ACS Chem Biol 8: 1435-41 (2013) Article DOI: 10.1021/cb400177q BindingDB Entry DOI: 10.7270/Q2KH0KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM50565350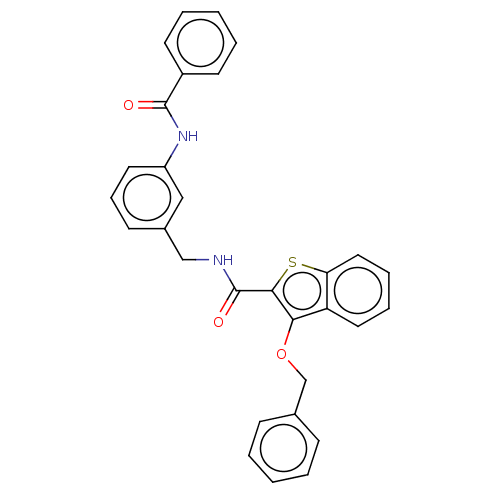 (CHEMBL4796113) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human SNEP2 (365 to 589 residues) expressed in Escherichia coli (DE3) cells using RanGAP-SUMO2 substrate preincubated for 1... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112553 BindingDB Entry DOI: 10.7270/Q2H135RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM103829 (SPI-04 | US11041859, Code SPI-04 | US9791447, Comp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
City of Hope US Patent | Assay Description The binding of SPI-01 to the enzyme-substrate complex was investigated. CSP analysis was carried out on the 40 kDa complex of 15N-labeled full length... | US Patent US9791447 (2017) BindingDB Entry DOI: 10.7270/Q2J67K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM103829 (SPI-04 | US11041859, Code SPI-04 | US9791447, Comp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitors confer the non-competitive inhibitory mechanism, as shown by nuclear magnetic resonance (NMR) and quantitative enzyme kinetic analysis... | Citation and Details BindingDB Entry DOI: 10.7270/Q29W0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM50565382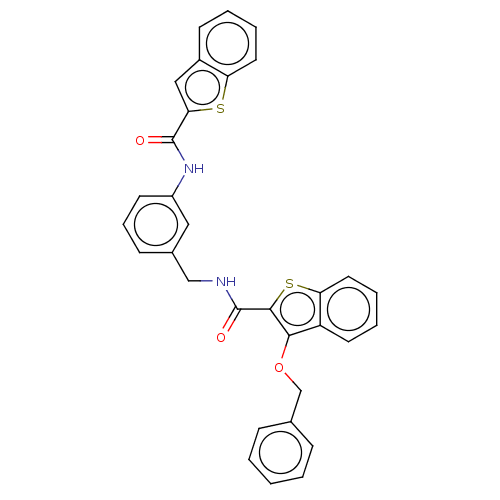 (CHEMBL4778045) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human SNEP2 (365 to 589 residues) expressed in Escherichia coli (DE3) cells using RanGAP-SUMO2 substrate preincubated for 1... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112553 BindingDB Entry DOI: 10.7270/Q2H135RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM50421693 (CHEMBL5269877) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM348885 (NSC8676 | US9791447, Compound SPI-03) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
City of Hope US Patent | Assay Description The binding of SPI-01 to the enzyme-substrate complex was investigated. CSP analysis was carried out on the 40 kDa complex of 15N-labeled full length... | US Patent US9791447 (2017) BindingDB Entry DOI: 10.7270/Q2J67K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM103828 (SPI-03 | US11041859, Code SPI-03) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitors confer the non-competitive inhibitory mechanism, as shown by nuclear magnetic resonance (NMR) and quantitative enzyme kinetic analysis... | Citation and Details BindingDB Entry DOI: 10.7270/Q29W0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM50565396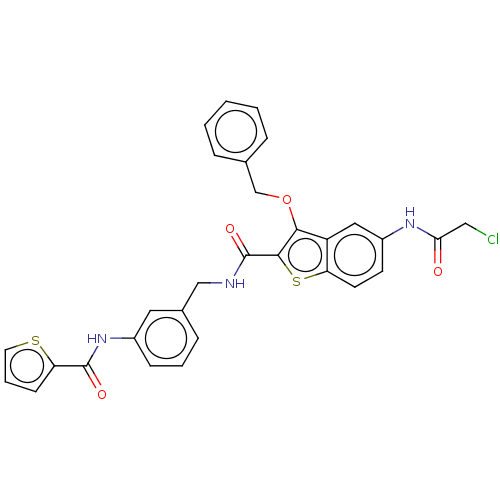 (CHEMBL4783695) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human SNEP2 (365 to 589 residues) expressed in Escherichia coli (DE3) cells using RanGAP-SUMO2 substrate preincubated for 1... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112553 BindingDB Entry DOI: 10.7270/Q2H135RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM103828 (SPI-03 | US11041859, Code SPI-03) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM103835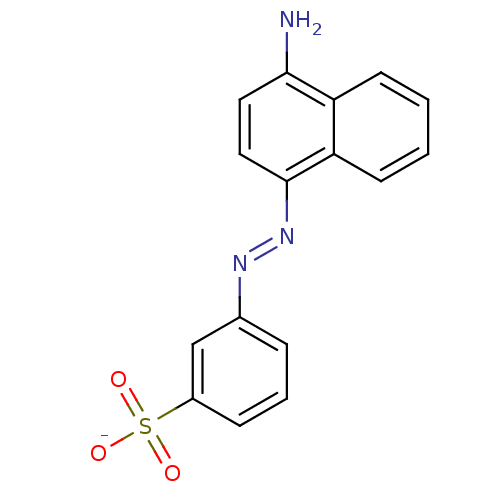 (SPI-10 | US11041859, Code SPI-10 | US9791447, Comp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
City of Hope US Patent | Assay Description The binding of SPI-01 to the enzyme-substrate complex was investigated. CSP analysis was carried out on the 40 kDa complex of 15N-labeled full length... | US Patent US9791447 (2017) BindingDB Entry DOI: 10.7270/Q2J67K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM103835 (SPI-10 | US11041859, Code SPI-10 | US9791447, Comp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitors confer the non-competitive inhibitory mechanism, as shown by nuclear magnetic resonance (NMR) and quantitative enzyme kinetic analysis... | Citation and Details BindingDB Entry DOI: 10.7270/Q29W0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM50565378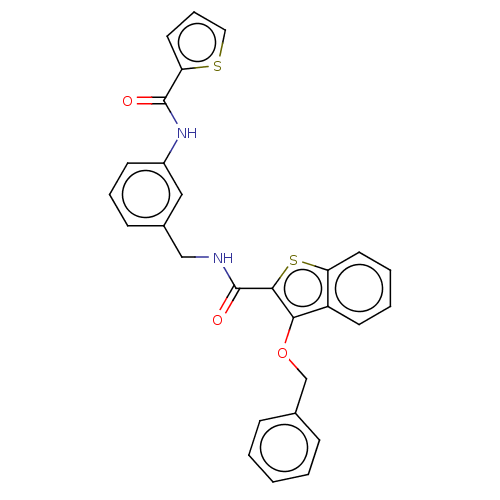 (CHEMBL4793952) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human SNEP2 (365 to 589 residues) expressed in Escherichia coli (DE3) cells using RanGAP-SUMO2 substrate preincubated for 1... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112553 BindingDB Entry DOI: 10.7270/Q2H135RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM50421692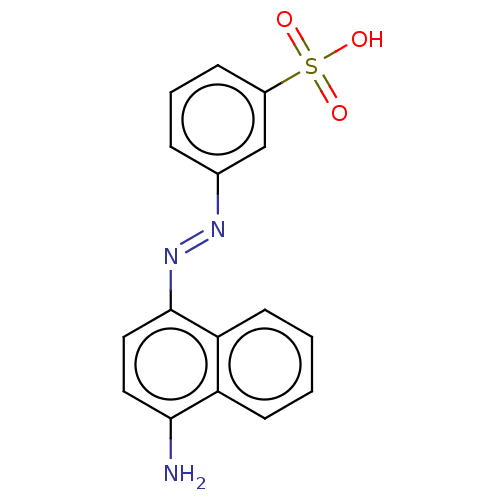 (CHEMBL5287963) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM103827 (SPI-02 | US11041859, Code SPI-02 | US9791447, Comp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Beckman Research Institute of the City of Hope | Assay Description Assays were performedby incubating SENPs with various concentrations of the inhibitor (0−60 μM) at RT for 10 min in assay buffer (50 mM Tr... | ACS Chem Biol 8: 1435-41 (2013) Article DOI: 10.1021/cb400177q BindingDB Entry DOI: 10.7270/Q2KH0KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM50164846 (CHEMBL3799152) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged SENP2 (363 to 589 residues) expressed in Escherichia coli BL21 (DE3) using RanGAP-SUMO as substrate incuba... | Bioorg Med Chem Lett 26: 2124-8 (2016) Article DOI: 10.1016/j.bmcl.2016.03.080 BindingDB Entry DOI: 10.7270/Q2BR8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM50164846 (CHEMBL3799152) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged SENP2 (363 to 589 residues) expressed in Escherichia coli BL21 (DE3) using RanGAP-SUMO as substrate incuba... | Bioorg Med Chem Lett 26: 2124-8 (2016) Article DOI: 10.1016/j.bmcl.2016.03.080 BindingDB Entry DOI: 10.7270/Q2BR8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM103826 (SPI-01 | US11041859, Code SPI-01 | US9791447, Comp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
City of Hope US Patent | Assay Description The binding of SPI-01 to the enzyme-substrate complex was investigated. CSP analysis was carried out on the 40 kDa complex of 15N-labeled full length... | US Patent US9791447 (2017) BindingDB Entry DOI: 10.7270/Q2J67K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM103826 (SPI-01 | US11041859, Code SPI-01 | US9791447, Comp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitors confer the non-competitive inhibitory mechanism, as shown by nuclear magnetic resonance (NMR) and quantitative enzyme kinetic analysis... | Citation and Details BindingDB Entry DOI: 10.7270/Q29W0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM103826 (SPI-01 | US11041859, Code SPI-01 | US9791447, Comp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM103828 (SPI-03 | US11041859, Code SPI-03) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Beckman Research Institute of the City of Hope | Assay Description Assays were performedby incubating SENPs with various concentrations of the inhibitor (0−60 μM) at RT for 10 min in assay buffer (50 mM Tr... | ACS Chem Biol 8: 1435-41 (2013) Article DOI: 10.1021/cb400177q BindingDB Entry DOI: 10.7270/Q2KH0KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2/Small ubiquitin-related modifier 1 (Homo sapiens (Human)) | BDBM103827 (SPI-02 | US11041859, Code SPI-02 | US9791447, Comp...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitors confer the non-competitive inhibitory mechanism, as shown by nuclear magnetic resonance (NMR) and quantitative enzyme kinetic analysis... | Citation and Details BindingDB Entry DOI: 10.7270/Q29W0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM103827 (SPI-02 | US11041859, Code SPI-02 | US9791447, Comp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.42E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Beckman Research Institute of the City of Hope | Assay Description Assays were performedby incubating SENPs with various concentrations of the inhibitor (0−60 μM) at RT for 10 min in assay buffer (50 mM Tr... | ACS Chem Biol 8: 1435-41 (2013) Article DOI: 10.1021/cb400177q BindingDB Entry DOI: 10.7270/Q2KH0KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM50565352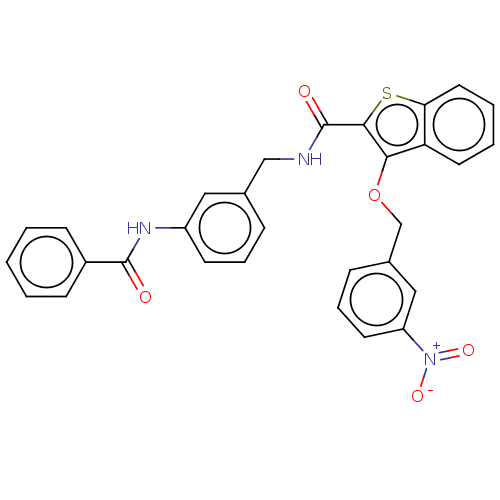 (CHEMBL4791044) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human SNEP2 (365 to 589 residues) expressed in Escherichia coli (DE3) cells using RanGAP-SUMO2 substrate preincubated for 1... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112553 BindingDB Entry DOI: 10.7270/Q2H135RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM50565397 (CHEMBL4796293) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human SNEP2 (365 to 589 residues) expressed in Escherichia coli (DE3) cells using RanGAP-SUMO2 substrate preincubated for 1... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112553 BindingDB Entry DOI: 10.7270/Q2H135RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2/Small ubiquitin-related modifier 1 (Homo sapiens (Human)) | BDBM348888 (NSC70551 | US11041859, Code SPI-06 | US9791447, Co...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitors confer the non-competitive inhibitory mechanism, as shown by nuclear magnetic resonance (NMR) and quantitative enzyme kinetic analysis... | Citation and Details BindingDB Entry DOI: 10.7270/Q29W0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM103831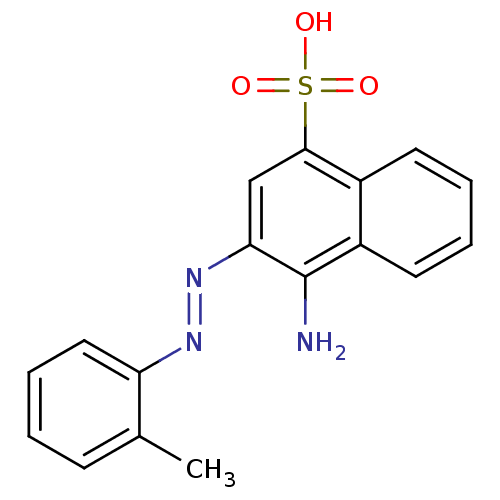 (SPI-06) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.62E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Beckman Research Institute of the City of Hope | Assay Description Assays were performedby incubating SENPs with various concentrations of the inhibitor (0−60 μM) at RT for 10 min in assay buffer (50 mM Tr... | ACS Chem Biol 8: 1435-41 (2013) Article DOI: 10.1021/cb400177q BindingDB Entry DOI: 10.7270/Q2KH0KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM81511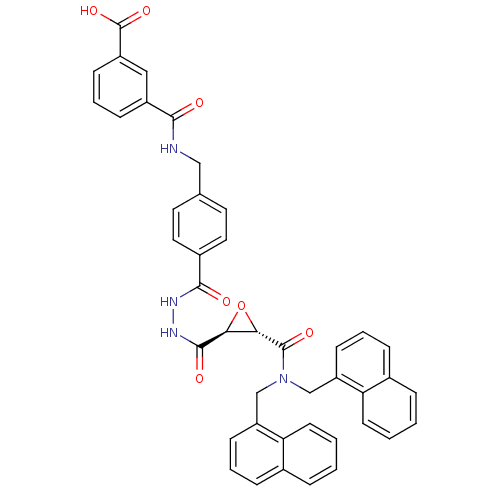 (VEA-260) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM348888 (NSC70551 | US11041859, Code SPI-06 | US9791447, Co...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
City of Hope US Patent | Assay Description The binding of SPI-01 to the enzyme-substrate complex was investigated. CSP analysis was carried out on the 40 kDa complex of 15N-labeled full length... | US Patent US9791447 (2017) BindingDB Entry DOI: 10.7270/Q2J67K27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM348888 (NSC70551 | US11041859, Code SPI-06 | US9791447, Co...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitors confer the non-competitive inhibitory mechanism, as shown by nuclear magnetic resonance (NMR) and quantitative enzyme kinetic analysis... | Citation and Details BindingDB Entry DOI: 10.7270/Q29W0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM50591764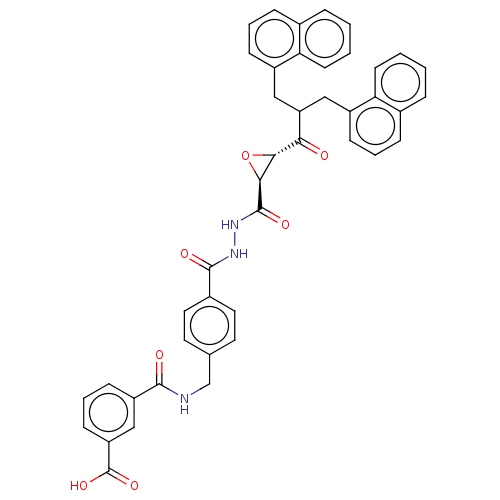 (CHEMBL5207368) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114227 BindingDB Entry DOI: 10.7270/Q2T43Z3H | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM50591766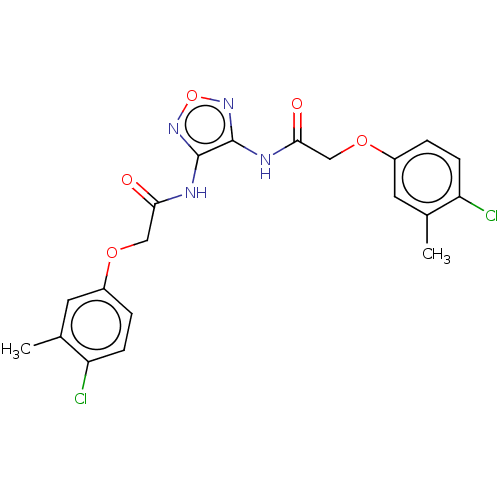 (CHEMBL5188918) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114227 BindingDB Entry DOI: 10.7270/Q2T43Z3H | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM103831 (SPI-06) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 135 total ) | Next | Last >> |