

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sulfotransferase 2A1 (Homo sapiens (Human)) | BDBM50176062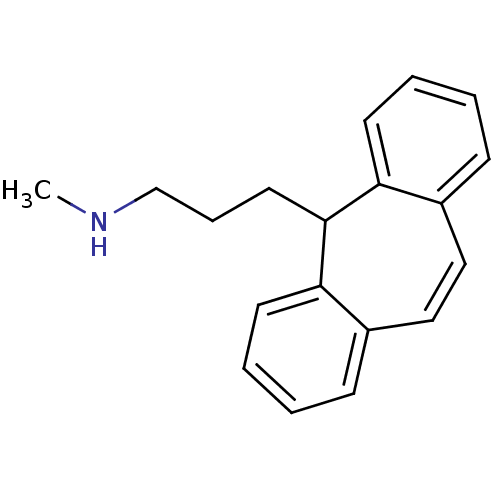 (3-(5H-dibenzo[a,d][7]annulen-5-yl)-N-methylpropan-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | 7.60E+4 | -21.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 2 |
Albert Einstein College of Medicine | Assay Description Reaction conditions were as follows: SULT1A1 or 2A1 (50 nM), PnP (3.0 or 100 uM, respectively; 2 x Km PnP), amoxipine or protriptyline (0, 50, 100, o... | J Biol Chem 288: 34494-501 (2013) Article DOI: 10.1074/jbc.M113.510974 BindingDB Entry DOI: 10.7270/Q2XP73S3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sulfotransferase 2A1 (Homo sapiens (Human)) | BDBM22870 (13-chloro-10-(piperazin-1-yl)-2-oxa-9-azatricyclo[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.25E+5 | -20.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 2 |
Albert Einstein College of Medicine | Assay Description Reaction conditions were as follows: SULT1A1 or 2A1 (50 nM), PnP (3.0 or 100 uM, respectively; 2 x Km PnP), amoxipine or protriptyline (0, 50, 100, o... | J Biol Chem 288: 34494-501 (2013) Article DOI: 10.1074/jbc.M113.510974 BindingDB Entry DOI: 10.7270/Q2XP73S3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sulfotransferase 2A1 (Homo sapiens (Human)) | BDBM50380592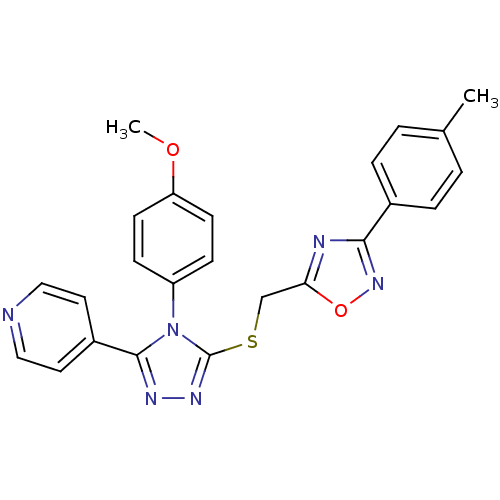 (CHEMBL1552719) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Affinity for CCK2 receptor assessed by inhibition of pentagastrin-stimulated acid secretion in perfused rat stomach | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sulfotransferase 2A1 (Homo sapiens (Human)) | BDBM50427989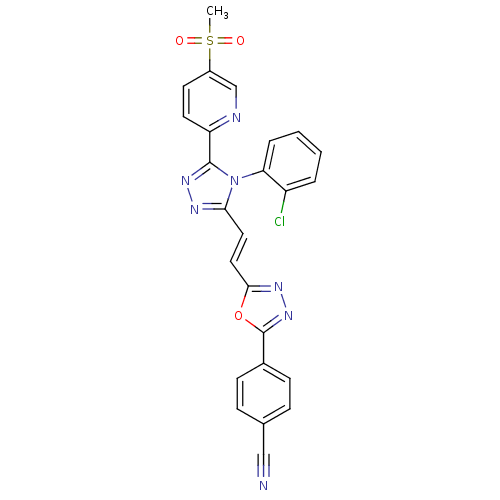 (CHEMBL2325503) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Affinity for CCK2 receptor assessed by inhibition of pentagastrin-stimulated acid secretion in perfused rat stomach | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sulfotransferase 2A1 (Rattus norvegicus) | BDBM19459 (5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sulfotransferase 2A1 (Rattus norvegicus) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sulfotransferase 2A1 (Rattus norvegicus) | BDBM7462 (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sulfotransferase 2A1 (Rattus norvegicus) | BDBM23420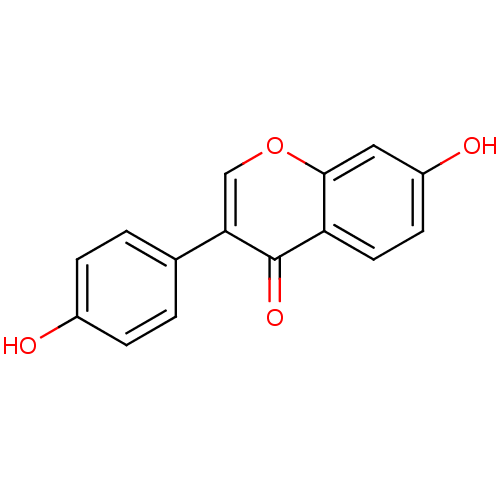 (7,4′-Dihydroxy-isoflavone (3a) | 7-hydroxy-3...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sulfotransferase 2A1 (Rattus norvegicus) | BDBM50051828 (2-(4-hydroxyphenyl)-3-methyl-1-(2,3,4,5,6-pentaflu...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibitory activity against dehydroepiandrosterone sulfatase (DHA-STS) | J Med Chem 39: 1349-51 (1996) Article DOI: 10.1021/jm950931z BindingDB Entry DOI: 10.7270/Q218375Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||