

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Equilibrative nucleoside transporter 1 (Rattus norvegicus) | BDBM50241166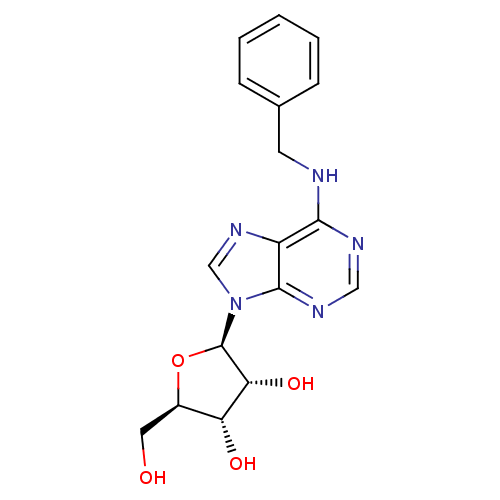 ((2R,3R,4S,5R)-2-(6-(benzylamino)-9H-purin-9-yl)-5-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Inhibition of [3H]S-(4-Nitrobenzyl)-6-thioinosine binding to adenosine uptake sites in rat brain membranes | J Med Chem 37: 3614-21 (1994) BindingDB Entry DOI: 10.7270/Q2348M1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Equilibrative nucleoside transporter 1 (Rattus norvegicus) | BDBM50367298 (Cardene | NICARDIPINE) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Ability to displace radioligand from Na+ independent Adenosine transporter in rat | J Med Chem 39: 2980-9 (1996) Article DOI: 10.1021/jm9600205 BindingDB Entry DOI: 10.7270/Q2833SQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Equilibrative nucleoside transporter 1 (Rattus norvegicus) | BDBM21221 ((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Inhibition of [3H]S-(4-Nitrobenzyl)-6-thioinosine binding to adenosine uptake sites in rat brain membranes | J Med Chem 37: 3614-21 (1994) BindingDB Entry DOI: 10.7270/Q2348M1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Equilibrative nucleoside transporter 1 (Rattus norvegicus) | BDBM50118812 ((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Inhibition of [3H]S-(4-Nitrobenzyl)-6-thioinosine binding to adenosine uptake sites in rat brain membranes | J Med Chem 37: 3614-21 (1994) BindingDB Entry DOI: 10.7270/Q2348M1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Equilibrative nucleoside transporter 1 (Rattus norvegicus) | BDBM50407234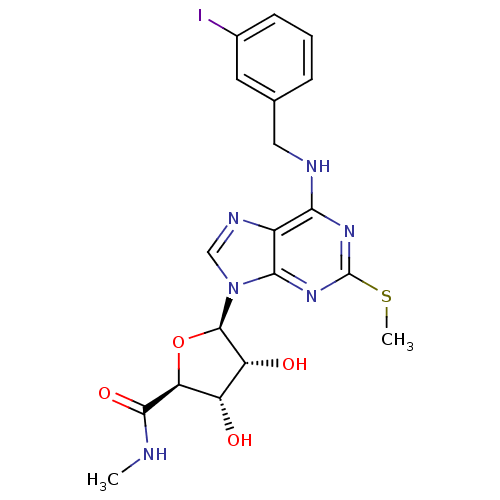 (CHEMBL2113557) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Inhibition of [3H]S-(4-Nitrobenzyl)-6-thioinosine binding to adenosine uptake sites in rat brain membranes | J Med Chem 37: 3614-21 (1994) BindingDB Entry DOI: 10.7270/Q2348M1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Equilibrative nucleoside transporter 1 (Rattus norvegicus) | BDBM23617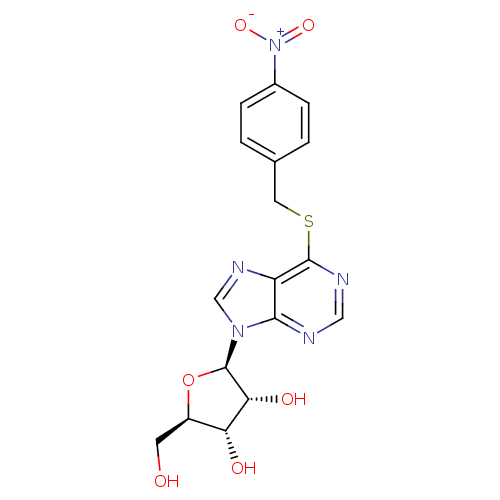 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-{[(4-nitrophe...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Carlo Bo Curated by ChEMBL | Assay Description Inhibitory concentration against human Adenosine A3 receptor expressed in HEK293 cells using 0.1 nM [3H]AB-MECA | J Med Chem 48: 6887-96 (2005) Article DOI: 10.1021/jm058018d BindingDB Entry DOI: 10.7270/Q28C9X1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||