

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50469353 (CHEMBL4283353) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNC Eshelman School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of TYRO3 (unknown origin) using 5'-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by MCE assay | J Med Chem 61: 10242-10254 (2018) Article DOI: 10.1021/acs.jmedchem.8b01229 BindingDB Entry DOI: 10.7270/Q2K076ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM497267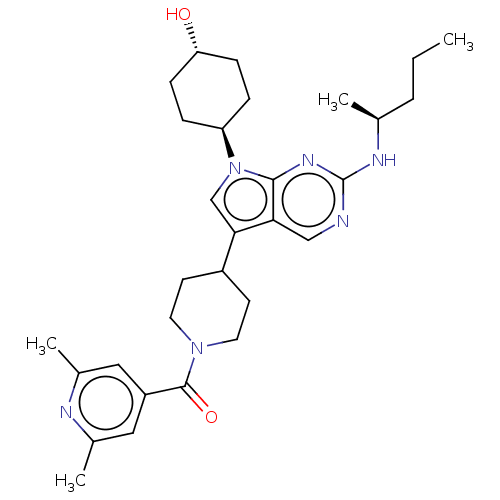 ((2,6-dimethylpyridin-4- yl)(4-(7-((1R,4S)-4- hydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATP competitive inhibition of TYRO3 (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113534 BindingDB Entry DOI: 10.7270/Q2M90DGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50425829 (CHEMBL2312304) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of tracer K5 binding to NanoLuc-fused TYRO3 (unknown origin) expressed in HEK293T cells measured after 2 hrs by NanoBRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00845 BindingDB Entry DOI: 10.7270/Q2WH2TSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50463483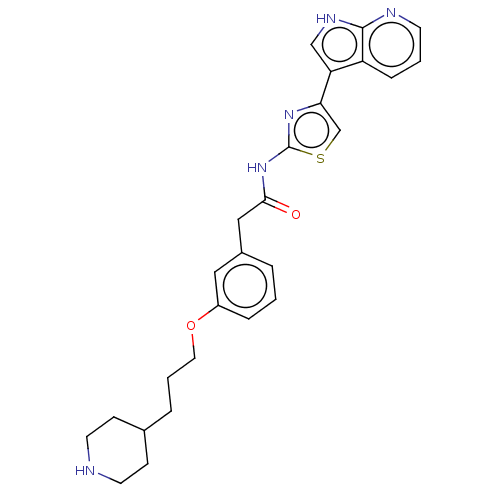 (CHEMBL4245242) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of TYRO3 (unknown origin) | Bioorg Med Chem Lett 28: 2622-2626 (2018) Article DOI: 10.1016/j.bmcl.2018.06.040 BindingDB Entry DOI: 10.7270/Q2ZC85HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50463479 (CHEMBL4249925) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of TYRO3 (unknown origin) | Bioorg Med Chem Lett 28: 2622-2626 (2018) Article DOI: 10.1016/j.bmcl.2018.06.040 BindingDB Entry DOI: 10.7270/Q2ZC85HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50557863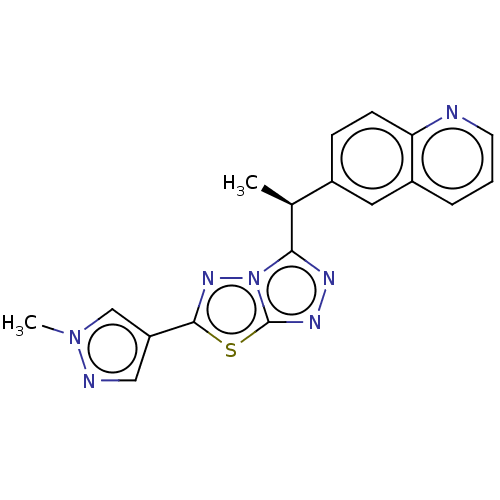 (CHEMBL4791034) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of SKY (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00094 BindingDB Entry DOI: 10.7270/Q28919JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515838 (US11053225, Compound 139) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515828 (US11053225, Compound 129 | US11053225, Compound 19...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515820 (US11053225, Compound 101 | US11053225, Compound 19...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515870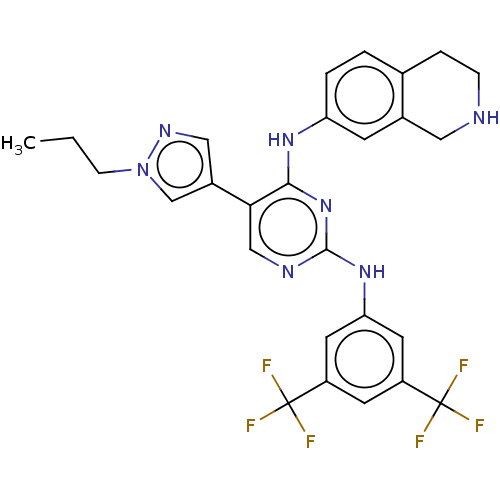 (US11053225, Compound 172) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515869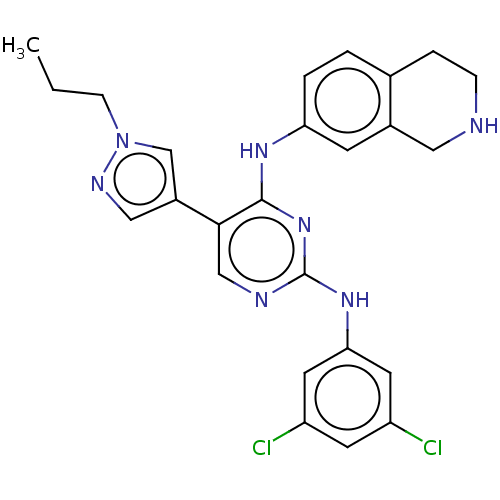 (US11053225, Compound 171) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515810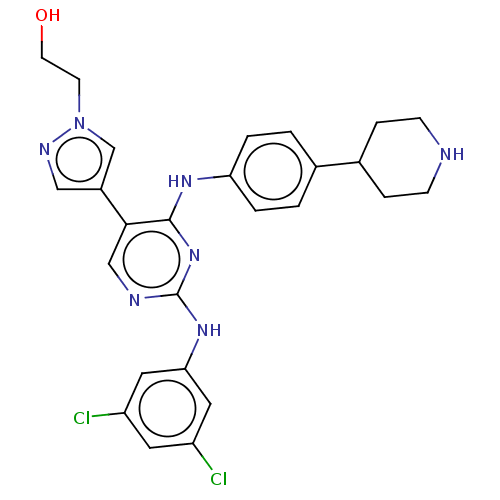 (US11053225, Compound 201) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515814 (US11053225, Compound 84) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515817 (US11053225, Compound 89) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515826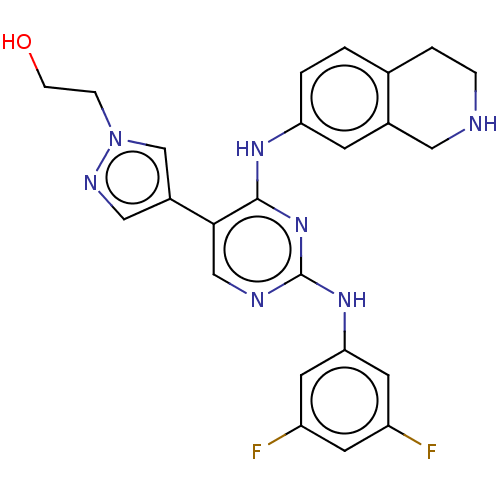 (US11053225, Compound 127) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515794 (US11053225, Compound 75) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515836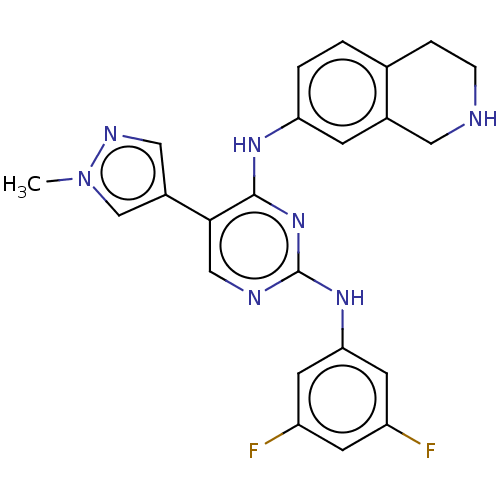 (US11053225, Compound 137) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515865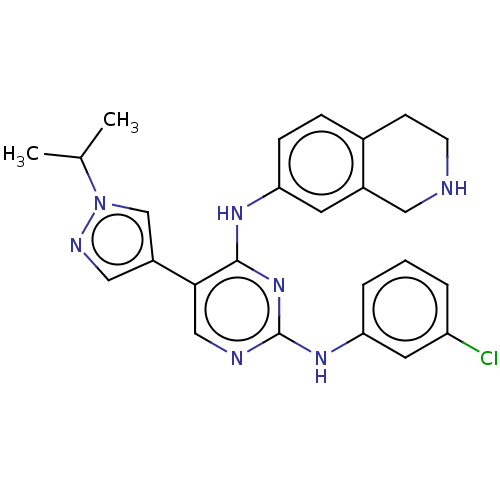 (US11053225, Compound 167) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515847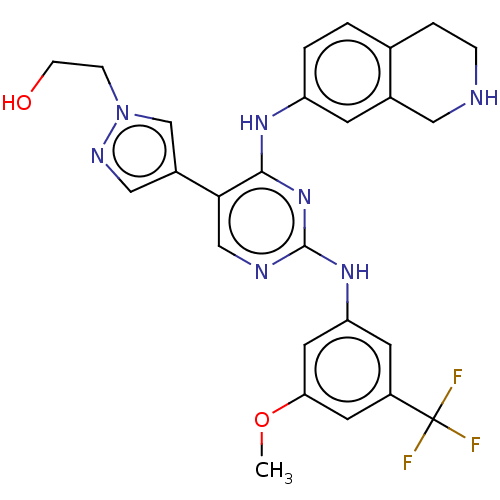 (US11053225, Compound 148) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515871 (US11053225, Compound 173) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515846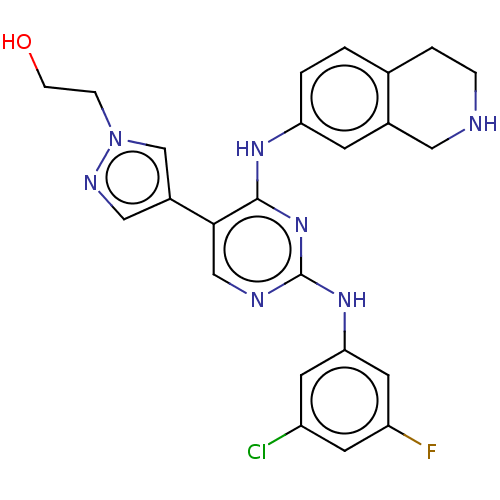 (US11053225, Compound 147) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515812 (US11053225, Compound 205) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515860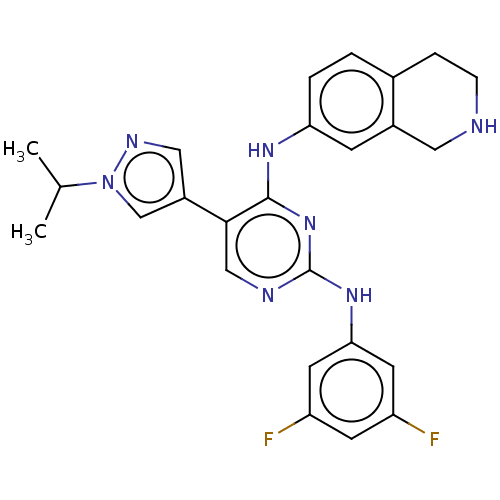 (US11053225, Compound 162) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515830 (US11053225, Compound 131) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515879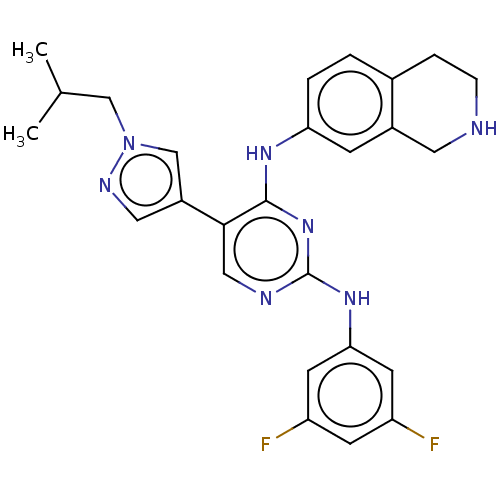 (US11053225, Compound 181) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515858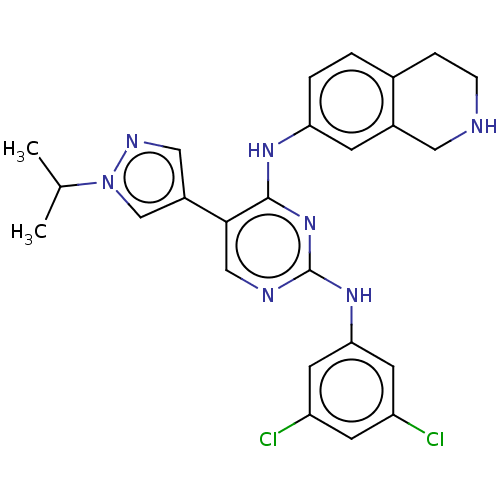 (US11053225, Compound 160) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515877 (US11053225, Compound 179) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515857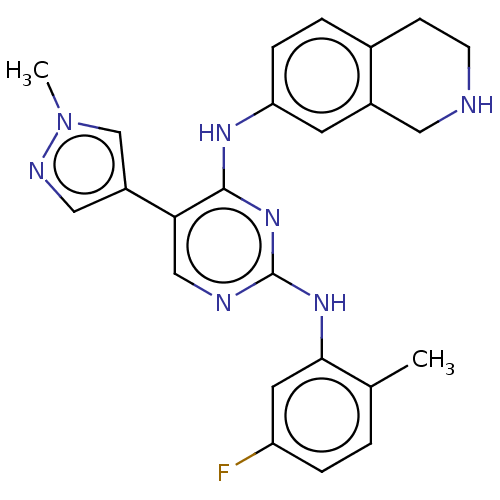 (US11053225, Compound 159) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515867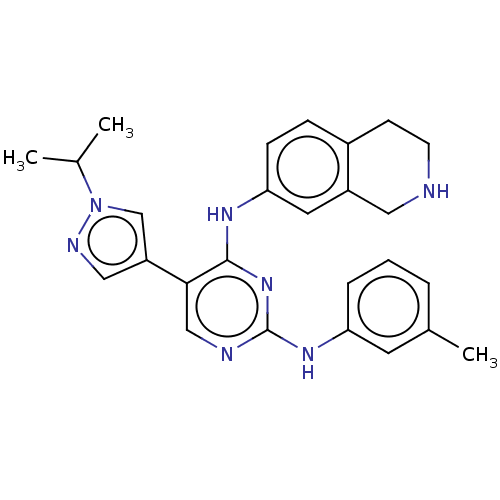 (US11053225, Compound 169) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515799 (US11053225, Compound 103) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515831 (US11053225, Compound 132) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515861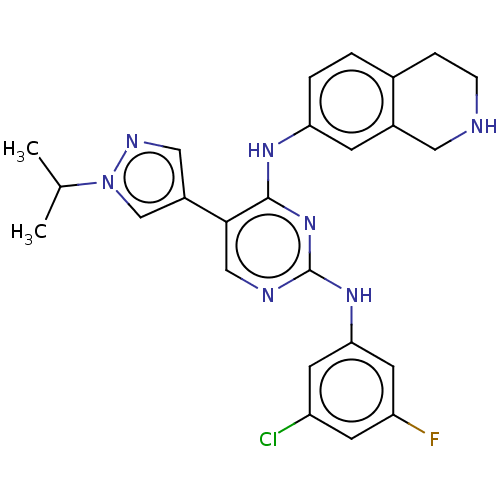 (US11053225, Compound 163) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515876 (US11053225, Compound 178) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515880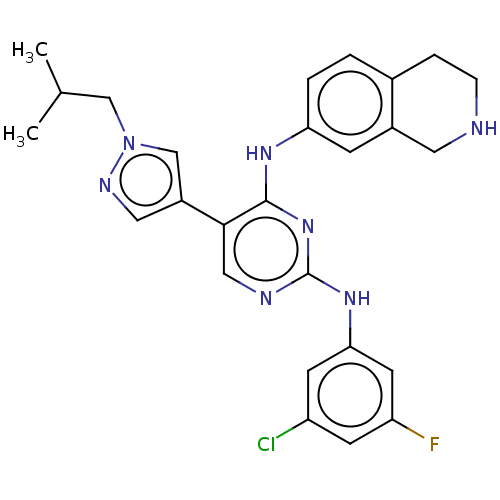 (US11053225, Compound 182) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515803 (US11053225, Compound 110) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515844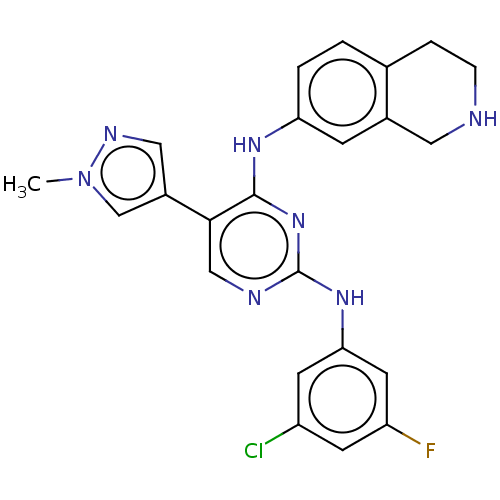 (US11053225, Compound 145) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515873 (US11053225, Compound 175) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515864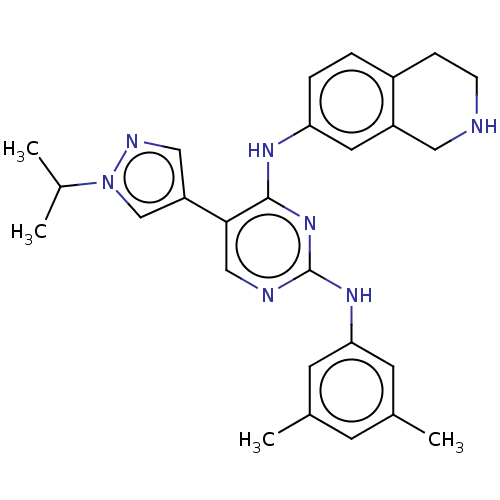 (US11053225, Compound 166) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515854 (US11053225, Compound 156) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515805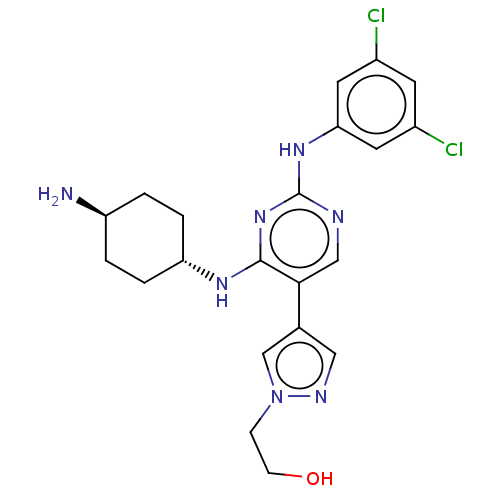 (US11053225, Compound 113) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515821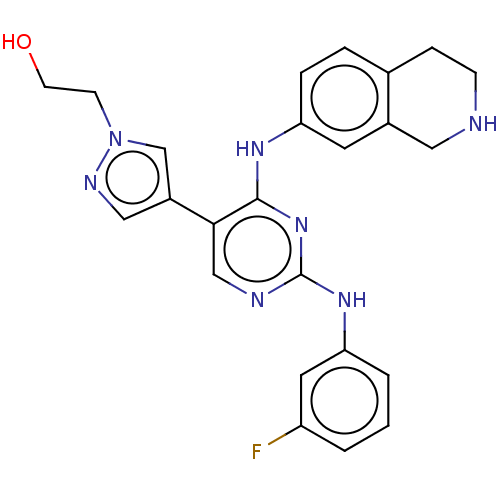 (US11053225, Compound 102) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515804 (US11053225, Compound 111) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515890 (US11053225, Compound 194) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515820 (US11053225, Compound 101 | US11053225, Compound 19...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50425862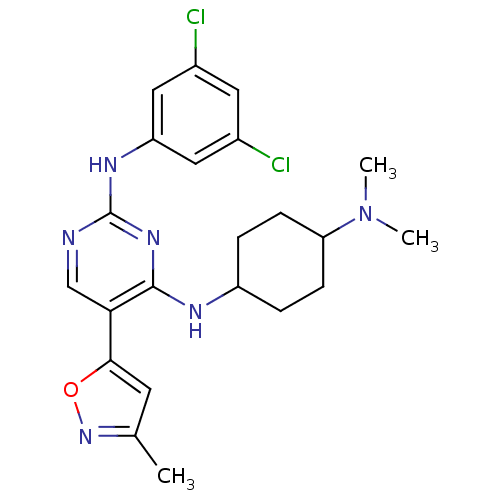 (CHEMBL2312654) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP | Bioorg Med Chem Lett 23: 1051-5 (2013) Article DOI: 10.1016/j.bmcl.2012.12.028 BindingDB Entry DOI: 10.7270/Q2XW4M38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515798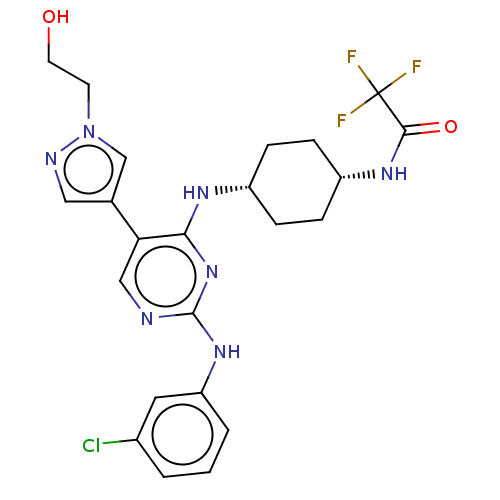 (US11053225, Compound 100) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515848 (US11053225, Compound 149) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50571440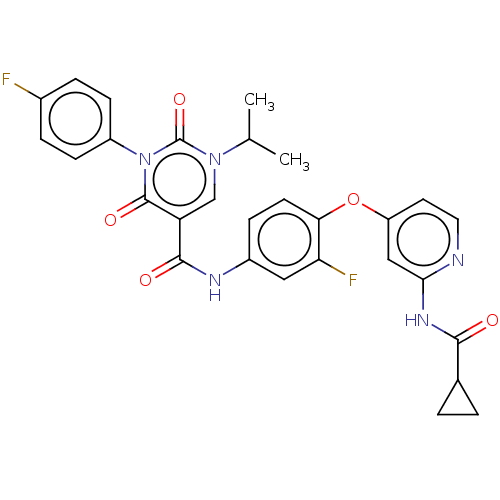 (CHEMBL4873571) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human RSE (451 to end residues) using KVEKIGEGTYGVVYK as substrate incubated for 40 mins in presence of [gamma33P]ATP by sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02093 BindingDB Entry DOI: 10.7270/Q2PR80SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515884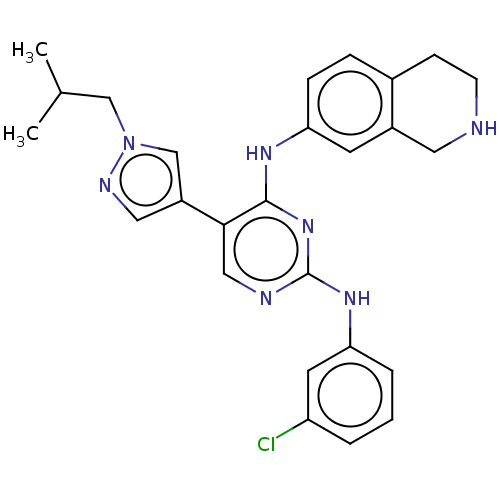 (US11053225, Compound 186) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515811 (US11053225, Compound 204) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2706 total ) | Next | Last >> |