

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50240845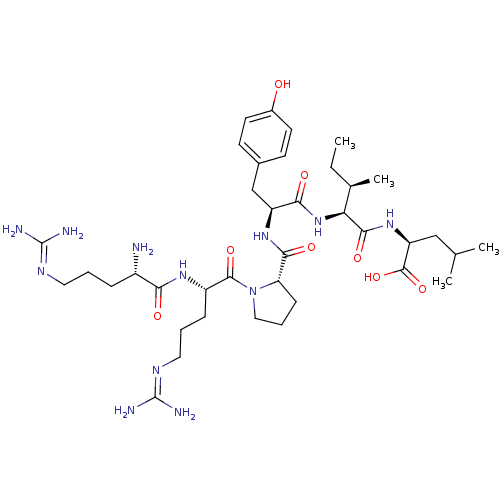 ((S)-2-{(2S,3R)-2-[(S)-2-({(S)-1-[(S)-2-((S)-2-Amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Agonist activity at rat NTS1 stably expressed in CHOK1 cells assessed as induction of calcium release by FLIPR assay | J Med Chem 57: 5318-32 (2014) Article DOI: 10.1021/jm5003843 BindingDB Entry DOI: 10.7270/Q2PR7XJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50130880 (CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-T...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Agonist activity at rat NTS1 stably expressed in CHOK1 cells assessed as induction of calcium release by FLIPR assay | J Med Chem 57: 5318-32 (2014) Article DOI: 10.1021/jm5003843 BindingDB Entry DOI: 10.7270/Q2PR7XJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50048908 (CHEMBL415788) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Agonist activity at human NTS1 in Sprague-Dawley rat ileum assessed as inhibition of carbachol-induced contraction | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50124132 (CHEMBL3622802) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Agonist activity at human NTS1 in Sprague-Dawley rat ileum assessed as inhibition of carbachol-induced contraction | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50548041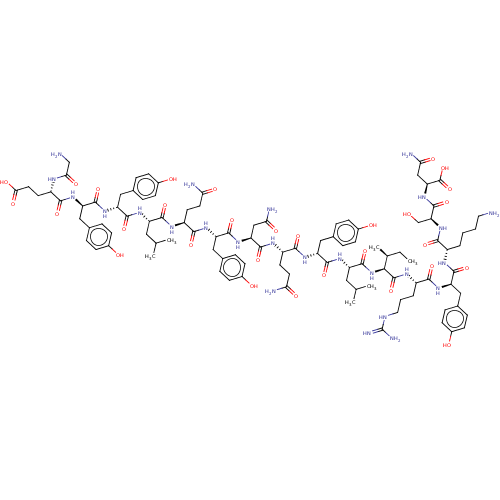 (CHEMBL4794546) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at rat NTR1 expressed in CHO cells assessed as effect on [3H] phosphoinositide accumulation using neurotensin (1 to 13 residues) as ... | Citation and Details Article DOI: 10.1039/d0md00173b BindingDB Entry DOI: 10.7270/Q20005PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50124135 (CHEMBL3622803) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Agonist activity at human NTS1 in Sprague-Dawley rat ileum assessed as inhibition of carbachol-induced contraction | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50322368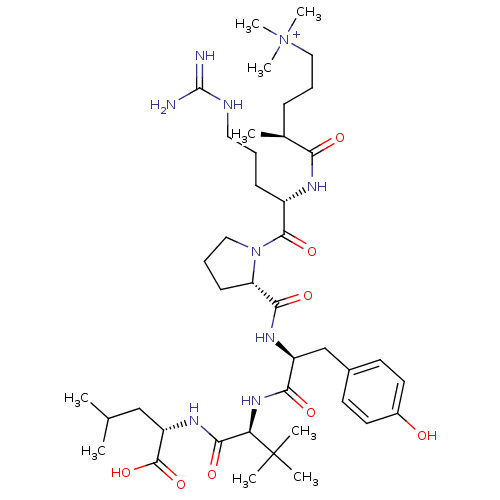 ((S)-5-((S)-1-((S)-2-((S)-1-((S)-1-((S)-1-carboxy-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
Medical University of South Carolina Campus Curated by ChEMBL | Assay Description Agonist activity at rat NTR1 expressed in LTK cells assessed as calcium mobilization | J Med Chem 53: 4623-32 (2010) Article DOI: 10.1021/jm100092s BindingDB Entry DOI: 10.7270/Q2DF6RDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50124138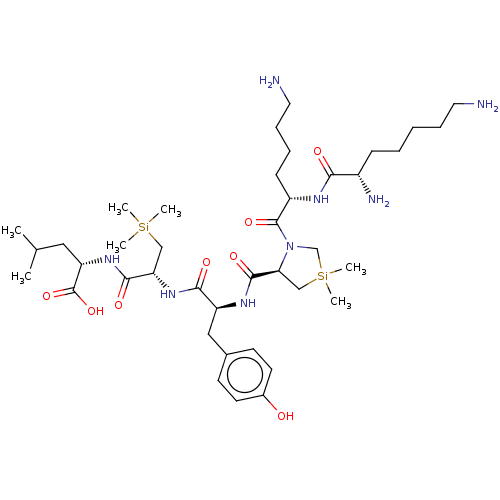 (CHEMBL3622804) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Agonist activity at human NTS1 in Sprague-Dawley rat ileum assessed as inhibition of carbachol-induced contraction | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50124141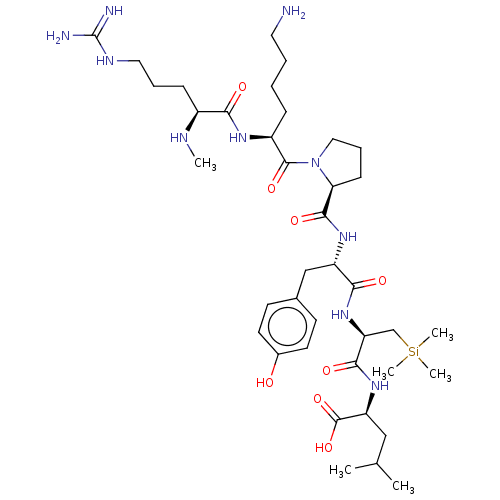 (CHEMBL3622805) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Agonist activity at human NTS1 in Sprague-Dawley rat ileum assessed as inhibition of carbachol-induced contraction | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50124146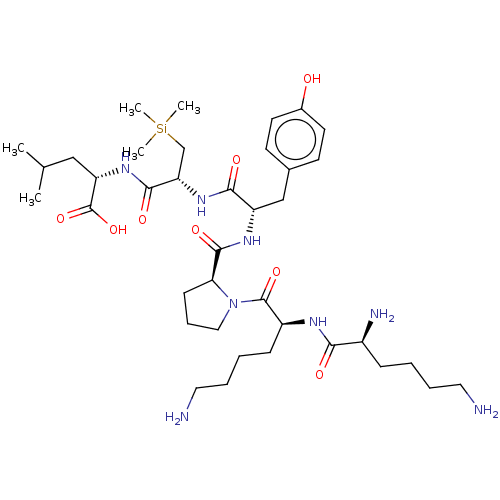 (CHEMBL3622801) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Agonist activity at human NTS1 in Sprague-Dawley rat ileum assessed as inhibition of carbachol-induced contraction | J Med Chem 58: 7785-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00841 BindingDB Entry DOI: 10.7270/Q2MW2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||