 Found 446 hits of ic50 for UniProtKB: P43681
Found 446 hits of ic50 for UniProtKB: P43681 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50170589
((3S)-3-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(2...)Show SMILES C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CNC(=O)CN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C65H97N23O24S4/c1-27(75-61(108)42(24-115)86-54(101)29(3)76-64(111)44-5-4-12-88(44)65(112)38(14-31-19-71-26-73-31)83-60(107)39(21-89)84-63(110)43(25-116)87-62(109)41(23-114)77-49(95)20-72-48(94)18-66)52(99)74-28(2)53(100)79-35(15-46(68)92)58(105)81-36(16-47(69)93)57(104)78-33(10-11-45(67)91)55(102)82-37(17-50(96)97)59(106)80-34(13-30-6-8-32(90)9-7-30)56(103)85-40(22-113)51(70)98/h6-9,19,26-29,33-44,89-90,113-116H,4-5,10-18,20-25,66H2,1-3H3,(H2,67,91)(H2,68,92)(H2,69,93)(H2,70,98)(H,71,73)(H,72,94)(H,74,99)(H,75,108)(H,76,111)(H,77,95)(H,78,104)(H,79,100)(H,80,106)(H,81,105)(H,82,102)(H,83,107)(H,84,110)(H,85,103)(H,86,101)(H,87,109)(H,96,97)/t27-,28-,29-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Nicotinic acetylcholine receptor alpha4-beta2 |
J Med Chem 48: 4705-45 (2005)
Article DOI: 10.1021/jm040219e
BindingDB Entry DOI: 10.7270/Q29W0G79 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50170586
((3S)-3-{[(2S)-1-[(2S)-2-[(2S,3R)-2-[(2S)-2-[(2S)-2...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CS)NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CS)C=O Show InChI InChI=1S/C65H92N16O21S4/c1-32(53(90)78-52(33(2)84)62(99)74-41(23-49(67)86)64(101)79-18-6-11-46(79)60(97)72-39(24-51(88)89)55(92)69-36(26-82)28-103)68-54(91)38(21-34-9-4-3-5-10-34)71-58(95)45(31-106)77-61(98)47-12-7-19-80(47)65(102)48-13-8-20-81(48)63(100)40(22-35-14-16-37(85)17-15-35)73-56(93)42(27-83)75-59(96)44(30-105)76-57(94)43(29-104)70-50(87)25-66/h3-5,9-10,14-17,26,32-33,36,38-48,52,83-85,103-106H,6-8,11-13,18-25,27-31,66H2,1-2H3,(H2,67,86)(H,68,91)(H,69,92)(H,70,87)(H,71,95)(H,72,97)(H,73,93)(H,74,99)(H,75,96)(H,76,94)(H,77,98)(H,78,90)(H,88,89)/t32-,33+,36+,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,52-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Nicotinic acetylcholine receptor alpha4-beta2; Range is 0.3-1.5 nM |
J Med Chem 48: 4705-45 (2005)
Article DOI: 10.1021/jm040219e
BindingDB Entry DOI: 10.7270/Q29W0G79 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50170586

((3S)-3-{[(2S)-1-[(2S)-2-[(2S,3R)-2-[(2S)-2-[(2S)-2...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CS)NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CS)C=O Show InChI InChI=1S/C65H92N16O21S4/c1-32(53(90)78-52(33(2)84)62(99)74-41(23-49(67)86)64(101)79-18-6-11-46(79)60(97)72-39(24-51(88)89)55(92)69-36(26-82)28-103)68-54(91)38(21-34-9-4-3-5-10-34)71-58(95)45(31-106)77-61(98)47-12-7-19-80(47)65(102)48-13-8-20-81(48)63(100)40(22-35-14-16-37(85)17-15-35)73-56(93)42(27-83)75-59(96)44(30-105)76-57(94)43(29-104)70-50(87)25-66/h3-5,9-10,14-17,26,32-33,36,38-48,52,83-85,103-106H,6-8,11-13,18-25,27-31,66H2,1-2H3,(H2,67,86)(H,68,91)(H,69,92)(H,70,87)(H,71,95)(H,72,97)(H,73,93)(H,74,99)(H,75,96)(H,76,94)(H,77,98)(H,78,90)(H,88,89)/t32-,33+,36+,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,52-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Nicotinic acetylcholine receptor alpha4-beta2; Range is 0.3-1.5 nM |
J Med Chem 48: 4705-45 (2005)
Article DOI: 10.1021/jm040219e
BindingDB Entry DOI: 10.7270/Q29W0G79 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50548705
(CHEMBL4740159)Show SMILES C[C@@H]1CC2C3Cn4c(cccc4=O)C(CN2C)C13 |r,TLB:0:1:4:13.15.14,16:15:2.1:4,THB:1:17:5.6.7:3.15.14,5:4:2.1:13.15.14,7:13:2.1:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR by radioligand competition analysis |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115820
BindingDB Entry DOI: 10.7270/Q2WW7N96 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50140089
(CHEMBL437423 | GCCSHPACAGNNQHIC*)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C61H96N24O20S4/c1-5-26(2)47(60(104)81-37(20-106)48(66)92)84-54(98)32(11-29-16-67-24-70-29)77-51(95)31(8-9-42(63)87)76-53(97)34(14-44(65)89)78-52(96)33(13-43(64)88)74-46(91)18-69-49(93)27(3)72-56(100)39(22-108)82-50(94)28(4)73-59(103)41-7-6-10-85(41)61(105)35(12-30-17-68-25-71-30)79-55(99)36(19-86)80-58(102)40(23-109)83-57(101)38(21-107)75-45(90)15-62/h16-17,24-28,31-41,47,86,106-109H,5-15,18-23,62H2,1-4H3,(H2,63,87)(H2,64,88)(H2,65,89)(H2,66,92)(H,67,70)(H,68,71)(H,69,93)(H,72,100)(H,73,103)(H,74,91)(H,75,90)(H,76,97)(H,77,95)(H,78,96)(H,79,99)(H,80,102)(H,81,104)(H,82,94)(H,83,101)(H,84,98)/t26-,27-,28-,31-,32-,33-,34-,35-,36-,37-,38-,39-,40-,41-,47-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Nicotinic acetylcholine receptor alpha4-beta2 |
J Med Chem 48: 4705-45 (2005)
Article DOI: 10.1021/jm040219e
BindingDB Entry DOI: 10.7270/Q29W0G79 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of alpha-4-beta-2 nAChR |
Bioorg Med Chem Lett 17: 6245-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.026
BindingDB Entry DOI: 10.7270/Q24J0GBN |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chile
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR overexpressed in human SHEP cells after 75 mins by liquid scintillation spectrometric analy... |
Bioorg Med Chem 21: 2687-94 (2013)
Article DOI: 10.1016/j.bmc.2013.03.024
BindingDB Entry DOI: 10.7270/Q2MW2JJQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50170592
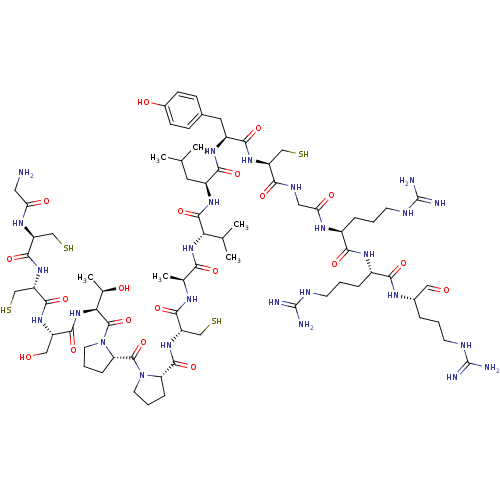
(CHEMBL411146)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)[C@@H](C)O)C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CS)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C=O Show InChI InChI=1S/C74H124N26O20S4/c1-36(2)26-45(62(111)91-46(27-40-17-19-42(104)20-18-40)63(112)94-48(32-121)59(108)85-29-55(106)88-43(13-8-22-83-73(78)79)61(110)90-44(14-9-23-84-74(80)81)60(109)87-41(30-101)12-7-21-82-72(76)77)92-69(118)56(37(3)4)97-58(107)38(5)86-65(114)50(34-123)96-68(117)52-15-10-24-99(52)70(119)53-16-11-25-100(53)71(120)57(39(6)103)98-64(113)47(31-102)93-67(116)51(35-124)95-66(115)49(33-122)89-54(105)28-75/h17-20,30,36-39,41,43-53,56-57,102-104,121-124H,7-16,21-29,31-35,75H2,1-6H3,(H,85,108)(H,86,114)(H,87,109)(H,88,106)(H,89,105)(H,90,110)(H,91,111)(H,92,118)(H,93,116)(H,94,112)(H,95,115)(H,96,117)(H,97,107)(H,98,113)(H4,76,77,82)(H4,78,79,83)(H4,80,81,84)/t38-,39+,41-,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,53-,56-,57-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Nicotinic acetylcholine receptor alpha4-beta2; Range is 0.3-1.5 nM |
J Med Chem 48: 4705-45 (2005)
Article DOI: 10.1021/jm040219e
BindingDB Entry DOI: 10.7270/Q29W0G79 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50329473

((S)-N-(1-(3-(4-(3-aminopropylamino)butylamino)prop...)Show SMILES CCCC(=O)N[C@@H](CC1CCCCC1)C(=O)NCCCNCCCCNCCCN |r| Show InChI InChI=1S/C23H47N5O2/c1-2-10-22(29)28-21(19-20-11-4-3-5-12-20)23(30)27-18-9-17-26-15-7-6-14-25-16-8-13-24/h20-21,25-26H,2-19,24H2,1H3,(H,27,30)(H,28,29)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Antagonist activity at alpha4beta2 nAChR (unknown origin) expressed in Xenopus laevis oocytes assessed as inhibition of Ach-induced channel activatio... |
J Med Chem 62: 6214-6222 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00519
BindingDB Entry DOI: 10.7270/Q2F47SGG |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50548704
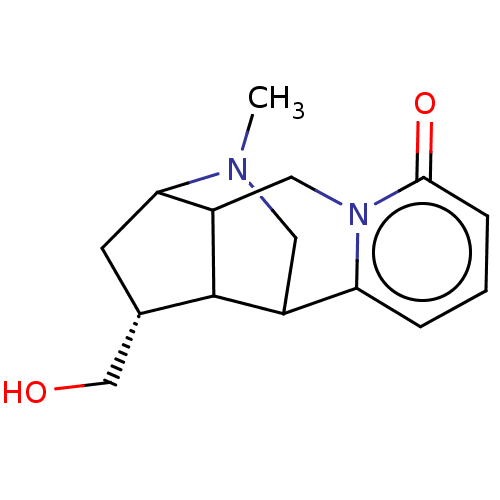
(CHEMBL4791046)Show SMILES CN1CC2C3[C@H](CO)CC1C3Cn1c2cccc1=O |r,TLB:0:1:8.5:10,5:4:11.12.13:9.1.2,11:10:8.5:3.1.2,THB:6:5:10:3.1.2,13:3:8.5:10| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR by radioligand competition analysis |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115820
BindingDB Entry DOI: 10.7270/Q2WW7N96 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50433566

(CHEMBL2381566)Show SMILES CN1CCC[C@H]1COC(=O)c1ccc(OCc2ccccc2)cc1 |r| Show InChI InChI=1S/C20H23NO3/c1-21-13-5-8-18(21)15-24-20(22)17-9-11-19(12-10-17)23-14-16-6-3-2-4-7-16/h2-4,6-7,9-12,18H,5,8,13-15H2,1H3/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chile
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR overexpressed in human SHEP cells after 75 mins by liquid scintillation spectrometric analy... |
Bioorg Med Chem 21: 2687-94 (2013)
Article DOI: 10.1016/j.bmc.2013.03.024
BindingDB Entry DOI: 10.7270/Q2MW2JJQ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50382474

(CHEMBL2024096 | US9303017, Sazetidine-A)Show InChI InChI=1S/C15H20N2O2/c18-8-4-2-1-3-5-13-9-15(11-16-10-13)19-12-14-6-7-17-14/h9-11,14,17-18H,1-2,4,6-8,12H2/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha4beta2 nAChR expressed in SH-EP1 cells assessed as inhibition of carbamylcholine induced 86Rb+ ion efflux preincuba... |
J Med Chem 55: 717-24 (2012)
Article DOI: 10.1021/jm201157c
BindingDB Entry DOI: 10.7270/Q21N8244 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50548703
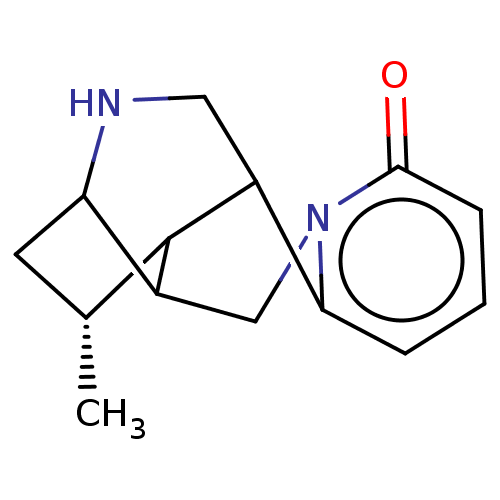
(CHEMBL4793576)Show SMILES C[C@@H]1CC2NCC3C1C2Cn1c3cccc1=O |r,TLB:1:7:9.10.11:3.4.5,THB:0:1:8:6.4.5,9:8:2.1:6.4.5,11:6:2.1:8| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR by radioligand competition analysis |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115820
BindingDB Entry DOI: 10.7270/Q2WW7N96 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]cytisine from human alpha4beta2 nAChR by radioligand competition analysis |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115820
BindingDB Entry DOI: 10.7270/Q2WW7N96 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50170584
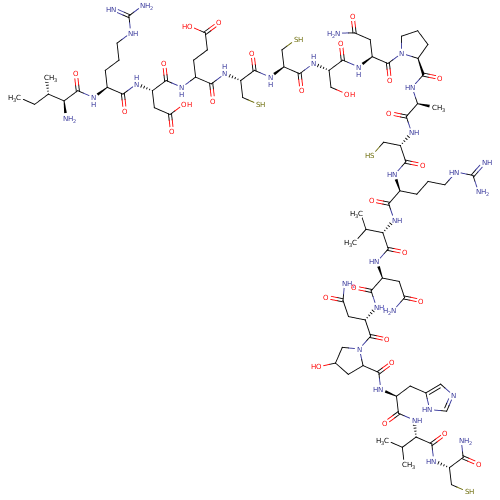
(CHEMBL411145)Show SMILES CC[C@H](C)[C@H](N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)NC(CCC(O)=O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1CC(O)CC1C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C83H137N31O28S4/c1-8-36(6)60(87)77(138)100-40(12-9-17-94-82(89)90)65(126)101-45(25-59(122)123)69(130)99-42(15-16-58(120)121)66(127)109-52(32-146)74(135)110-51(31-145)73(134)106-48(28-115)71(132)105-46(23-56(85)118)80(141)113-19-11-14-53(113)75(136)97-37(7)64(125)108-50(30-144)72(133)98-41(13-10-18-95-83(91)92)67(128)111-61(34(2)3)78(139)103-44(22-55(84)117)68(129)104-47(24-57(86)119)81(142)114-27-39(116)21-54(114)76(137)102-43(20-38-26-93-33-96-38)70(131)112-62(35(4)5)79(140)107-49(29-143)63(88)124/h26,33-37,39-54,60-62,115-116,143-146H,8-25,27-32,87H2,1-7H3,(H2,84,117)(H2,85,118)(H2,86,119)(H2,88,124)(H,93,96)(H,97,136)(H,98,133)(H,99,130)(H,100,138)(H,101,126)(H,102,137)(H,103,139)(H,104,129)(H,105,132)(H,106,134)(H,107,140)(H,108,125)(H,109,127)(H,110,135)(H,111,128)(H,112,131)(H,120,121)(H,122,123)(H4,89,90,94)(H4,91,92,95)/t36-,37-,39?,40-,41-,42?,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54?,60-,61-,62-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Nicotinic acetylcholine receptor alpha4-beta2; Range is 3-5 nM |
J Med Chem 48: 4705-45 (2005)
Article DOI: 10.1021/jm040219e
BindingDB Entry DOI: 10.7270/Q29W0G79 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50515189
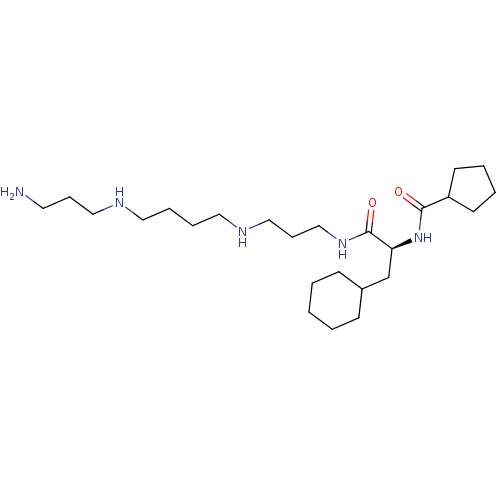
(CHEMBL4463134)Show SMILES NCCCNCCCCNCCCNC(=O)[C@H](CC1CCCCC1)NC(=O)C1CCCC1 |r| Show InChI InChI=1S/C25H49N5O2/c26-14-8-17-27-15-6-7-16-28-18-9-19-29-25(32)23(20-21-10-2-1-3-11-21)30-24(31)22-12-4-5-13-22/h21-23,27-28H,1-20,26H2,(H,29,32)(H,30,31)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Antagonist activity at alpha4beta2 nAChR (unknown origin) expressed in Xenopus laevis oocytes assessed as inhibition of Ach-induced channel activatio... |
J Med Chem 62: 6214-6222 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00519
BindingDB Entry DOI: 10.7270/Q2F47SGG |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50437504
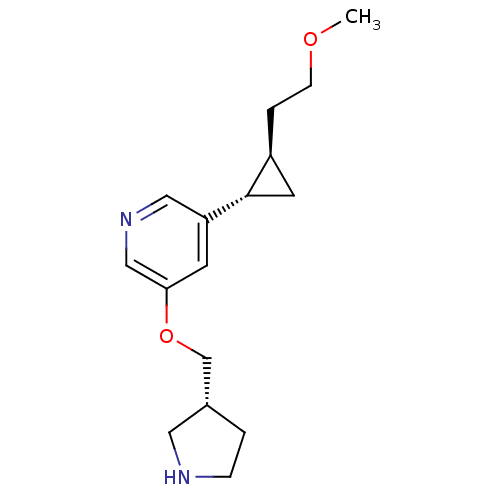
(CHEMBL2409631)Show SMILES COCC[C@H]1C[C@@H]1c1cncc(OC[C@@H]2CCNC2)c1 |r| Show InChI InChI=1S/C16H24N2O2/c1-19-5-3-13-7-16(13)14-6-15(10-18-9-14)20-11-12-2-4-17-8-12/h6,9-10,12-13,16-17H,2-5,7-8,11H2,1H3/t12-,13+,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha4beta2 nACHR expressed in human SHEP1 cells assessed as inhibition of carbamylcholine-induced 86Rb+ ion efflux prei... |
J Med Chem 56: 5495-504 (2014)
Article DOI: 10.1021/jm400510u
BindingDB Entry DOI: 10.7270/Q23J3FCH |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50382472
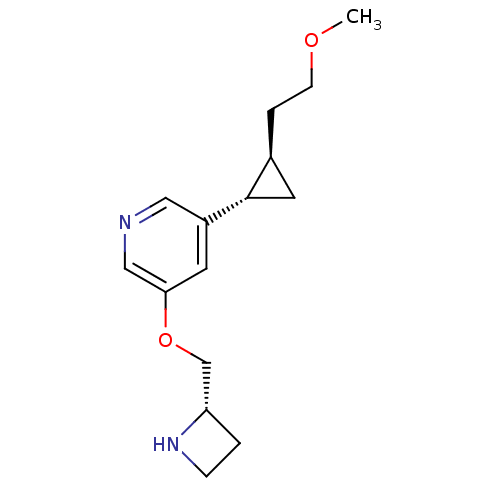
(CHEMBL2024094)Show SMILES COCC[C@H]1C[C@@H]1c1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C15H22N2O2/c1-18-5-3-11-7-15(11)12-6-14(9-16-8-12)19-10-13-2-4-17-13/h6,8-9,11,13,15,17H,2-5,7,10H2,1H3/t11-,13-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha4beta2 nACHR expressed in human SHEP1 cells assessed as inhibition of carbamylcholine-induced 86Rb+ ion efflux prei... |
J Med Chem 56: 5495-504 (2014)
Article DOI: 10.1021/jm400510u
BindingDB Entry DOI: 10.7270/Q23J3FCH |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50382472

(CHEMBL2024094)Show SMILES COCC[C@H]1C[C@@H]1c1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C15H22N2O2/c1-18-5-3-11-7-15(11)12-6-14(9-16-8-12)19-10-13-2-4-17-13/h6,8-9,11,13,15,17H,2-5,7,10H2,1H3/t11-,13-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha4beta2 nAChR expressed in SH-EP1 cells assessed as inhibition of carbamylcholine induced 86Rb+ ion efflux preincuba... |
J Med Chem 55: 717-24 (2012)
Article DOI: 10.1021/jm201157c
BindingDB Entry DOI: 10.7270/Q21N8244 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50382474

(CHEMBL2024096 | US9303017, Sazetidine-A)Show InChI InChI=1S/C15H20N2O2/c18-8-4-2-1-3-5-13-9-15(11-16-10-13)19-12-14-6-7-17-14/h9-11,14,17-18H,1-2,4,6-8,12H2/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University; Duke University
US Patent
| Assay Description
IC50(10′): The functional properties of the ligands were determined by 86Rb+ efflux assays in cells expressing α3β4 and α4β... |
US Patent US9303017 (2016)
BindingDB Entry DOI: 10.7270/Q25X27SF |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50302927
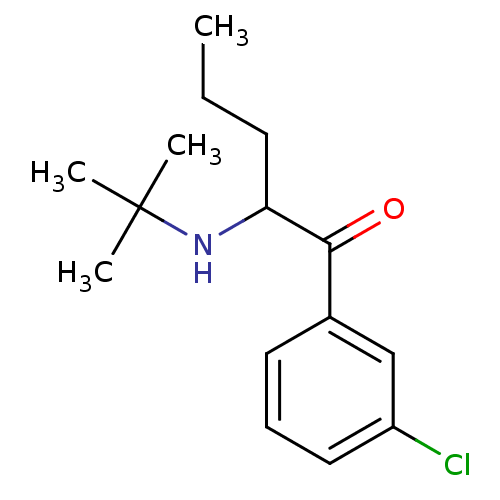
(2-(N-tert-Butylamino)-3'-chloropentanophenone | 2-...)Show InChI InChI=1S/C15H22ClNO/c1-5-7-13(17-15(2,3)4)14(18)11-8-6-9-12(16)10-11/h6,8-10,13,17H,5,7H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha4beta2 nAChR receptor expressed in human SH-SY5Y cells assessed as inhibition of carbamylcholine-induced 86Rb+ effl... |
J Med Chem 53: 2204-14 (2010)
Article DOI: 10.1021/jm9017465
BindingDB Entry DOI: 10.7270/Q2FN1693 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50140087
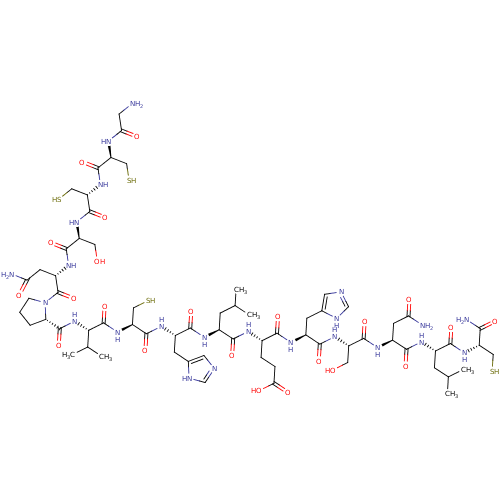
(CHEMBL265198 | GCCSNPVCHLEHSNLC*)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(C)C)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C67H107N23O22S4/c1-29(2)12-35(55(100)77-34(9-10-51(96)97)54(99)80-38(15-33-20-73-28-75-33)58(103)84-41(21-91)60(105)82-39(16-48(69)93)59(104)79-36(13-30(3)4)56(101)86-43(23-113)53(71)98)78-57(102)37(14-32-19-72-27-74-32)81-63(108)46(26-116)88-66(111)52(31(5)6)89-65(110)47-8-7-11-90(47)67(112)40(17-49(70)94)83-61(106)42(22-92)85-64(109)45(25-115)87-62(107)44(24-114)76-50(95)18-68/h19-20,27-31,34-47,52,91-92,113-116H,7-18,21-26,68H2,1-6H3,(H2,69,93)(H2,70,94)(H2,71,98)(H,72,74)(H,73,75)(H,76,95)(H,77,100)(H,78,102)(H,79,104)(H,80,99)(H,81,108)(H,82,105)(H,83,106)(H,84,103)(H,85,109)(H,86,101)(H,87,107)(H,88,111)(H,89,110)(H,96,97)/t34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,52-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Nicotinic acetylcholine receptor alpha4-beta2; Range is 0.5-8 nM |
J Med Chem 48: 4705-45 (2005)
Article DOI: 10.1021/jm040219e
BindingDB Entry DOI: 10.7270/Q29W0G79 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398321
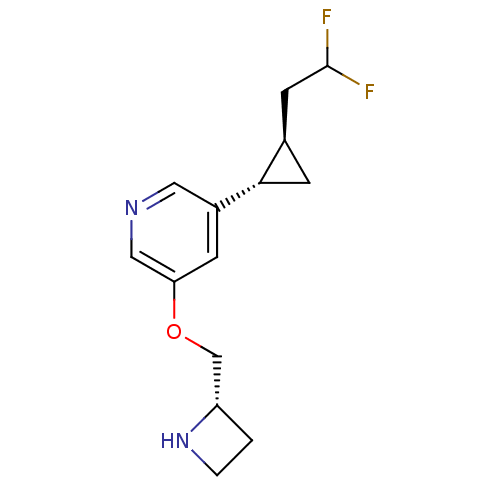
(CHEMBL2177344)Show SMILES FC(F)C[C@H]1C[C@@H]1c1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C14H18F2N2O/c15-14(16)5-9-4-13(9)10-3-12(7-17-6-10)19-8-11-1-2-18-11/h3,6-7,9,11,13-14,18H,1-2,4-5,8H2/t9-,11+,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Antagonist activity at high sensitivity and low sensitivity alpha4beta2-nAChR expressed in human SH-EP1 cells assessed as inhibition of carbamylcholi... |
J Med Chem 55: 8028-37 (2012)
Article DOI: 10.1021/jm3008739
BindingDB Entry DOI: 10.7270/Q2NC62BW |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50302929
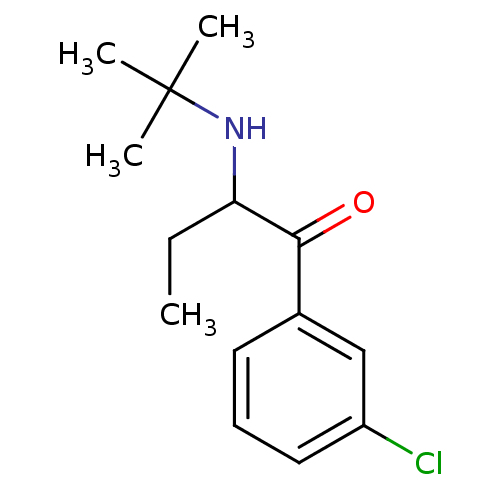
(2-(N-tert-Butylamino)-3'-chlorobutyrophenone | 2-(...)Show InChI InChI=1S/C14H20ClNO/c1-5-12(16-14(2,3)4)13(17)10-7-6-8-11(15)9-10/h6-9,12,16H,5H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha4beta2 nAChR receptor expressed in human SH-SY5Y cells assessed as inhibition of carbamylcholine-induced 86Rb+ effl... |
J Med Chem 53: 2204-14 (2010)
Article DOI: 10.1021/jm9017465
BindingDB Entry DOI: 10.7270/Q2FN1693 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50302917
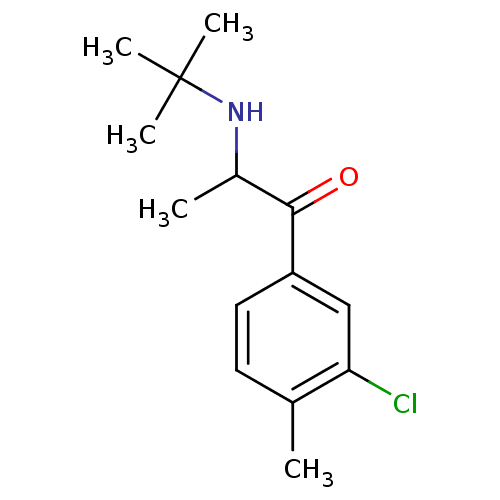
(2-(N-tert-Butylamino)-3'-chloro-4'-methylpropiophe...)Show InChI InChI=1S/C14H20ClNO/c1-9-6-7-11(8-12(9)15)13(17)10(2)16-14(3,4)5/h6-8,10,16H,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha4beta2 nAChR receptor expressed in human SH-SY5Y cells assessed as inhibition of carbamylcholine-induced 86Rb+ effl... |
J Med Chem 53: 2204-14 (2010)
Article DOI: 10.1021/jm9017465
BindingDB Entry DOI: 10.7270/Q2FN1693 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50382466
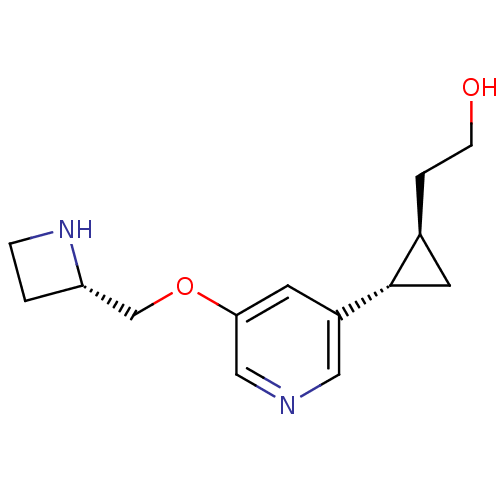
(CHEMBL2024087)Show SMILES OCC[C@H]1C[C@@H]1c1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C14H20N2O2/c17-4-2-10-6-14(10)11-5-13(8-15-7-11)18-9-12-1-3-16-12/h5,7-8,10,12,14,16-17H,1-4,6,9H2/t10-,12-,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha4beta2 nAChR expressed in SH-EP1 cells assessed as inhibition of carbamylcholine induced 86Rb+ ion efflux preincuba... |
J Med Chem 55: 717-24 (2012)
Article DOI: 10.1021/jm201157c
BindingDB Entry DOI: 10.7270/Q21N8244 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50170600
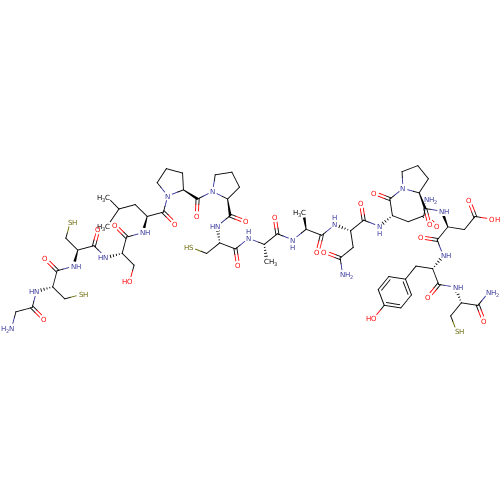
((3S)-3-{[(2S)-1-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CS)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C65H99N19O22S4/c1-29(2)18-37(76-57(98)39(24-85)78-60(101)43(28-110)80-59(100)41(26-108)72-49(89)23-66)63(104)84-17-7-10-46(84)65(106)83-16-6-9-45(83)62(103)81-42(27-109)58(99)71-30(3)52(93)70-31(4)53(94)73-35(20-47(67)87)55(96)77-38(21-48(68)88)64(105)82-15-5-8-44(82)61(102)75-36(22-50(90)91)56(97)74-34(19-32-11-13-33(86)14-12-32)54(95)79-40(25-107)51(69)92/h11-14,29-31,34-46,85-86,107-110H,5-10,15-28,66H2,1-4H3,(H2,67,87)(H2,68,88)(H2,69,92)(H,70,93)(H,71,99)(H,72,89)(H,73,94)(H,74,97)(H,75,102)(H,76,98)(H,77,96)(H,78,101)(H,79,95)(H,80,100)(H,81,103)(H,90,91)/t30-,31-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Nicotinic acetylcholine receptor alpha4-beta2 |
J Med Chem 48: 4705-45 (2005)
Article DOI: 10.1021/jm040219e
BindingDB Entry DOI: 10.7270/Q29W0G79 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50302924
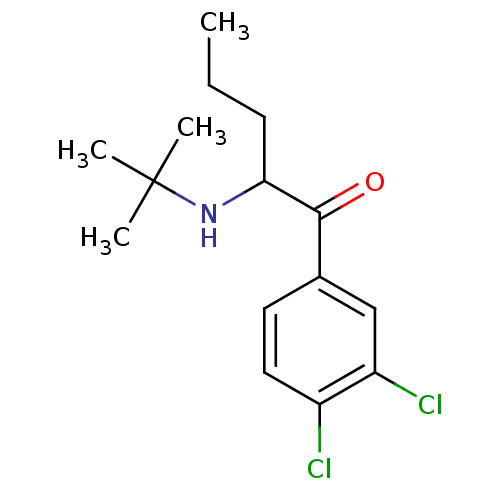
(2-(tert-Butylamino)-3',4'-dichloropentanophenone |...)Show InChI InChI=1S/C15H21Cl2NO/c1-5-6-13(18-15(2,3)4)14(19)10-7-8-11(16)12(17)9-10/h7-9,13,18H,5-6H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha4beta2 nAChR receptor expressed in human SH-SY5Y cells assessed as inhibition of carbamylcholine-induced 86Rb+ effl... |
J Med Chem 53: 2204-14 (2010)
Article DOI: 10.1021/jm9017465
BindingDB Entry DOI: 10.7270/Q2FN1693 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50302914
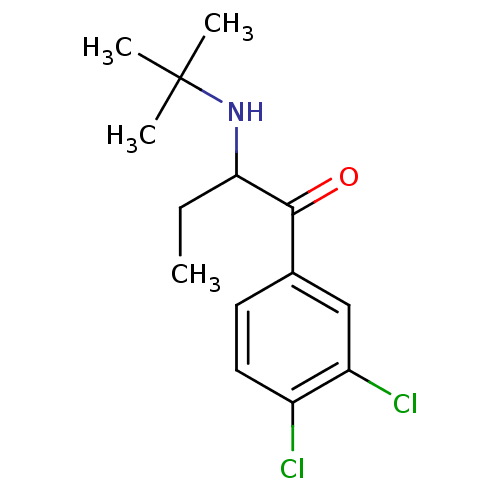
(2-(tert-Butylamino)-3',4'-dichlorobutyrophenone | ...)Show InChI InChI=1S/C14H19Cl2NO/c1-5-12(17-14(2,3)4)13(18)9-6-7-10(15)11(16)8-9/h6-8,12,17H,5H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha4beta2 nAChR receptor expressed in human SH-SY5Y cells assessed as inhibition of carbamylcholine-induced 86Rb+ effl... |
J Med Chem 53: 2204-14 (2010)
Article DOI: 10.1021/jm9017465
BindingDB Entry DOI: 10.7270/Q2FN1693 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50398320
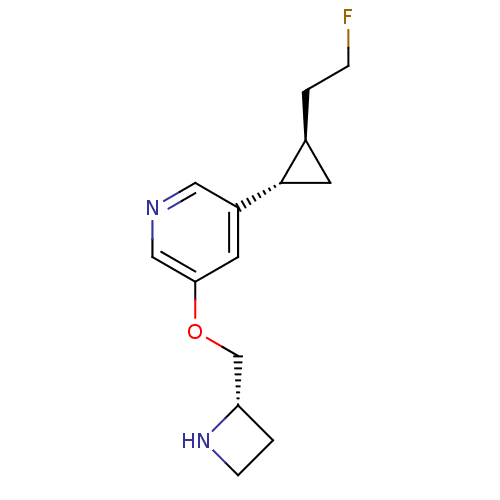
(CHEMBL2177345)Show SMILES FCC[C@H]1C[C@@H]1c1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C14H19FN2O/c15-3-1-10-6-14(10)11-5-13(8-16-7-11)18-9-12-2-4-17-12/h5,7-8,10,12,14,17H,1-4,6,9H2/t10-,12-,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Antagonist activity at high sensitivity and low sensitivity alpha4beta2-nAChR expressed in human SH-EP1 cells assessed as inhibition of carbamylcholi... |
J Med Chem 55: 8028-37 (2012)
Article DOI: 10.1021/jm3008739
BindingDB Entry DOI: 10.7270/Q2NC62BW |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50428079
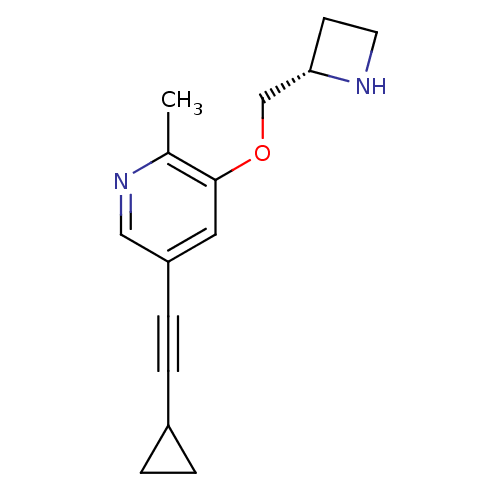
(CHEMBL2323569 | US9303017, 25, YL-1-199)Show InChI InChI=1S/C15H18N2O/c1-11-15(18-10-14-6-7-16-14)8-13(9-17-11)5-4-12-2-3-12/h8-9,12,14,16H,2-3,6-7,10H2,1H3/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University; Duke University
US Patent
| Assay Description
IC50(10′): The functional properties of the ligands were determined by 86Rb+ efflux assays in cells expressing α3β4 and α4β... |
US Patent US9303017 (2016)
BindingDB Entry DOI: 10.7270/Q25X27SF |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50382474

(CHEMBL2024096 | US9303017, Sazetidine-A)Show InChI InChI=1S/C15H20N2O2/c18-8-4-2-1-3-5-13-9-15(11-16-10-13)19-12-14-6-7-17-14/h9-11,14,17-18H,1-2,4,6-8,12H2/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
Desensitization of human alpha4beta2 nACHR expressed in HEK293 cells assessed as inhibition of 86Rb+ efflux preincubated for 10 mins measured after 2... |
J Med Chem 56: 3000-11 (2013)
Article DOI: 10.1021/jm4000374
BindingDB Entry DOI: 10.7270/Q21Z45R1 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50382474

(CHEMBL2024096 | US9303017, Sazetidine-A)Show InChI InChI=1S/C15H20N2O2/c18-8-4-2-1-3-5-13-9-15(11-16-10-13)19-12-14-6-7-17-14/h9-11,14,17-18H,1-2,4,6-8,12H2/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha4beta2 nAChR assessed as inhibition of nicotine-induced [86Rb+] efflux preincubated for 10 mins before nicotine exp... |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50515186
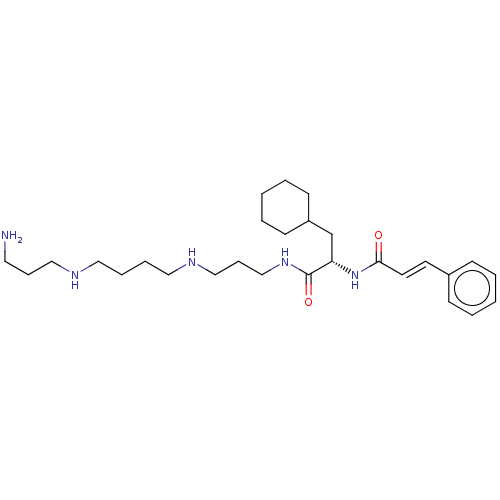
(CHEMBL4462550)Show SMILES NCCCNCCCCNCCCNC(=O)[C@H](CC1CCCCC1)NC(=O)\C=C\c1ccccc1 |r| Show InChI InChI=1S/C28H47N5O2/c29-17-9-20-30-18-7-8-19-31-21-10-22-32-28(35)26(23-25-13-5-2-6-14-25)33-27(34)16-15-24-11-3-1-4-12-24/h1,3-4,11-12,15-16,25-26,30-31H,2,5-10,13-14,17-23,29H2,(H,32,35)(H,33,34)/b16-15+/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Antagonist activity at alpha4beta2 nAChR (unknown origin) expressed in Xenopus laevis oocytes assessed as inhibition of Ach-induced channel activatio... |
J Med Chem 62: 6214-6222 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00519
BindingDB Entry DOI: 10.7270/Q2F47SGG |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50048392
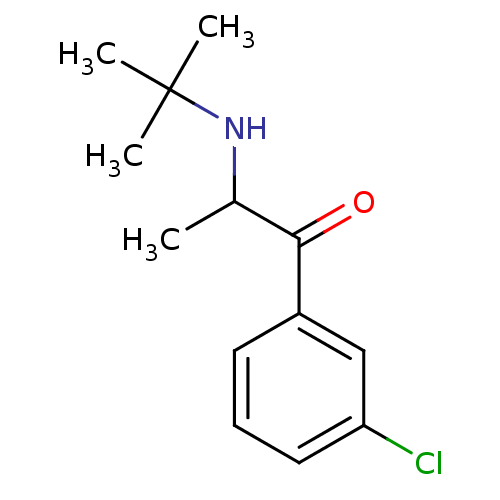
(2-(tert-butylamino)-1-(3-chlorophenyl)propan-1-one...)Show InChI InChI=1S/C13H18ClNO/c1-9(15-13(2,3)4)12(16)10-6-5-7-11(14)8-10/h5-9,15H,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha4beta2 nAChR receptor expressed in human SH-SY5Y cells assessed as inhibition of carbamylcholine-induced 86Rb+ effl... |
J Med Chem 53: 2204-14 (2010)
Article DOI: 10.1021/jm9017465
BindingDB Entry DOI: 10.7270/Q2FN1693 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50382474

(CHEMBL2024096 | US9303017, Sazetidine-A)Show InChI InChI=1S/C15H20N2O2/c18-8-4-2-1-3-5-13-9-15(11-16-10-13)19-12-14-6-7-17-14/h9-11,14,17-18H,1-2,4,6-8,12H2/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University; Duke University
US Patent
| Assay Description
IC50(10′): The functional properties of the ligands were determined by 86Rb+ efflux assays in cells expressing α3β4 and α4β... |
US Patent US9303017 (2016)
BindingDB Entry DOI: 10.7270/Q25X27SF |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50302930
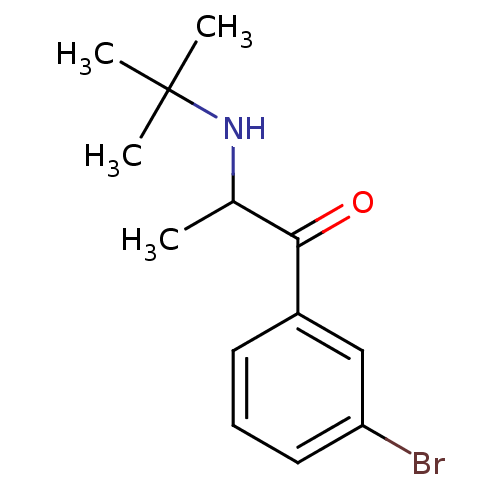
(1-(3-bromophenyl)-2-(tert-butylamino)propan-1-one ...)Show InChI InChI=1S/C13H18BrNO/c1-9(15-13(2,3)4)12(16)10-6-5-7-11(14)8-10/h5-9,15H,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha4beta2 nAChR receptor expressed in human SH-SY5Y cells assessed as inhibition of carbamylcholine-induced 86Rb+ effl... |
J Med Chem 53: 2204-14 (2010)
Article DOI: 10.1021/jm9017465
BindingDB Entry DOI: 10.7270/Q2FN1693 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
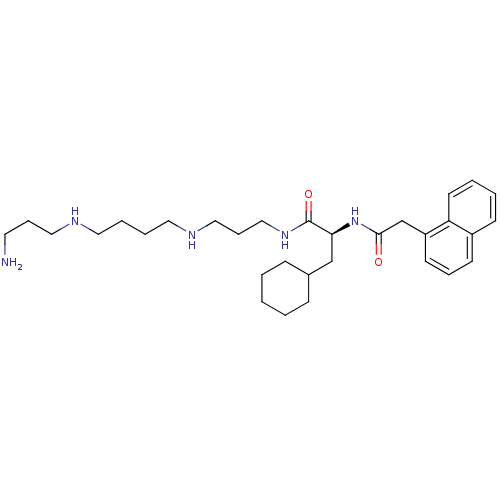
(Homo sapiens (Human)) | BDBM50329477
((S)-N-(3-(4-(3-aminopropylamino)butylamino)propyl)...)Show SMILES NCCCNCCCCNCCCNC(=O)[C@H](CC1CCCCC1)NC(=O)Cc1cccc2ccccc12 |r| Show InChI InChI=1S/C31H49N5O2/c32-17-9-20-33-18-6-7-19-34-21-10-22-35-31(38)29(23-25-11-2-1-3-12-25)36-30(37)24-27-15-8-14-26-13-4-5-16-28(26)27/h4-5,8,13-16,25,29,33-34H,1-3,6-7,9-12,17-24,32H2,(H,35,38)(H,36,37)/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Antagonist activity at alpha4beta2 nAChR (unknown origin) expressed in Xenopus laevis oocytes assessed as inhibition of Ach-induced channel activatio... |
J Med Chem 62: 6214-6222 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00519
BindingDB Entry DOI: 10.7270/Q2F47SGG |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50210334
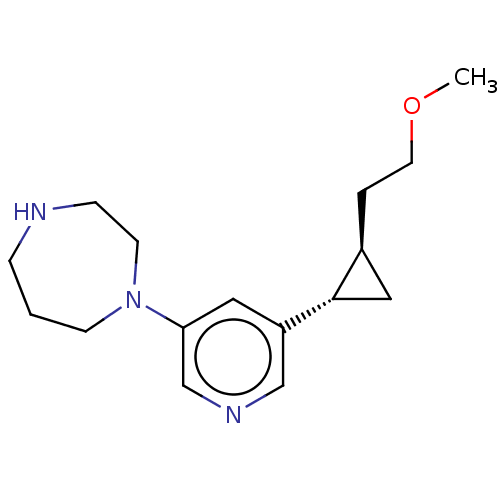
(CHEMBL3948977)Show SMILES [H][C@]1(CCOC)C[C@]1([H])c1cncc(c1)N1CCCNCC1 |r| Show InChI InChI=1S/C16H25N3O/c1-20-8-3-13-10-16(13)14-9-15(12-18-11-14)19-6-2-4-17-5-7-19/h9,11-13,16-17H,2-8,10H2,1H3/t13-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha4beta2 expressed in human SH-EP1 cells assessed as reduction in carbamylcholine induced 86Rb+ efflux preincubated f... |
Eur J Med Chem 124: 689-697 (2016)
Article DOI: 10.1016/j.ejmech.2016.09.016
BindingDB Entry DOI: 10.7270/Q2H41TDB |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50210335
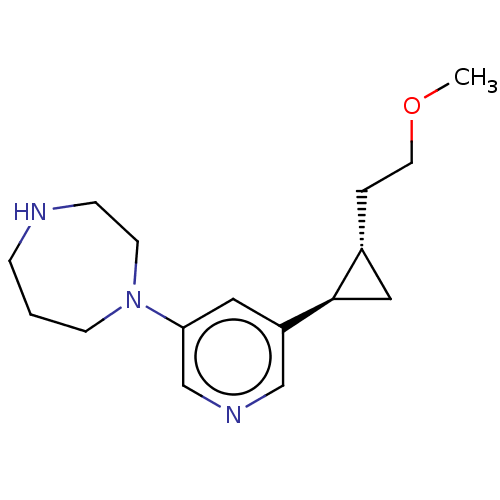
(CHEMBL3898434)Show SMILES [H][C@@]1(CCOC)C[C@@]1([H])c1cncc(c1)N1CCCNCC1 |r| Show InChI InChI=1S/C16H25N3O/c1-20-8-3-13-10-16(13)14-9-15(12-18-11-14)19-6-2-4-17-5-7-19/h9,11-13,16-17H,2-8,10H2,1H3/t13-,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha4beta2 expressed in human SH-EP1 cells assessed as reduction in carbamylcholine induced 86Rb+ efflux preincubated f... |
Eur J Med Chem 124: 689-697 (2016)
Article DOI: 10.1016/j.ejmech.2016.09.016
BindingDB Entry DOI: 10.7270/Q2H41TDB |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50419266
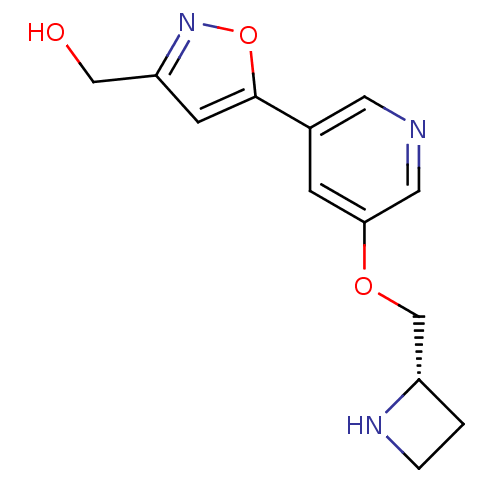
(CHEMBL1835610)Show InChI InChI=1S/C13H15N3O3/c17-7-11-4-13(19-16-11)9-3-12(6-14-5-9)18-8-10-1-2-15-10/h3-6,10,15,17H,1-2,7-8H2/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha4beta2 nAChR expressed in SH-EP1 cells assessed as inhibition of carbamylcholine-induced 86Rb+ ion efflux by liquid... |
J Med Chem 54: 7280-8 (2011)
Article DOI: 10.1021/jm200855b
BindingDB Entry DOI: 10.7270/Q2RB750M |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50210335

(CHEMBL3898434)Show SMILES [H][C@@]1(CCOC)C[C@@]1([H])c1cncc(c1)N1CCCNCC1 |r| Show InChI InChI=1S/C16H25N3O/c1-20-8-3-13-10-16(13)14-9-15(12-18-11-14)19-6-2-4-17-5-7-19/h9,11-13,16-17H,2-8,10H2,1H3/t13-,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha4beta2 expressed in human SH-EP1 cells assessed as reduction in carbamylcholine induced 86Rb+ efflux preincubated f... |
Eur J Med Chem 124: 689-697 (2016)
Article DOI: 10.1016/j.ejmech.2016.09.016
BindingDB Entry DOI: 10.7270/Q2H41TDB |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50442930
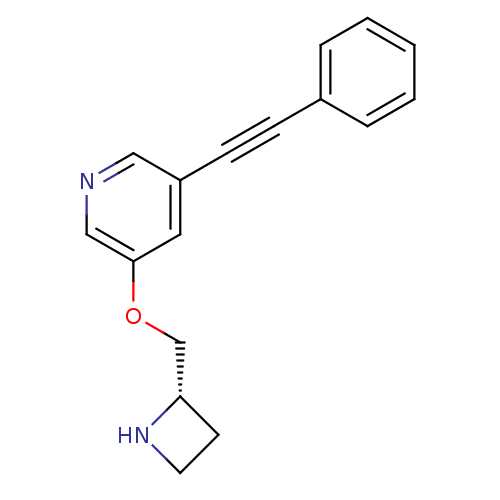
(CHEMBL3086991)Show InChI InChI=1S/C17H16N2O/c1-2-4-14(5-3-1)6-7-15-10-17(12-18-11-15)20-13-16-8-9-19-16/h1-5,10-12,16,19H,8-9,13H2/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha4beta2 nAChR assessed as inhibition of nicotine-induced [86Rb+] efflux preincubated for 10 mins before nicotine exp... |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50210334

(CHEMBL3948977)Show SMILES [H][C@]1(CCOC)C[C@]1([H])c1cncc(c1)N1CCCNCC1 |r| Show InChI InChI=1S/C16H25N3O/c1-20-8-3-13-10-16(13)14-9-15(12-18-11-14)19-6-2-4-17-5-7-19/h9,11-13,16-17H,2-8,10H2,1H3/t13-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha4beta2 expressed in human SH-EP1 cells assessed as reduction in carbamylcholine induced 86Rb+ efflux preincubated f... |
Eur J Med Chem 124: 689-697 (2016)
Article DOI: 10.1016/j.ejmech.2016.09.016
BindingDB Entry DOI: 10.7270/Q2H41TDB |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50302941
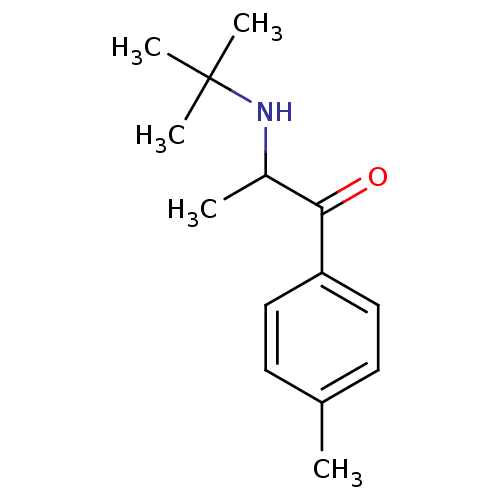
(2-(N-tert-Butylamino)-4'-methylpropiophenone | 2-(...)Show InChI InChI=1S/C14H21NO/c1-10-6-8-12(9-7-10)13(16)11(2)15-14(3,4)5/h6-9,11,15H,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha4beta2 nAChR receptor expressed in human SH-SY5Y cells assessed as inhibition of carbamylcholine-induced 86Rb+ effl... |
J Med Chem 53: 2204-14 (2010)
Article DOI: 10.1021/jm9017465
BindingDB Entry DOI: 10.7270/Q2FN1693 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50428085
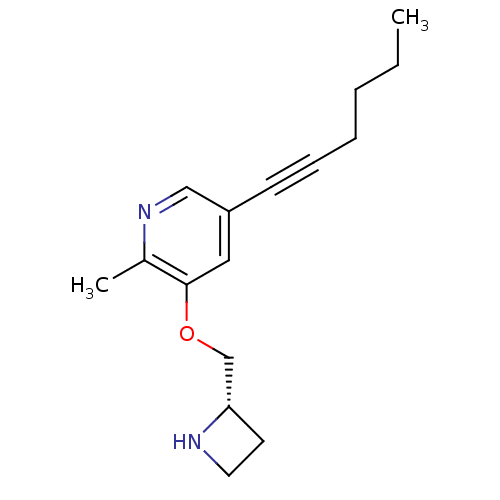
(CHEMBL2323566 | US9303017, (S)-15, YL-1-127)Show InChI InChI=1S/C16H22N2O/c1-3-4-5-6-7-14-10-16(13(2)18-11-14)19-12-15-8-9-17-15/h10-11,15,17H,3-5,8-9,12H2,1-2H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University; Duke University
US Patent
| Assay Description
IC50(10′): The functional properties of the ligands were determined by 86Rb+ efflux assays in cells expressing α3β4 and α4β... |
US Patent US9303017 (2016)
BindingDB Entry DOI: 10.7270/Q25X27SF |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
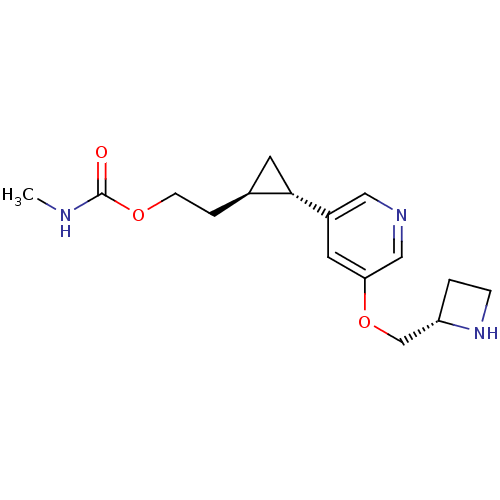
(Homo sapiens (Human)) | BDBM50382467
(CHEMBL2024089)Show SMILES CNC(=O)OCC[C@H]1C[C@@H]1c1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C16H23N3O3/c1-17-16(20)21-5-3-11-7-15(11)12-6-14(9-18-8-12)22-10-13-2-4-19-13/h6,8-9,11,13,15,19H,2-5,7,10H2,1H3,(H,17,20)/t11-,13-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18.2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha4beta2 nAChR expressed in SH-EP1 cells assessed as inhibition of carbamylcholine induced 86Rb+ ion efflux preincuba... |
J Med Chem 55: 717-24 (2012)
Article DOI: 10.1021/jm201157c
BindingDB Entry DOI: 10.7270/Q21N8244 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
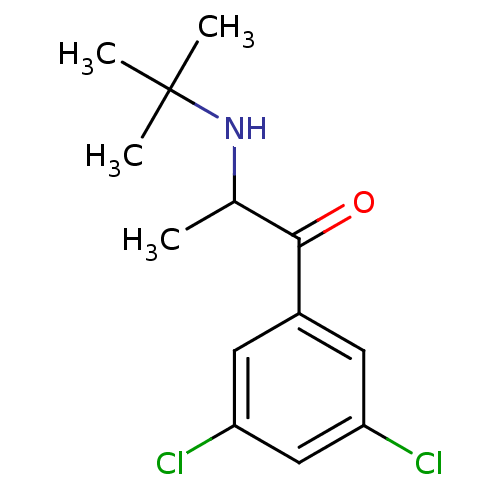
(Homo sapiens (Human)) | BDBM50302938
(2-(tert-Butylamino)-3',5'-dichloropropiophenone | ...)Show InChI InChI=1S/C13H17Cl2NO/c1-8(16-13(2,3)4)12(17)9-5-10(14)7-11(15)6-9/h5-8,16H,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha4beta2 nAChR receptor expressed in human SH-SY5Y cells assessed as inhibition of carbamylcholine-induced 86Rb+ effl... |
J Med Chem 53: 2204-14 (2010)
Article DOI: 10.1021/jm9017465
BindingDB Entry DOI: 10.7270/Q2FN1693 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
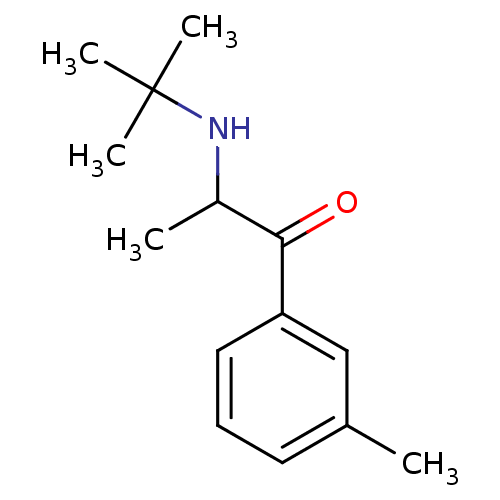
(Homo sapiens (Human)) | BDBM50302942
(2-(N-tert-Butylamino)-3'-methylpropiophenone | 2-(...)Show InChI InChI=1S/C14H21NO/c1-10-7-6-8-12(9-10)13(16)11(2)15-14(3,4)5/h6-9,11,15H,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha4beta2 nAChR receptor expressed in human SH-SY5Y cells assessed as inhibition of carbamylcholine-induced 86Rb+ effl... |
J Med Chem 53: 2204-14 (2010)
Article DOI: 10.1021/jm9017465
BindingDB Entry DOI: 10.7270/Q2FN1693 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
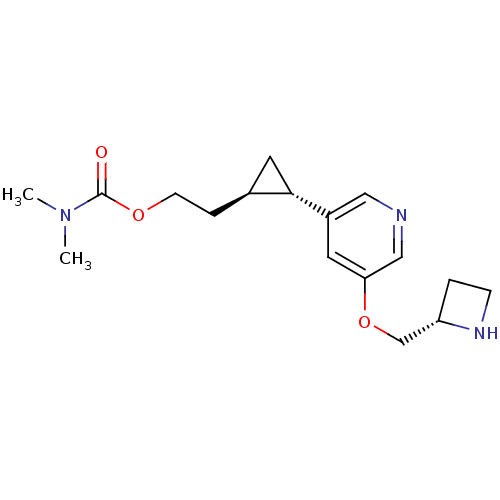
(Homo sapiens (Human)) | BDBM50382468
(CHEMBL2024090)Show SMILES CN(C)C(=O)OCC[C@H]1C[C@@H]1c1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C17H25N3O3/c1-20(2)17(21)22-6-4-12-8-16(12)13-7-15(10-18-9-13)23-11-14-3-5-19-14/h7,9-10,12,14,16,19H,3-6,8,11H2,1-2H3/t12-,14-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19.1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha4beta2 nAChR expressed in SH-EP1 cells assessed as inhibition of carbamylcholine induced 86Rb+ ion efflux preincuba... |
J Med Chem 55: 717-24 (2012)
Article DOI: 10.1021/jm201157c
BindingDB Entry DOI: 10.7270/Q21N8244 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data