

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycogen phosphorylase, liver form (Rattus norvegicus) | BDBM50135550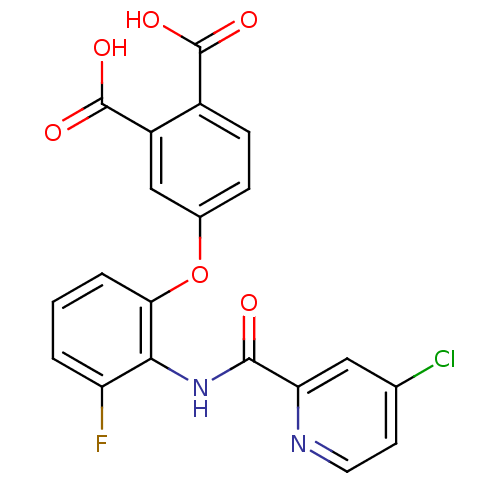 (4-{2-[(4-Chloro-pyridine-2-carbonyl)-amino]-3-fluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against rat liver glycogen phosphorylase | Bioorg Med Chem Lett 13: 4125-8 (2003) BindingDB Entry DOI: 10.7270/Q26M367F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Rattus norvegicus) | BDBM50135552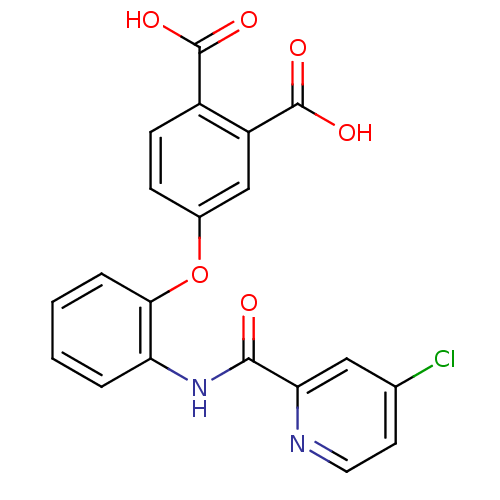 (4-{2-[(4-Chloro-pyridine-2-carbonyl)-amino]-phenox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against rat liver glycogen phosphorylase | Bioorg Med Chem Lett 13: 4125-8 (2003) BindingDB Entry DOI: 10.7270/Q26M367F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Rattus norvegicus) | BDBM50135563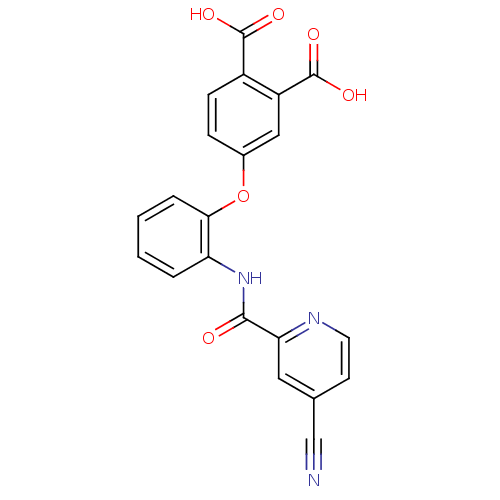 (4-{2-[(4-Cyano-pyridine-2-carbonyl)-amino]-phenoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against rat liver glycogen phosphorylase | Bioorg Med Chem Lett 13: 4125-8 (2003) BindingDB Entry DOI: 10.7270/Q26M367F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Rattus norvegicus) | BDBM50135556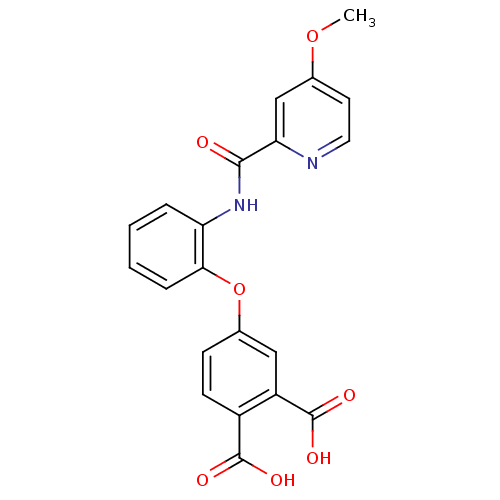 (4-{2-[(4-Methoxy-pyridine-2-carbonyl)-amino]-pheno...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against rat liver glycogen phosphorylase | Bioorg Med Chem Lett 13: 4125-8 (2003) BindingDB Entry DOI: 10.7270/Q26M367F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Rattus norvegicus) | BDBM50135549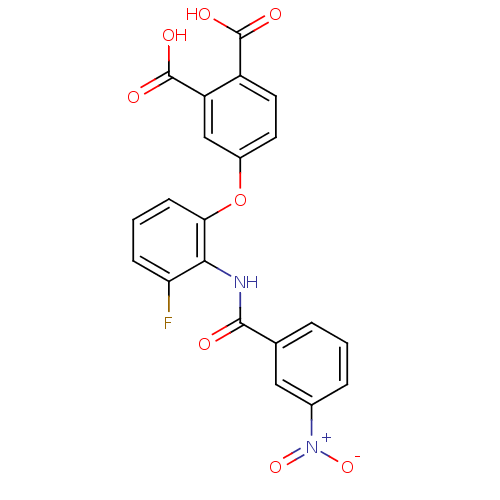 (4-[3-Fluoro-2-(3-nitro-benzoylamino)-phenoxy]-phth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 302 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against rat liver glycogen phosphorylase | Bioorg Med Chem Lett 13: 4125-8 (2003) BindingDB Entry DOI: 10.7270/Q26M367F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Rattus norvegicus) | BDBM50194701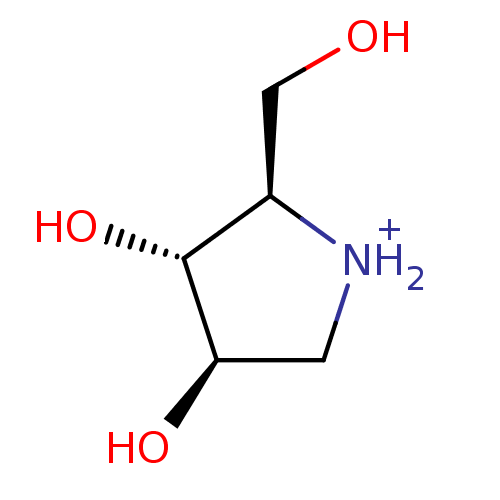 ((2R,3R,4R)-2-Hydroxymethylpyrrolidin-3,4-diol hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation Curated by ChEMBL | Assay Description Inhibition of phosphorylated form of rat liver glycogen phosphorylase | J Med Chem 49: 5687-701 (2006) Article DOI: 10.1021/jm060496g BindingDB Entry DOI: 10.7270/Q25Q4VQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Rattus norvegicus) | BDBM50182801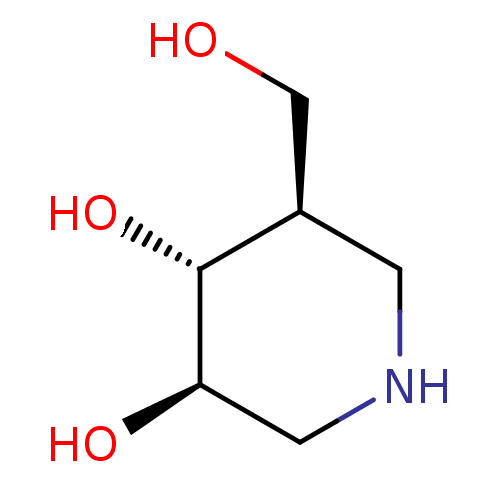 ((3R,4R,5R)-5-(Hydroxymethyl)piperidine-3,4-diol, 8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation Curated by ChEMBL | Assay Description Inhibition of phosphorylated form of rat liver glycogen phosphorylase | J Med Chem 49: 5687-701 (2006) Article DOI: 10.1021/jm060496g BindingDB Entry DOI: 10.7270/Q25Q4VQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Rattus norvegicus) | BDBM50016703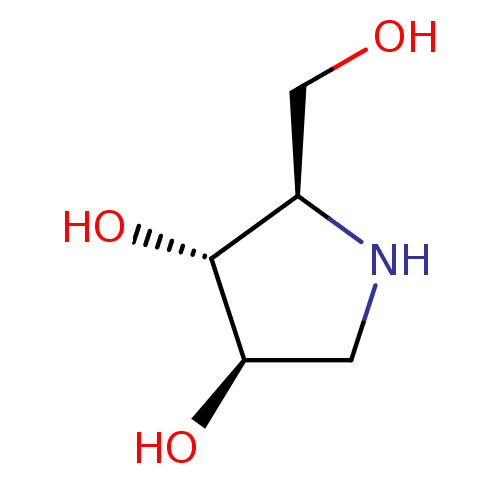 (2-Hydroxymethyl-pyrrolidine-3,4-diol | BDBM5003148...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of glycogen phosphorylase in rat hepatocytes assessed as reduction in basal glycogenolysis | Drug Metab Dispos 41: 878-87 (2013) Article DOI: 10.1124/dmd.112.050591 BindingDB Entry DOI: 10.7270/Q2TB18M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Rattus norvegicus) | BDBM50016703 (2-Hydroxymethyl-pyrrolidine-3,4-diol | BDBM5003148...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of glycogen phosphorylase in rat hepatocytes assessed as reduction in glucagom-induced glycogenolysis | Drug Metab Dispos 41: 878-87 (2013) Article DOI: 10.1124/dmd.112.050591 BindingDB Entry DOI: 10.7270/Q2TB18M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Rattus norvegicus) | BDBM50194702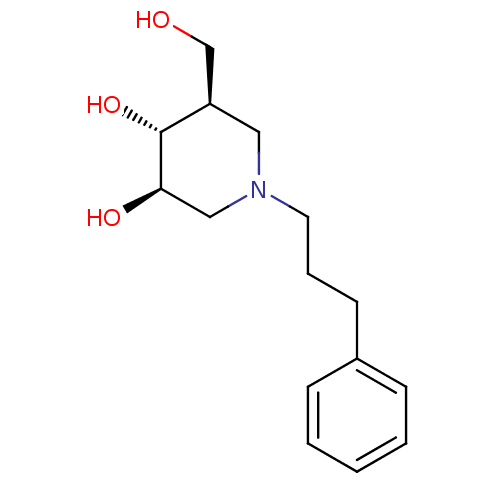 ((3R,4R,5R)-5-(HYDROXYMETHYL)-1-(3-PHENYLPROPYL)PIP...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation Curated by ChEMBL | Assay Description Inhibition of phosphorylated form of rat liver glycogen phosphorylase | J Med Chem 49: 5687-701 (2006) Article DOI: 10.1021/jm060496g BindingDB Entry DOI: 10.7270/Q25Q4VQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Rattus norvegicus) | BDBM50146091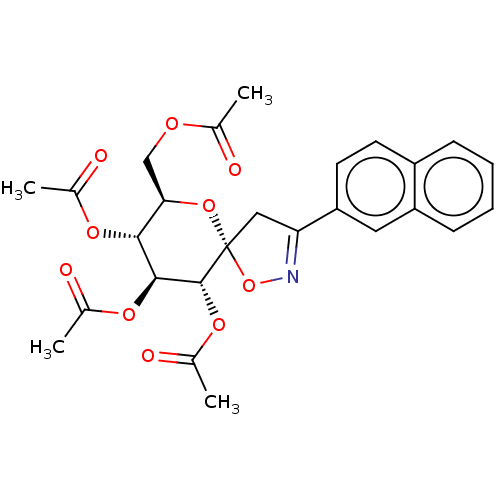 (CHEMBL3765349) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of glycogen phosphorylase in Wistar rat hepatocytes assessed as decrease in glucagon stimulated glucose release after 3 hrs by glucose oxi... | Eur J Med Chem 108: 444-54 (2016) Article DOI: 10.1016/j.ejmech.2015.12.004 BindingDB Entry DOI: 10.7270/Q2QR500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Rattus norvegicus) | BDBM50146082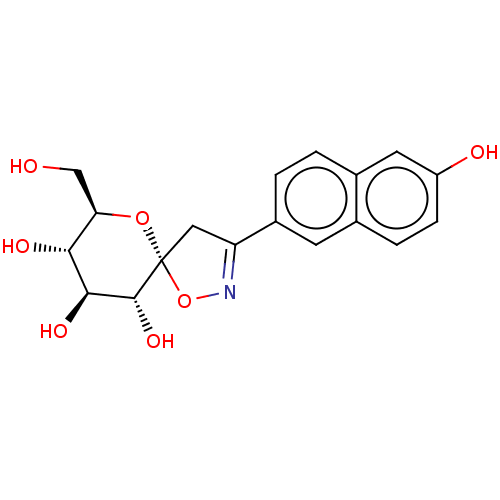 (CHEMBL3763876) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of glycogen phosphorylase in Wistar rat hepatocytes assessed as decrease in glucagon stimulated glucose release after 3 hrs by glucose oxi... | Eur J Med Chem 108: 444-54 (2016) Article DOI: 10.1016/j.ejmech.2015.12.004 BindingDB Entry DOI: 10.7270/Q2QR500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Rattus norvegicus) | BDBM34110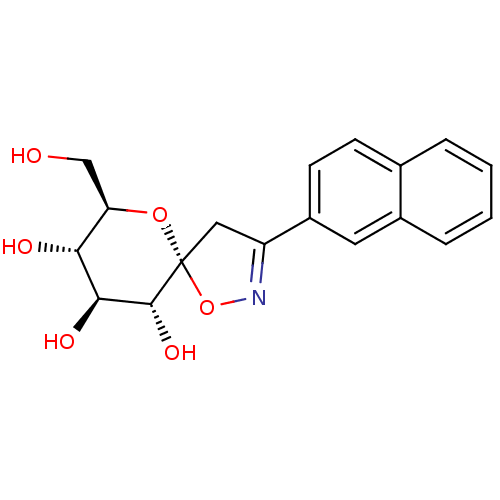 (glucopyranosylidene-spiro-isoxazoline, 4e) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of glycogen phosphorylase in Wistar rat hepatocytes assessed as decrease in glucagon stimulated glucose release after 3 hrs by glucose oxi... | Eur J Med Chem 108: 444-54 (2016) Article DOI: 10.1016/j.ejmech.2015.12.004 BindingDB Entry DOI: 10.7270/Q2QR500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Rattus norvegicus) | BDBM50294476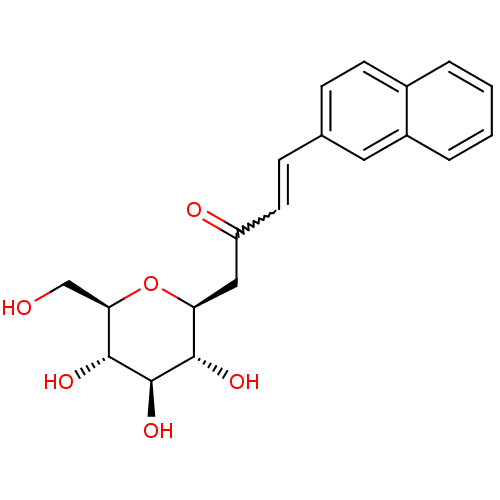 ((E)-4-(naphthalen-2-yl)-1-((2S,3R,4R,5S,6R)-3,4,5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of rat liver glycogen phosphatase | Bioorg Med Chem Lett 19: 2699-703 (2009) Article DOI: 10.1016/j.bmcl.2009.03.136 BindingDB Entry DOI: 10.7270/Q20V8CTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Rattus norvegicus) | BDBM50175892 (2alpha,3beta-dihydroxy-12-oleanen-28-oic acid | 2a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory concentration against rat liver glycogen phosphorylase | Bioorg Med Chem Lett 15: 4944-8 (2005) Article DOI: 10.1016/j.bmcl.2005.08.026 BindingDB Entry DOI: 10.7270/Q27M08R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Rattus norvegicus) | BDBM50146088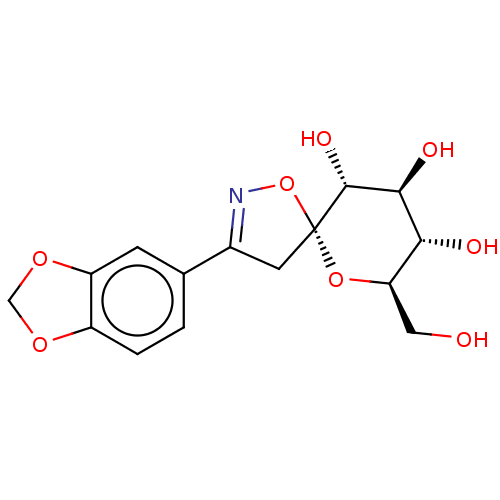 (CHEMBL3763929) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of glycogen phosphorylase in Wistar rat hepatocytes assessed as decrease in glucagon stimulated glucose release after 3 hrs by glucose oxi... | Eur J Med Chem 108: 444-54 (2016) Article DOI: 10.1016/j.ejmech.2015.12.004 BindingDB Entry DOI: 10.7270/Q2QR500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Rattus norvegicus) | BDBM50146090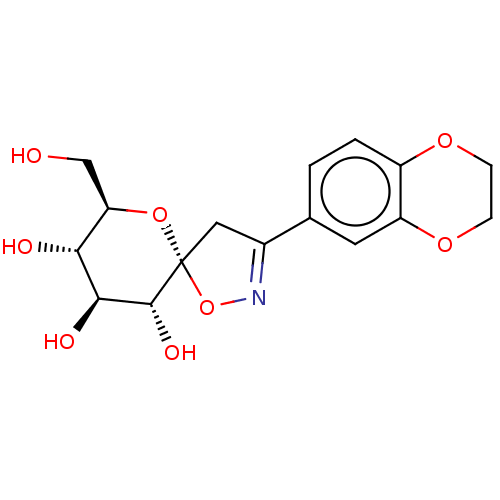 (CHEMBL3763976) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of glycogen phosphorylase in Wistar rat hepatocytes assessed as decrease in glucagon stimulated glucose release after 3 hrs by glucose oxi... | Eur J Med Chem 108: 444-54 (2016) Article DOI: 10.1016/j.ejmech.2015.12.004 BindingDB Entry DOI: 10.7270/Q2QR500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Rattus norvegicus) | BDBM50146085 (CHEMBL3764911) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of glycogen phosphorylase in Wistar rat hepatocytes assessed as decrease in glucagon stimulated glucose release after 3 hrs by glucose oxi... | Eur J Med Chem 108: 444-54 (2016) Article DOI: 10.1016/j.ejmech.2015.12.004 BindingDB Entry DOI: 10.7270/Q2QR500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Rattus norvegicus) | BDBM50146081 (CHEMBL3764195) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of glycogen phosphorylase in Wistar rat hepatocytes assessed as decrease in glucagon stimulated glucose release after 3 hrs by glucose oxi... | Eur J Med Chem 108: 444-54 (2016) Article DOI: 10.1016/j.ejmech.2015.12.004 BindingDB Entry DOI: 10.7270/Q2QR500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Rattus norvegicus) | BDBM50146086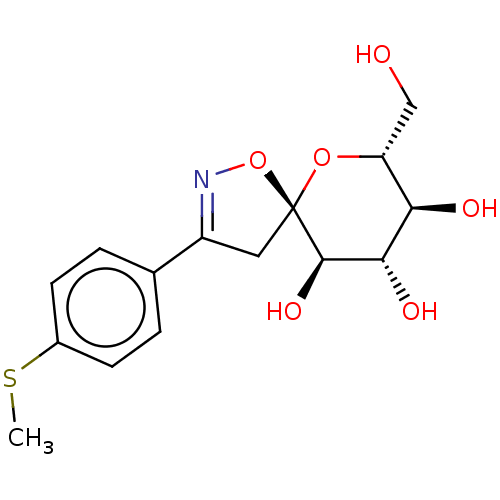 (CHEMBL3764775) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of glycogen phosphorylase in Wistar rat hepatocytes assessed as decrease in glucagon stimulated glucose release after 3 hrs by glucose oxi... | Eur J Med Chem 108: 444-54 (2016) Article DOI: 10.1016/j.ejmech.2015.12.004 BindingDB Entry DOI: 10.7270/Q2QR500M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Rattus norvegicus) | BDBM50222205 ((1S,2R,4aS,6aS,6bR,8aR,10R,11R,12aR,12bR,14bS)-10,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory concentration against rat liver glycogen phosphorylase | Bioorg Med Chem Lett 15: 4944-8 (2005) Article DOI: 10.1016/j.bmcl.2005.08.026 BindingDB Entry DOI: 10.7270/Q27M08R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Rattus norvegicus) | BDBM10849 (1,3,7-trimethyl-2,3,6,7-tetrahydro-1H-purine-2,6-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6.48E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory concentration against rat liver glycogen phosphorylase | Bioorg Med Chem Lett 15: 4944-8 (2005) Article DOI: 10.1016/j.bmcl.2005.08.026 BindingDB Entry DOI: 10.7270/Q27M08R5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||