

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50255753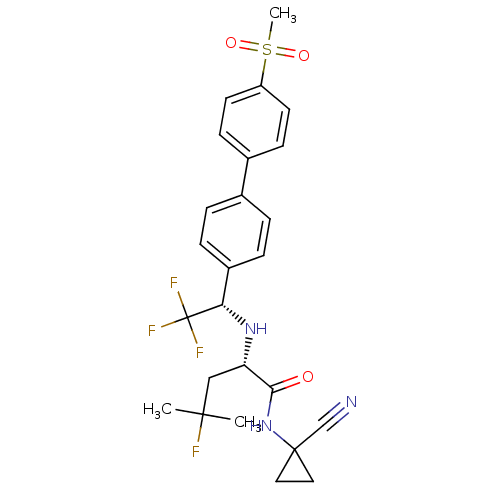 (CHEMBL481611 | MK-0822 | Odanacatib) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of cathepsin H | Bioorg Med Chem Lett 18: 923-8 (2008) Article DOI: 10.1016/j.bmcl.2007.12.047 BindingDB Entry DOI: 10.7270/Q21J9BM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50148298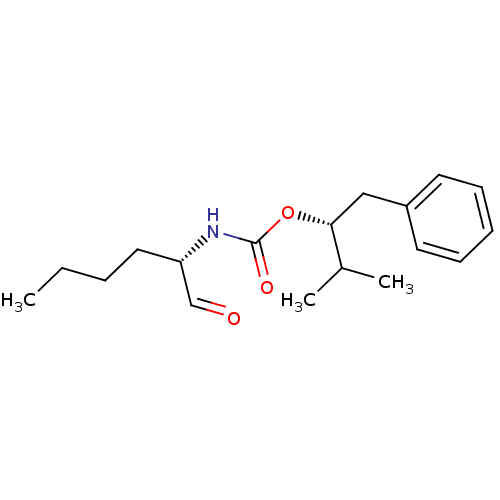 (((S)-1-Formyl-pentyl)-carbamic acid (R)-1-benzyl-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant human cathepsin H in a fluorescence assay | Bioorg Med Chem Lett 16: 978-83 (2006) Article DOI: 10.1016/j.bmcl.2005.10.108 BindingDB Entry DOI: 10.7270/Q2X929VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50163831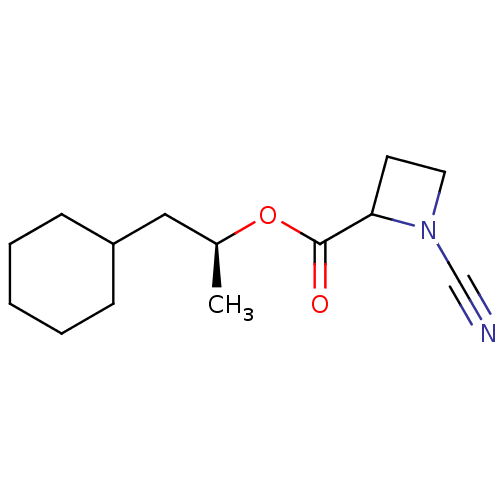 ((2S)-1-cyclohexylpropan-2-yl 1-cyanoazetidine-2-ca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against human cathepsin H using 50 uM L-Arg-beta-naphthalamide | Bioorg Med Chem Lett 15: 1815-9 (2005) Article DOI: 10.1016/j.bmcl.2005.02.033 BindingDB Entry DOI: 10.7270/Q2DR2V1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50148296 (((S)-1-Formyl-pentyl)-carbamic acid (S)-1-methyl-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant human cathepsin H in a fluorescence assay | Bioorg Med Chem Lett 16: 978-83 (2006) Article DOI: 10.1016/j.bmcl.2005.10.108 BindingDB Entry DOI: 10.7270/Q2X929VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM397464 (US10676470, Compound 43 | US10730826, Compound 73 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM397464 (US10676470, Compound 43 | US10730826, Compound 73 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM397464 (US10676470, Compound 43 | US10730826, Compound 73 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM397464 (US10676470, Compound 43 | US10730826, Compound 73 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50250152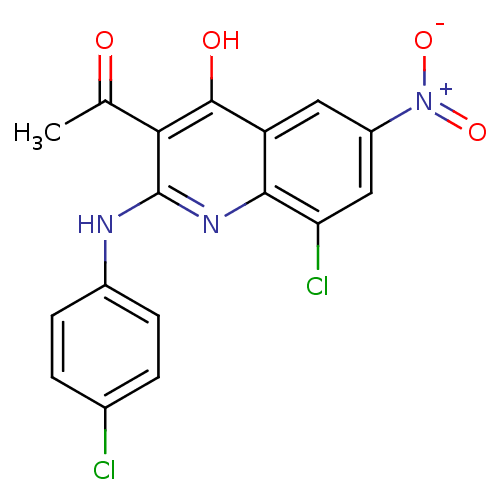 (3-acetyl-8-chloro-2-(4-chlorophenylamino)-6-nitroq...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin H after 30 mins by fluorometric end-point assay | J Med Chem 52: 3093-7 (2009) Article DOI: 10.1021/jm8014734 BindingDB Entry DOI: 10.7270/Q2KK9CQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50177501 (CHEMBL203663 | {(S)-1-[(morpholine-4-carbonyl)-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant human cathepsin H in a fluorescence assay | Bioorg Med Chem Lett 16: 978-83 (2006) Article DOI: 10.1016/j.bmcl.2005.10.108 BindingDB Entry DOI: 10.7270/Q2X929VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50157741 (CHEMBL374508 | E-64 | E64) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin H after 10 mins | J Biol Chem 282: 20836-46 (2007) Article DOI: 10.1074/jbc.M702615200 BindingDB Entry DOI: 10.7270/Q2125TJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50177494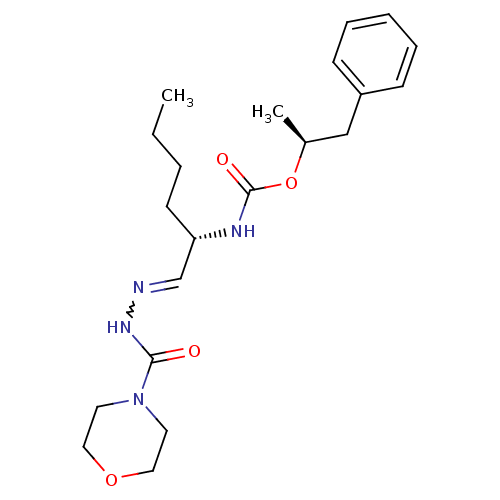 (CHEMBL204605 | {(S)-1-[(morpholine-4-carbonyl)-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant human cathepsin H in a fluorescence assay | Bioorg Med Chem Lett 16: 978-83 (2006) Article DOI: 10.1016/j.bmcl.2005.10.108 BindingDB Entry DOI: 10.7270/Q2X929VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50152524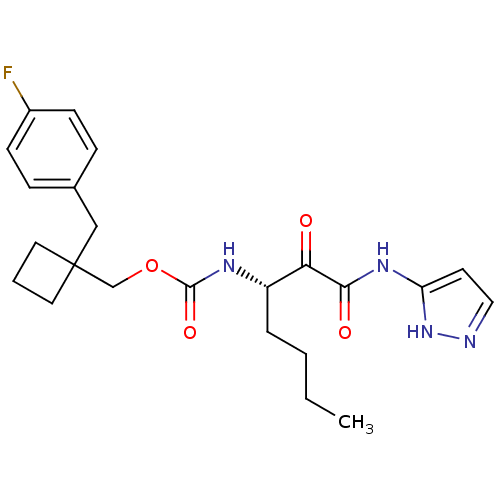 ((S)-(1-(4-fluorobenzyl)cyclobutyl)methyl 1-(1H-pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of 50 uM L-Arg-beta-naphthalamide binding to human cathepsin H in fluorescence assay | Bioorg Med Chem Lett 14: 4897-902 (2004) Article DOI: 10.1016/j.bmcl.2004.07.031 BindingDB Entry DOI: 10.7270/Q26W99JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50165424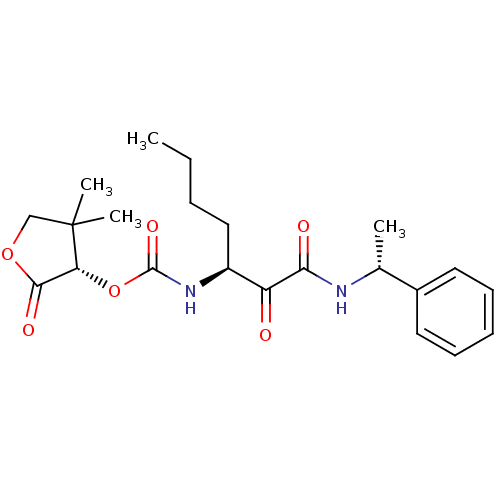 ((S)-4,4-dimethyl-2-oxo-tetrahydrofuran-3-yl (S)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against human cathepsin H by fluorescence assay using 50 uM L-Arg-b-naphthalamide | Bioorg Med Chem Lett 15: 2209-13 (2005) Article DOI: 10.1016/j.bmcl.2005.03.023 BindingDB Entry DOI: 10.7270/Q20C4V8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM453275 (BDBM453327 | US10730826, Compound 1a-non-racemic |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM553804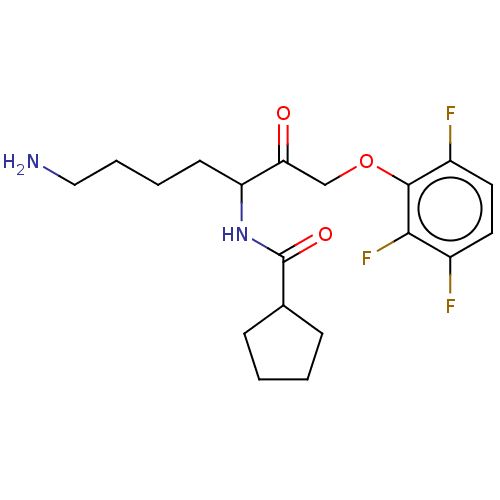 (US11325884, Compound 1a-non-racemic | US11325884, ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM453275 (BDBM453327 | US10730826, Compound 1a-non-racemic |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM453275 (BDBM453327 | US10730826, Compound 1a-non-racemic |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50163832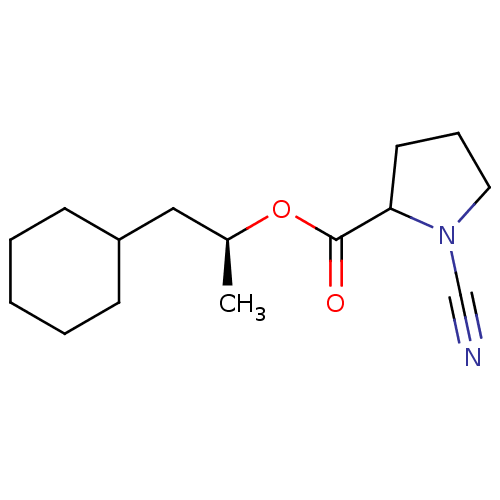 ((1S)-2-cyclohexyl-1-methylethyl (2S)-1-cyanopyrrol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against human cathepsin H using 50 uM L-Arg-beta-naphthalamide | Bioorg Med Chem Lett 15: 1815-9 (2005) Article DOI: 10.1016/j.bmcl.2005.02.033 BindingDB Entry DOI: 10.7270/Q2DR2V1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM453289 (US10730826, Compound 2a | US11325884, Compound 2a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM453289 (US10730826, Compound 2a | US11325884, Compound 2a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM453289 (US10730826, Compound 2a | US11325884, Compound 2a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM453289 (US10730826, Compound 2a | US11325884, Compound 2a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50152526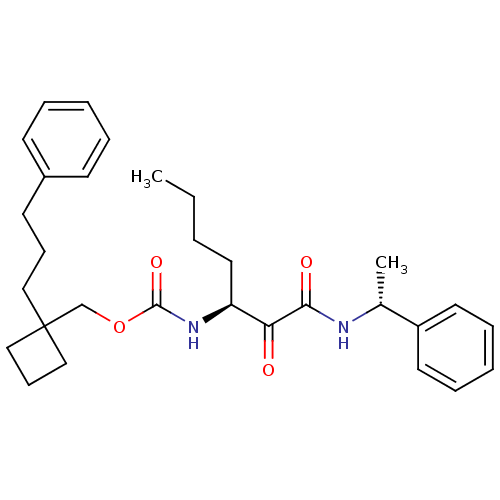 ((1-(3-phenylpropyl)cyclobutyl)methyl(S)-1,2-dioxo-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of 50 uM L-Arg-beta-naphthalamide binding to human cathepsin H in fluorescence assay | Bioorg Med Chem Lett 14: 4897-902 (2004) Article DOI: 10.1016/j.bmcl.2004.07.031 BindingDB Entry DOI: 10.7270/Q26W99JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50165425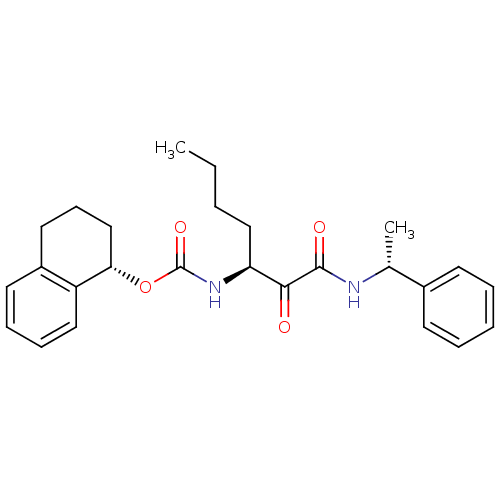 (CHEMBL195963 | [(S)-1-((R)-1-Phenyl-ethylaminooxal...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against human cathepsin H by fluorescence assay using 50 uM L-Arg-b-naphthalamide | Bioorg Med Chem Lett 15: 2209-13 (2005) Article DOI: 10.1016/j.bmcl.2005.03.023 BindingDB Entry DOI: 10.7270/Q20C4V8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50152521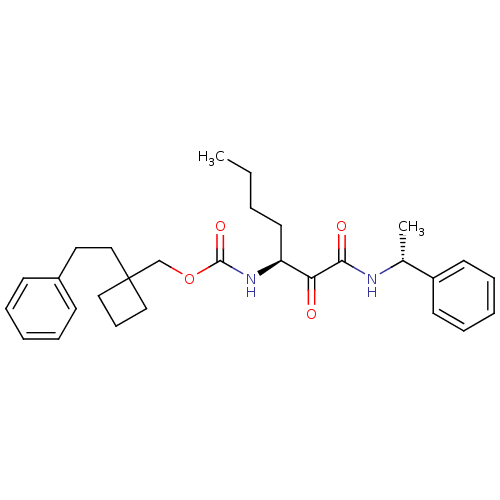 ((1-phenethylcyclobutyl)methyl(S)-1,2-dioxo-1-((R)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of 50 uM L-Arg-beta-naphthalamide binding to human cathepsin H in fluorescence assay | Bioorg Med Chem Lett 14: 4897-902 (2004) Article DOI: 10.1016/j.bmcl.2004.07.031 BindingDB Entry DOI: 10.7270/Q26W99JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50201701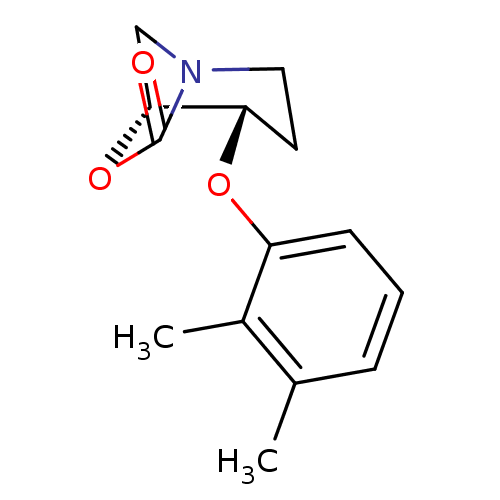 (CHEMBL390474 | cis-4-(2,3-dimethylphenoxy)-6-oxa-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin H | Bioorg Med Chem Lett 17: 1254-9 (2007) Article DOI: 10.1016/j.bmcl.2006.12.014 BindingDB Entry DOI: 10.7270/Q2QN66DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50152522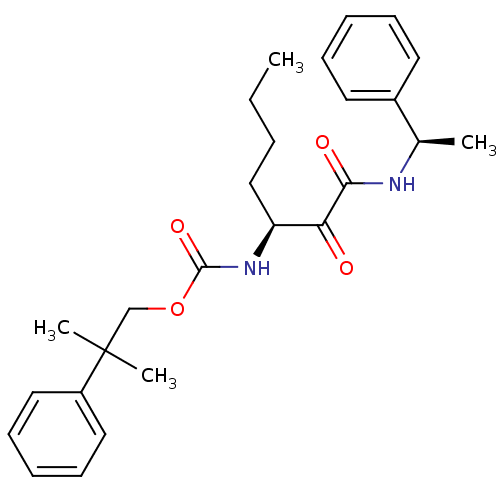 (2-methyl-2-phenylpropyl(S)-1,2-dioxo-1-((R)-1-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of 50 uM L-Arg-beta-naphthalamide binding to human cathepsin H in fluorescence assay | Bioorg Med Chem Lett 14: 4897-902 (2004) Article DOI: 10.1016/j.bmcl.2004.07.031 BindingDB Entry DOI: 10.7270/Q26W99JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50165420 (CHEMBL197509 | [(S)-1-((R)-1-Phenyl-ethylaminooxal...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against human cathepsin H by fluorescence assay using 50 uM L-Arg-b-naphthalamide | Bioorg Med Chem Lett 15: 2209-13 (2005) Article DOI: 10.1016/j.bmcl.2005.03.023 BindingDB Entry DOI: 10.7270/Q20C4V8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50152527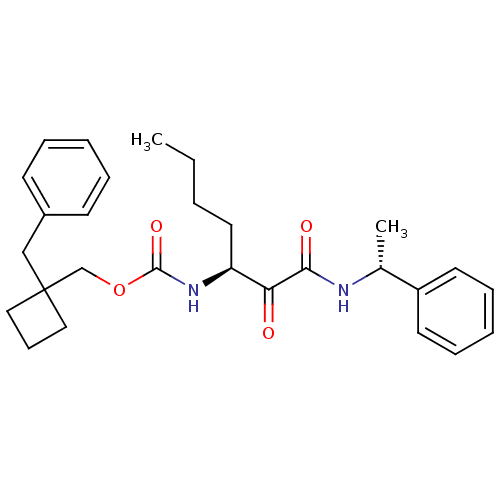 ((1-benzylcyclobutyl)methyl(S)-1,2-dioxo-1-((R)-1-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of 50 uM L-Arg-beta-naphthalamide binding to human cathepsin H in fluorescence assay | Bioorg Med Chem Lett 14: 4897-902 (2004) Article DOI: 10.1016/j.bmcl.2004.07.031 BindingDB Entry DOI: 10.7270/Q26W99JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50152523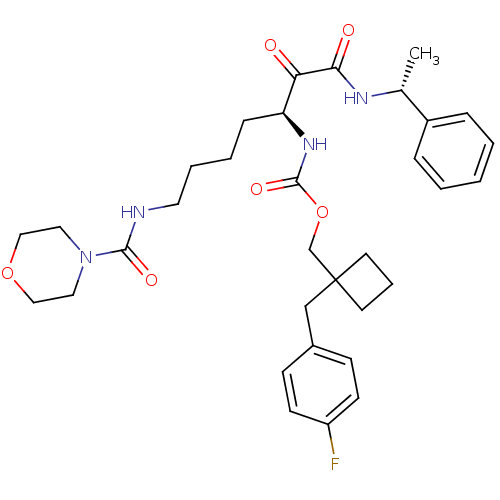 (CHEMBL180528 | [(S)-5-[(Morpholine-4-carbonyl)-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of 50 uM L-Arg-beta-naphthalamide binding to human cathepsin H in fluorescence assay | Bioorg Med Chem Lett 14: 4897-902 (2004) Article DOI: 10.1016/j.bmcl.2004.07.031 BindingDB Entry DOI: 10.7270/Q26W99JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50152530 (CHEMBL186650 | P2,P3 Ketoamide derivative) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of 50 uM L-Arg-beta-naphthalamide binding to human cathepsin H in fluorescence assay | Bioorg Med Chem Lett 14: 4897-902 (2004) Article DOI: 10.1016/j.bmcl.2004.07.031 BindingDB Entry DOI: 10.7270/Q26W99JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50152525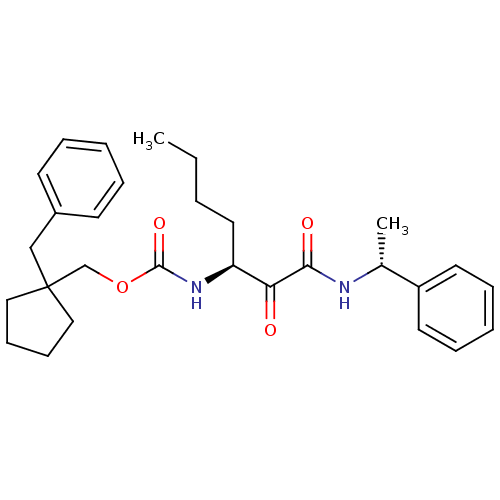 (CHEMBL184403 | [(S)-1-((R)-1-Phenyl-ethylaminooxal...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of 50 uM L-Arg-beta-naphthalamide binding to human cathepsin H in fluorescence assay | Bioorg Med Chem Lett 14: 4897-902 (2004) Article DOI: 10.1016/j.bmcl.2004.07.031 BindingDB Entry DOI: 10.7270/Q26W99JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50152532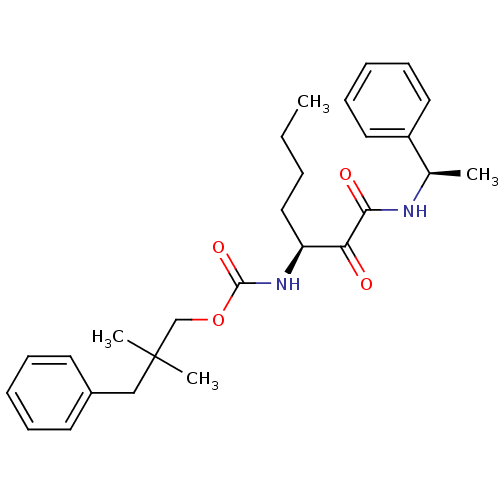 (2,2-dimethyl-3-phenylpropyl(S)-1,2-dioxo-1-((R)-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of 50 uM L-Arg-beta-naphthalamide binding to human cathepsin H in fluorescence assay | Bioorg Med Chem Lett 14: 4897-902 (2004) Article DOI: 10.1016/j.bmcl.2004.07.031 BindingDB Entry DOI: 10.7270/Q26W99JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50201700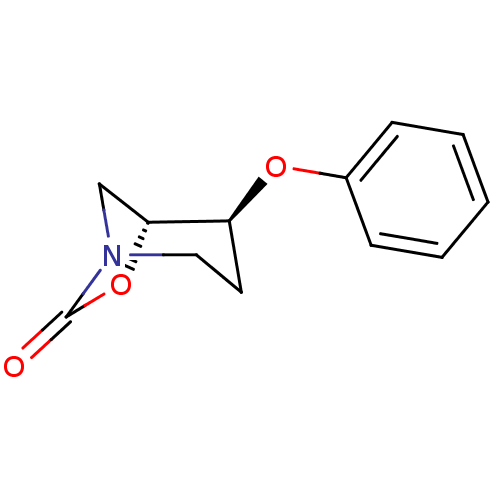 ((1R,2S)-2-phenoxy-7-oxa-5-aza-bicyclo[3.2.1]octan-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin H | Bioorg Med Chem Lett 17: 1254-9 (2007) Article DOI: 10.1016/j.bmcl.2006.12.014 BindingDB Entry DOI: 10.7270/Q2QN66DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50163838 ((1-Cyano-pyrrolidin-3-yl)-carbamic acid (S)-2-cycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against human cathepsin H using 50 uM L-Arg-beta-naphthalamide | Bioorg Med Chem Lett 15: 1815-9 (2005) Article DOI: 10.1016/j.bmcl.2005.02.033 BindingDB Entry DOI: 10.7270/Q2DR2V1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50270029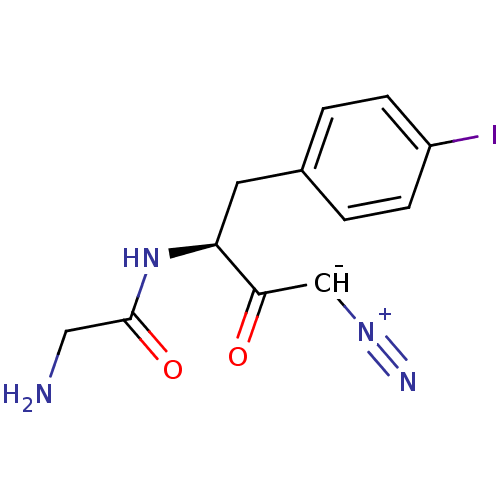 (2-Amino-N-[(S)-3-diazo-1-(4-iodo-benzyl)-2-oxo-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin H after 10 mins | J Biol Chem 282: 20836-46 (2007) Article DOI: 10.1074/jbc.M702615200 BindingDB Entry DOI: 10.7270/Q2125TJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50186088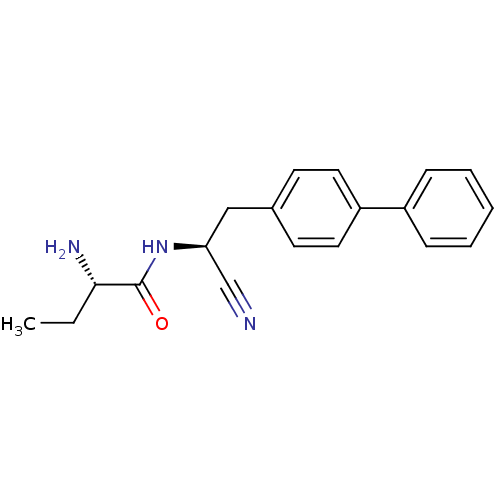 ((S)-2-amino-N-((S)-2-(biphenyl-4-yl)-1-cyanoethyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arpida A/S Curated by ChEMBL | Assay Description Inhibition of Cathepsin H | Bioorg Med Chem Lett 16: 3614-7 (2006) Article DOI: 10.1016/j.bmcl.2006.01.102 BindingDB Entry DOI: 10.7270/Q2639PC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50201699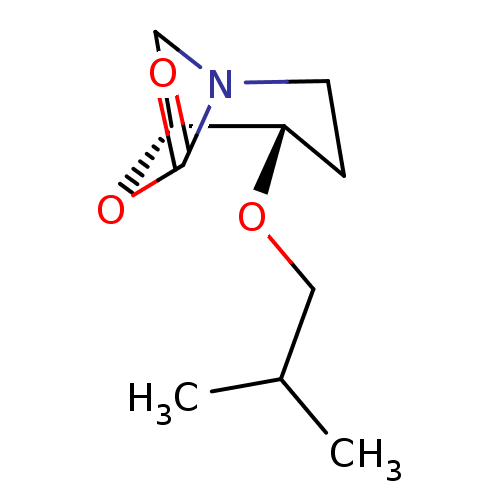 ((1R,2S)-2-isobutoxy-7-oxa-5-aza-bicyclo[3.2.1]octa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin H | Bioorg Med Chem Lett 17: 1254-9 (2007) Article DOI: 10.1016/j.bmcl.2006.12.014 BindingDB Entry DOI: 10.7270/Q2QN66DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50201705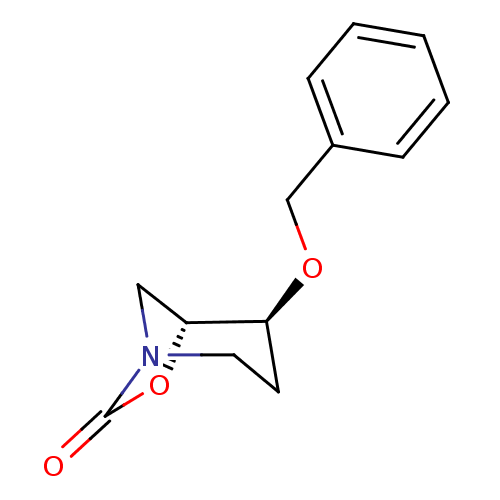 ((1R,2S)-2-(benzyloxy)-7-oxa-5-aza-bicyclo[3.2.1]oc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin H | Bioorg Med Chem Lett 17: 1254-9 (2007) Article DOI: 10.1016/j.bmcl.2006.12.014 BindingDB Entry DOI: 10.7270/Q2QN66DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50302107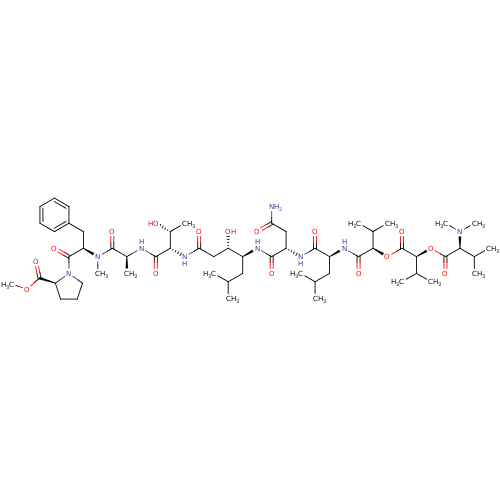 (CHEMBL567893 | Grassystatin A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of cathepsin H after 10 to 15 mins by fluorescence assay | J Med Chem 52: 5732-47 (2009) Article DOI: 10.1021/jm9009394 BindingDB Entry DOI: 10.7270/Q2BG2PXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM453318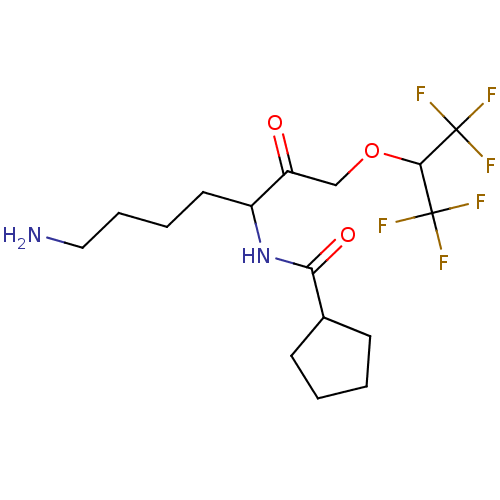 (BDBM553824 | US10730826, Compound 18a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM453329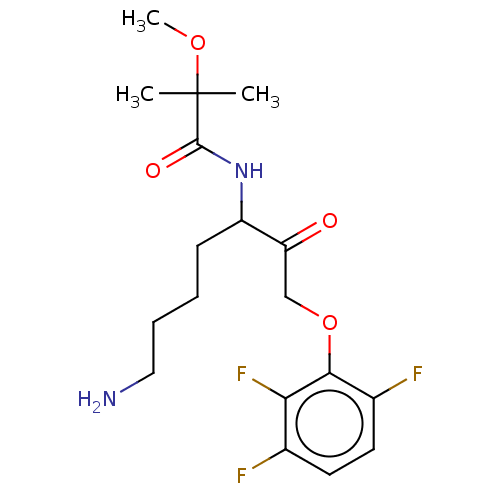 (BDBM553832 | US10730826, Compound 47a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM453330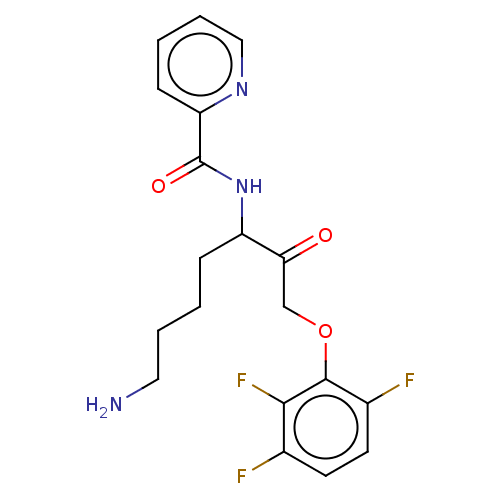 (BDBM553833 | US10730826, Compound 50a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM453331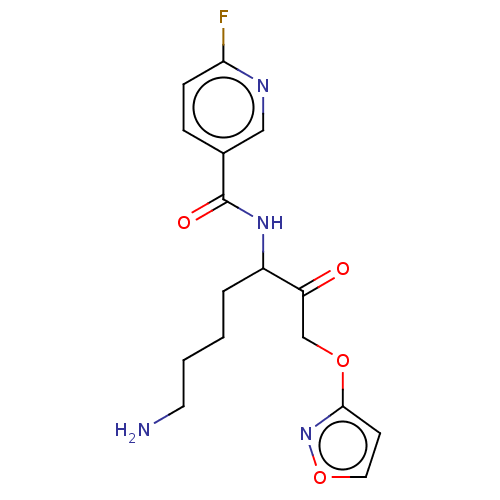 (BDBM553834 | US10730826, Compound 55a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM453332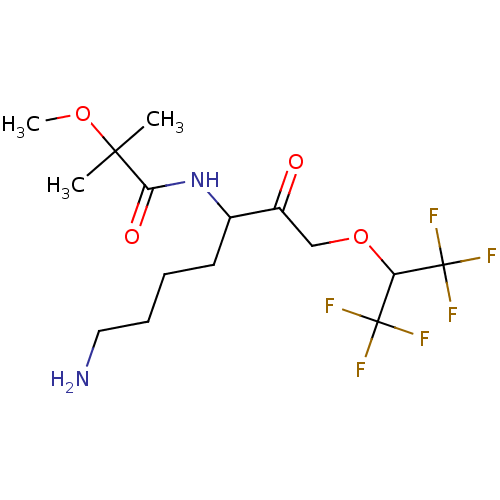 (BDBM553835 | US10730826, Compound 69a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM453318 (BDBM553824 | US10730826, Compound 18a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM453329 (BDBM553832 | US10730826, Compound 47a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM453330 (BDBM553833 | US10730826, Compound 50a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM453331 (BDBM553834 | US10730826, Compound 55a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 88 total ) | Next | Last >> |