

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122654 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Tested for inhibition of arachidonic acid(AA) release from CHO cells in Human | J Med Chem 46: 113-24 (2002) Article DOI: 10.1021/jm020180i BindingDB Entry DOI: 10.7270/Q26W9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122654 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human GnRH receptor expressed in CHO cells assessed as inhibition of GnRH-induced arachadonic acid release using [5,6,8,9,11,1... | J Med Chem 54: 4998-5012 (2011) Article DOI: 10.1021/jm200216q BindingDB Entry DOI: 10.7270/Q22N52MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189733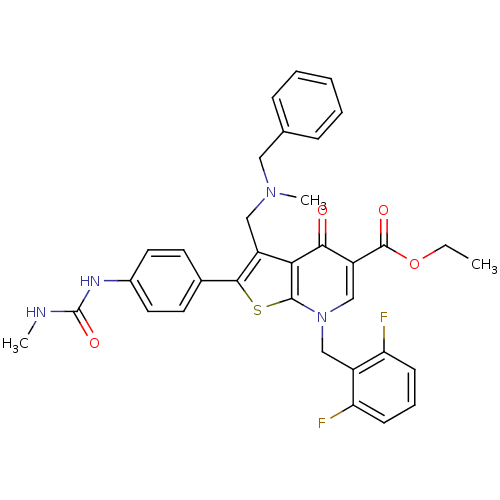 (CHEMBL379629 | ethyl 3-(N-benzyl-N-methylaminometh...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122652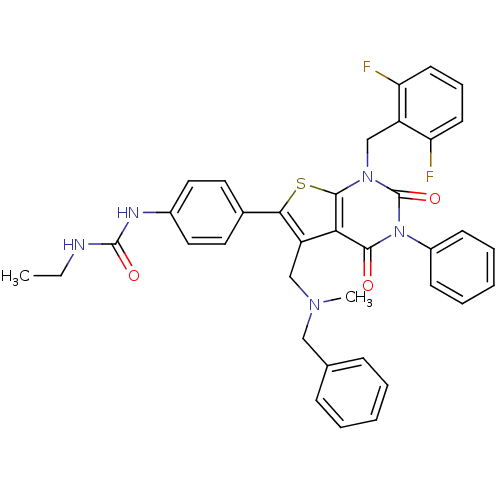 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Tested for inhibition of arachidonic acid(AA) release from CHO cells in Human | J Med Chem 46: 113-24 (2002) Article DOI: 10.1021/jm020180i BindingDB Entry DOI: 10.7270/Q26W9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189704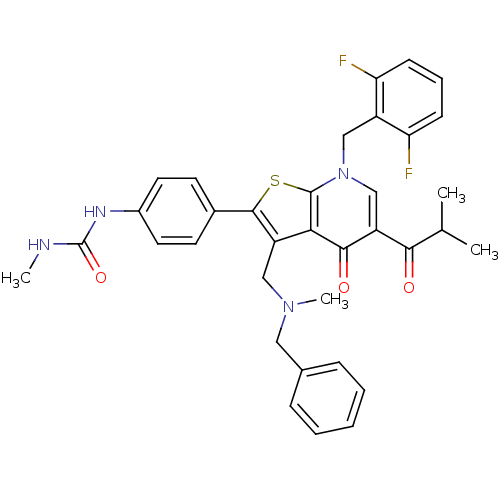 (3-(N-benzyl-N-methylaminomethyl)-7-(2,6-difluorobe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of LHRH-stimulated arachidonic acid release in CHO cells expressing human LHRH receptor | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189716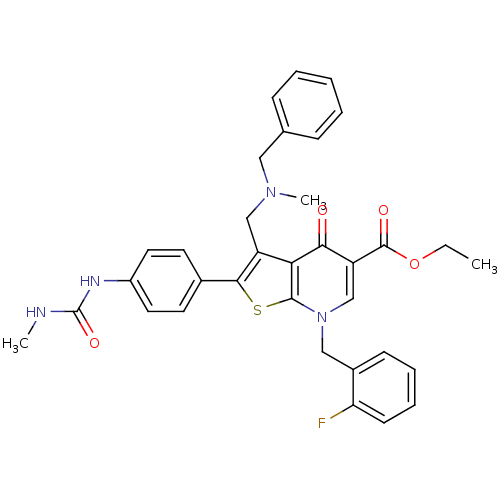 (CHEMBL210294 | ethyl 3-(N-benzyl-N-methylaminometh...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50347996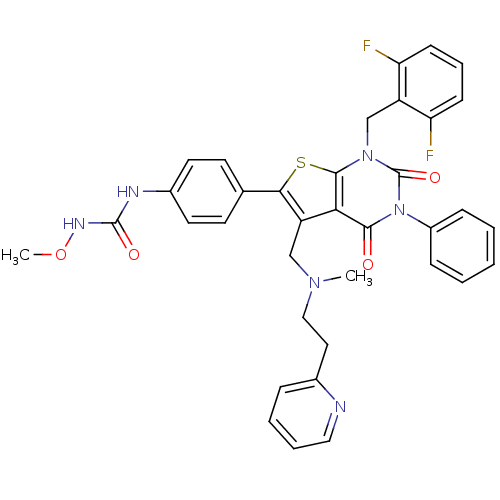 (CHEMBL1800663) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]leuprorelin from recombinant human GnRH receptor expressed in CHO cells after 60 mins by X-ray counter | J Med Chem 54: 4998-5012 (2011) Article DOI: 10.1021/jm200216q BindingDB Entry DOI: 10.7270/Q22N52MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50347996 (CHEMBL1800663) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human GnRH receptor expressed in CHO cells assessed as inhibition of GnRH-induced arachadonic acid release using [5,6,8,9,11,1... | J Med Chem 54: 4998-5012 (2011) Article DOI: 10.1021/jm200216q BindingDB Entry DOI: 10.7270/Q22N52MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50347986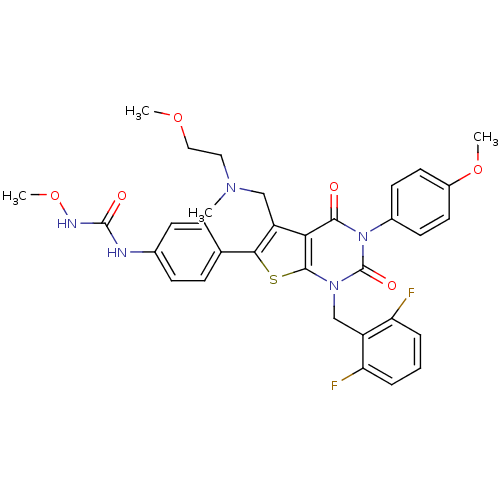 (CHEMBL1800155) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]leuprorelin from recombinant human GnRH receptor expressed in CHO cells after 60 mins by X-ray counter | J Med Chem 54: 4998-5012 (2011) Article DOI: 10.1021/jm200216q BindingDB Entry DOI: 10.7270/Q22N52MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50347985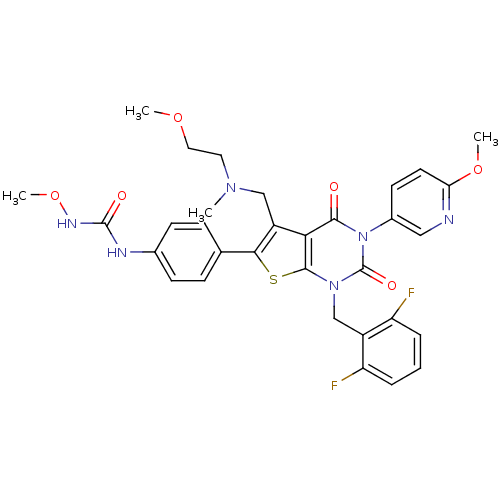 (CHEMBL1800156) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human GnRH receptor expressed in CHO cells assessed as inhibition of GnRH-induced arachadonic acid release using [5,6,8,9,11,1... | J Med Chem 54: 4998-5012 (2011) Article DOI: 10.1021/jm200216q BindingDB Entry DOI: 10.7270/Q22N52MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50347989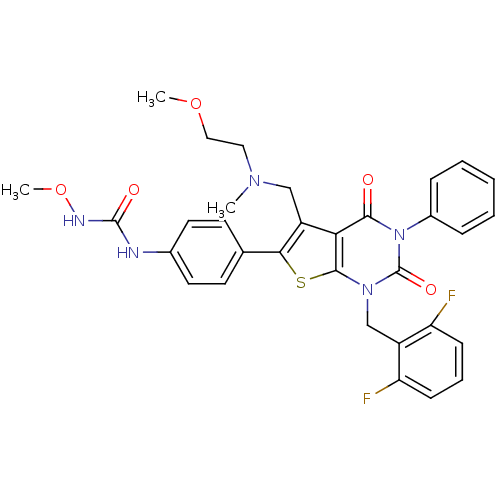 (CHEMBL1800668) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human GnRH receptor expressed in CHO cells assessed as inhibition of GnRH-induced arachadonic acid release using [5,6,8,9,11,1... | J Med Chem 54: 4998-5012 (2011) Article DOI: 10.1021/jm200216q BindingDB Entry DOI: 10.7270/Q22N52MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50347984 (CHEMBL1800157) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]leuprorelin from recombinant human GnRH receptor expressed in CHO cells after 60 mins by X-ray counter | J Med Chem 54: 4998-5012 (2011) Article DOI: 10.1021/jm200216q BindingDB Entry DOI: 10.7270/Q22N52MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50347982 (CHEMBL1800159) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]leuprorelin from recombinant human GnRH receptor expressed in CHO cells after 60 mins by X-ray counter | J Med Chem 54: 4998-5012 (2011) Article DOI: 10.1021/jm200216q BindingDB Entry DOI: 10.7270/Q22N52MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50347997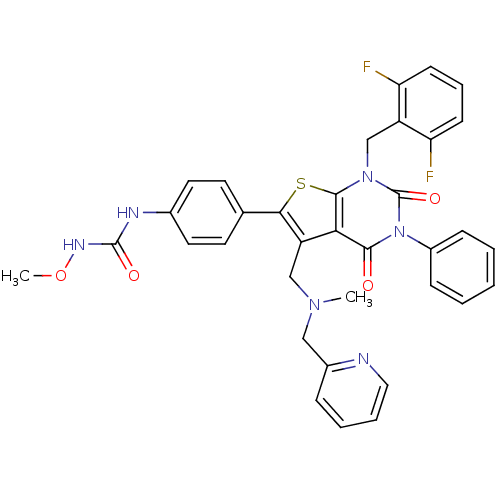 (CHEMBL1800661) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human GnRH receptor expressed in CHO cells assessed as inhibition of GnRH-induced arachadonic acid release using [5,6,8,9,11,1... | J Med Chem 54: 4998-5012 (2011) Article DOI: 10.1021/jm200216q BindingDB Entry DOI: 10.7270/Q22N52MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50347997 (CHEMBL1800661) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]leuprorelin from recombinant human GnRH receptor expressed in CHO cells after 60 mins by X-ray counter | J Med Chem 54: 4998-5012 (2011) Article DOI: 10.1021/jm200216q BindingDB Entry DOI: 10.7270/Q22N52MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50347994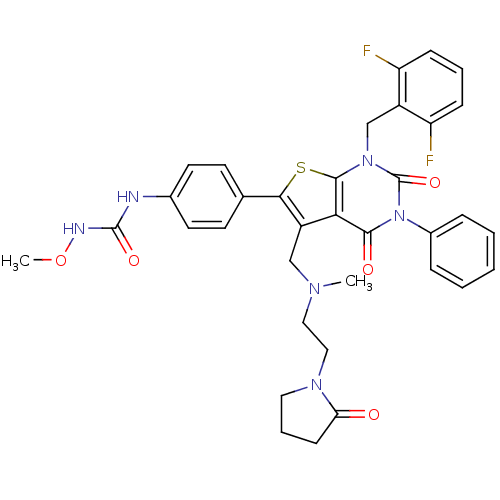 (CHEMBL1800666) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]leuprorelin from recombinant human GnRH receptor expressed in CHO cells after 60 mins by X-ray counter | J Med Chem 54: 4998-5012 (2011) Article DOI: 10.1021/jm200216q BindingDB Entry DOI: 10.7270/Q22N52MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122654 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Antagonist concentration required to inhibit specific binding of [125I]leuprorelin to human luteinizing releasing hormone receptor in cloned chinese ... | J Med Chem 46: 113-24 (2002) Article DOI: 10.1021/jm020180i BindingDB Entry DOI: 10.7270/Q26W9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM230879 (US9346822, 41) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description The monkey and human membrane fractions prepared were diluted with the assay buffer to yield a 200 g/ml dilution, each of which was then dispensed at... | US Patent US9346822 (2016) BindingDB Entry DOI: 10.7270/Q2251H2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189703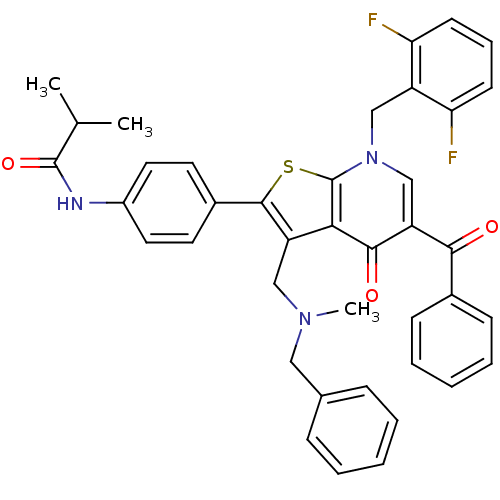 (CHEMBL208812 | N-(4-(7-(2,6-difluorobenzyl)-5-benz...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189701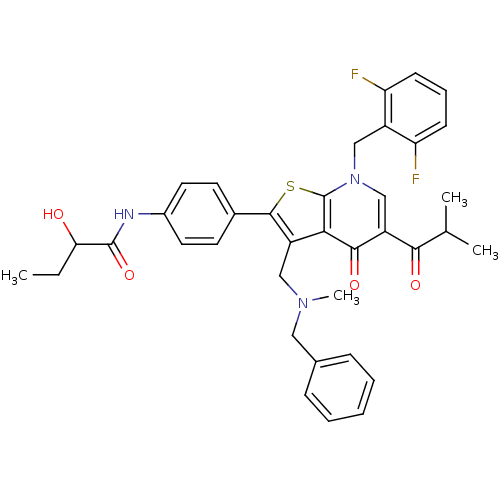 (3-(N-benzyl-N-methylaminomethyl)-7-(2,6-difluorobe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of LHRH-stimulated arachidonic acid release in CHO cells expressing human LHRH receptor | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189704 (3-(N-benzyl-N-methylaminomethyl)-7-(2,6-difluorobe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189730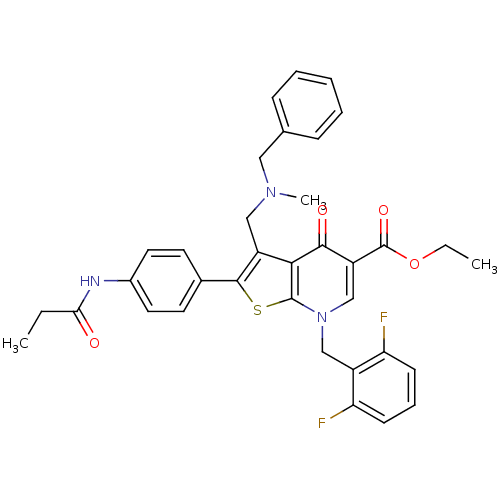 (CHEMBL210709 | ethyl 3-(N-benzyl-N-methylaminometh...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189701 (3-(N-benzyl-N-methylaminomethyl)-7-(2,6-difluorobe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50409694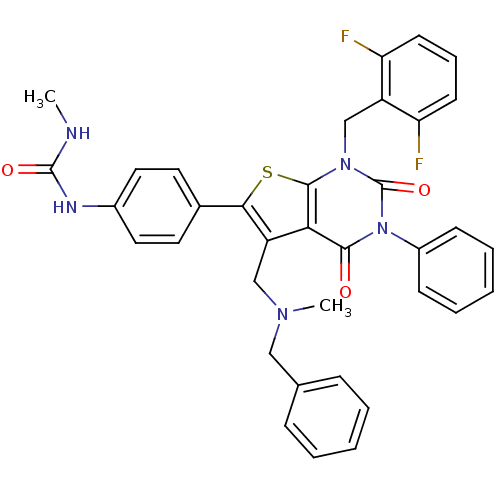 (CHEMBL2092994) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Antagonist concentration required to inhibit specific binding of [125I]leuprorelin to human luteinizing releasing hormone receptor in cloned chinese ... | J Med Chem 46: 113-24 (2002) Article DOI: 10.1021/jm020180i BindingDB Entry DOI: 10.7270/Q26W9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122654 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc Curated by ChEMBL | Assay Description Binding affinity at human GnRH receptor | J Med Chem 51: 3331-48 (2008) Article DOI: 10.1021/jm701249f BindingDB Entry DOI: 10.7270/Q2KD1XQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50347987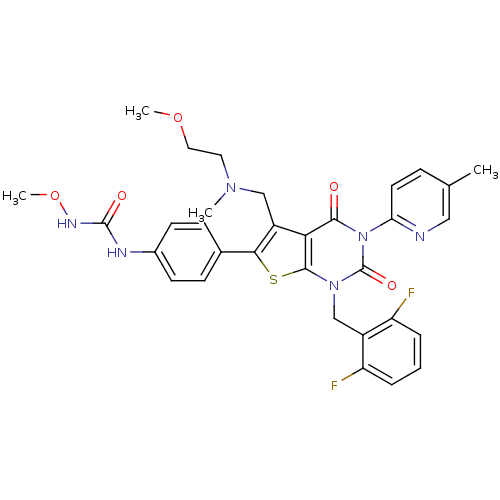 (CHEMBL1800153) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]leuprorelin from recombinant human GnRH receptor expressed in CHO cells after 60 mins by X-ray counter | J Med Chem 54: 4998-5012 (2011) Article DOI: 10.1021/jm200216q BindingDB Entry DOI: 10.7270/Q22N52MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50347989 (CHEMBL1800668) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]leuprorelin from recombinant human GnRH receptor expressed in CHO cells after 60 mins by X-ray counter | J Med Chem 54: 4998-5012 (2011) Article DOI: 10.1021/jm200216q BindingDB Entry DOI: 10.7270/Q22N52MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50347995 (CHEMBL1800664) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]leuprorelin from recombinant human GnRH receptor expressed in CHO cells after 60 mins by X-ray counter | J Med Chem 54: 4998-5012 (2011) Article DOI: 10.1021/jm200216q BindingDB Entry DOI: 10.7270/Q22N52MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122654 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]leuprorelin from recombinant human GnRH receptor expressed in CHO cells after 60 mins by X-ray counter | J Med Chem 54: 4998-5012 (2011) Article DOI: 10.1021/jm200216q BindingDB Entry DOI: 10.7270/Q22N52MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50155363 (7-Chloro-2-oxo-4-[2-(4-oxo-azetidin-2-yl)-ethoxy]-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human gonadotropin releasing hormone receptor expressed in CHO cells was determined by using [125I]-buserelin as radioligand | Bioorg Med Chem Lett 14: 5599-603 (2004) Article DOI: 10.1016/j.bmcl.2004.08.056 BindingDB Entry DOI: 10.7270/Q20V8C8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122652 (1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Antagonist concentration required to inhibit specific binding of [125I]leuprorelin to human luteinizing releasing hormone receptor in cloned chinese ... | J Med Chem 46: 113-24 (2002) Article DOI: 10.1021/jm020180i BindingDB Entry DOI: 10.7270/Q26W9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM405268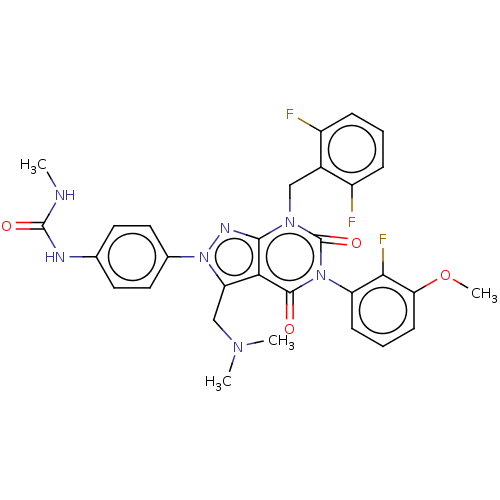 (1-(4-(7-(2,6-difluorobenzyl)-3-((dimethylamino)met...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co., Ltd US Patent | Assay Description Human: Test Example 1. Human GnRHr (GnRH Receptor) Activity Assay of the Present Compounds.In vitro GnRHr protein activity was tested by the followin... | US Patent US10344034 (2019) BindingDB Entry DOI: 10.7270/Q29Z978Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50526065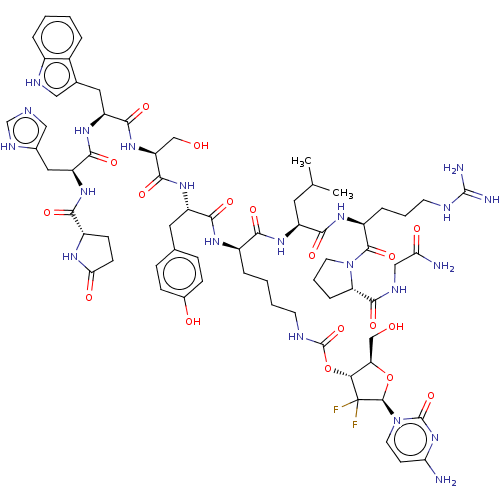 (CHEMBL4454362) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ioannina Curated by ChEMBL | Assay Description Displacement of [125I]-D-Tyr6-His5-GnRH from recombinant human GnRH receptor expressed in HEK293 cell membrane measured after 16 to 19 hrs by gamma c... | Eur J Med Chem 166: 256-266 (2019) Article DOI: 10.1016/j.ejmech.2019.01.041 BindingDB Entry DOI: 10.7270/Q27084VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM439612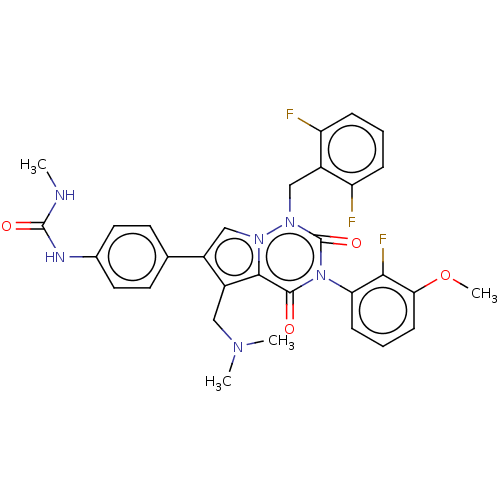 (1-(4-(1-(2,6-difluorobenzyl)-5-((dimethylamino)met...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co., ltd.; Jiangsu Hangrui Medicine Co., Ltd. US Patent | Assay Description In vitro GnRHr protein activity was tested by the following methods.This assay was used to determine the inhibition effect of the present compound on... | US Patent US10633388 (2020) BindingDB Entry DOI: 10.7270/Q2F76GM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50347983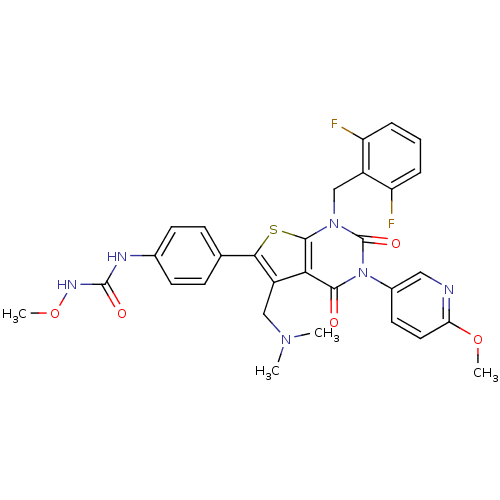 (CHEMBL1800158) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]leuprorelin from recombinant human GnRH receptor expressed in CHO cells after 60 mins by X-ray counter | J Med Chem 54: 4998-5012 (2011) Article DOI: 10.1021/jm200216q BindingDB Entry DOI: 10.7270/Q22N52MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50533873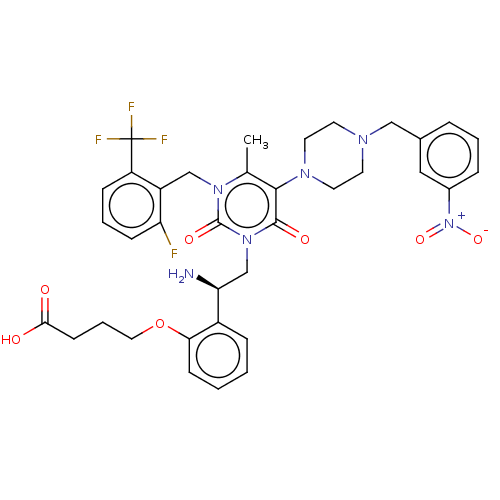 (CHEMBL4585638) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
SK Chemicals Company Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]D-Trp6-LHRH from human GnRH receptor expressed in CHO-K1 cell membranes incubated for 1 hr by competitive binding assay | J Med Chem 59: 9150-9172 (2016) Article DOI: 10.1021/acs.jmedchem.6b01071 BindingDB Entry DOI: 10.7270/Q2F1937S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50533953 (CHEMBL4474379) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
SK Chemicals Company Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]D-Trp6-LHRH from human GnRH receptor expressed in CHO-K1 cell membranes incubated for 1 hr by competitive binding assay | J Med Chem 59: 9150-9172 (2016) Article DOI: 10.1021/acs.jmedchem.6b01071 BindingDB Entry DOI: 10.7270/Q2F1937S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50533933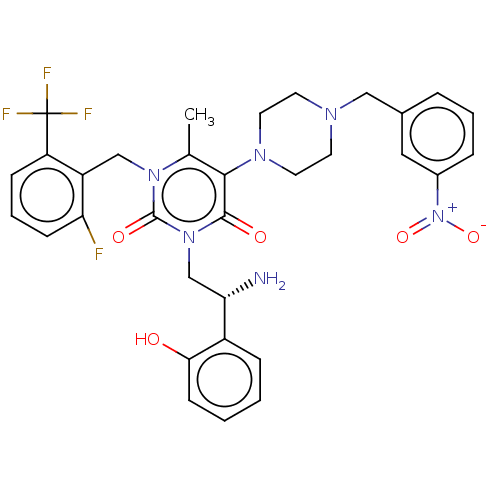 (CHEMBL4566719) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
SK Chemicals Company Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]D-Trp6-LHRH from human GnRH receptor expressed in CHO-K1 cell membranes incubated for 1 hr by competitive binding assay | J Med Chem 59: 9150-9172 (2016) Article DOI: 10.1021/acs.jmedchem.6b01071 BindingDB Entry DOI: 10.7270/Q2F1937S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50347986 (CHEMBL1800155) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human GnRH receptor expressed in CHO cells assessed as inhibition of GnRH-induced arachadonic acid release using [5,6,8,9,11,1... | J Med Chem 54: 4998-5012 (2011) Article DOI: 10.1021/jm200216q BindingDB Entry DOI: 10.7270/Q22N52MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50533944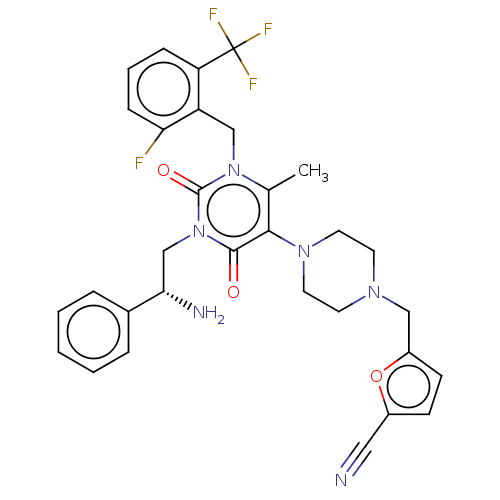 (CHEMBL4516712) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
SK Chemicals Company Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]D-Trp6-LHRH from human GnRH receptor expressed in CHO-K1 cell membranes incubated for 1 hr by competitive binding assay | J Med Chem 59: 9150-9172 (2016) Article DOI: 10.1021/acs.jmedchem.6b01071 BindingDB Entry DOI: 10.7270/Q2F1937S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50347987 (CHEMBL1800153) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human GnRH receptor expressed in CHO cells assessed as inhibition of GnRH-induced arachadonic acid release using [5,6,8,9,11,1... | J Med Chem 54: 4998-5012 (2011) Article DOI: 10.1021/jm200216q BindingDB Entry DOI: 10.7270/Q22N52MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50067485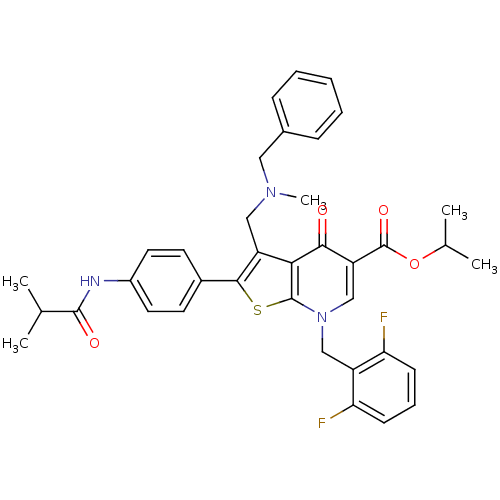 (3-[(Benzyl-methyl-amino)-methyl]-7-(2,6-difluoro-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description The Compound was tested for the concentration to inhibit 50% of [125 I ]leuprorelin binding to the cloned human Leutinizing releasing hormone recepto... | J Med Chem 41: 4190-5 (1998) Article DOI: 10.1021/jm9803673 BindingDB Entry DOI: 10.7270/Q2CR5V19 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50067485 (3-[(Benzyl-methyl-amino)-methyl]-7-(2,6-difluoro-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Ability of compound to inhibit [I125-Tyr5,DLeu6,NMeLeu7,Pro9-NEt]GnRH agonist binding to the cloned human Gonadotropin-releasing hormone receptor was... | Bioorg Med Chem Lett 12: 2179-83 (2002) BindingDB Entry DOI: 10.7270/Q28S4P8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122660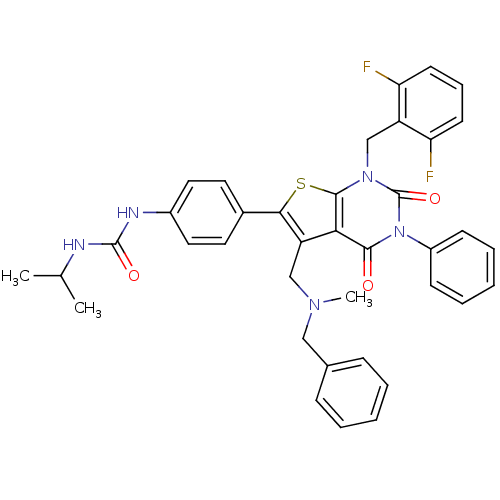 (1-{4-[5-[(Benzyl-methyl-amino)-methyl]-1-(2,6-difl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Antagonist concentration required to inhibit specific binding of [125I]leuprorelin to human luteinizing releasing hormone receptor in cloned chinese ... | J Med Chem 46: 113-24 (2002) Article DOI: 10.1021/jm020180i BindingDB Entry DOI: 10.7270/Q26W9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50122667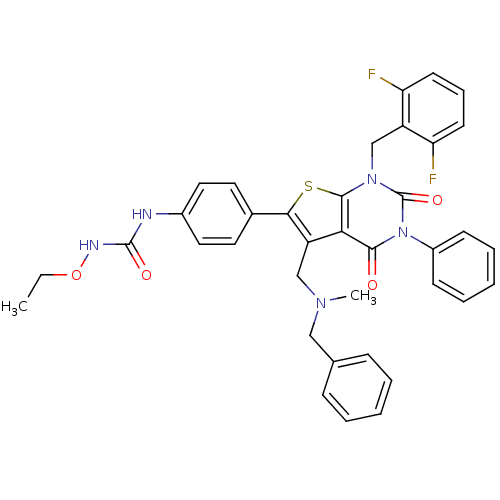 (1-{4-[5-[(Benzyl-methyl-amino)-methyl]-1-(2,6-difl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Antagonist concentration required to inhibit specific binding of [125I]leuprorelin to human luteinizing releasing hormone receptor in cloned chinese ... | J Med Chem 46: 113-24 (2002) Article DOI: 10.1021/jm020180i BindingDB Entry DOI: 10.7270/Q26W9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50067485 (3-[(Benzyl-methyl-amino)-methyl]-7-(2,6-difluoro-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Antagonist concentration required to inhibit specific binding of [125I]leuprorelin to human luteinizing releasing hormone receptor in cloned chinese ... | J Med Chem 46: 113-24 (2002) Article DOI: 10.1021/jm020180i BindingDB Entry DOI: 10.7270/Q26W9BTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189707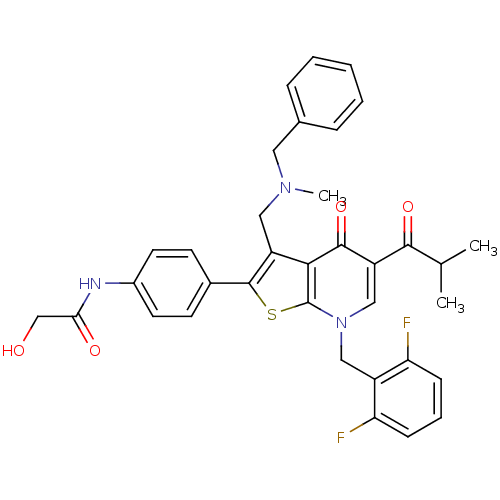 (3-(N-benzyl-N-methylaminomethyl)-7-(2,6-difluorobe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189720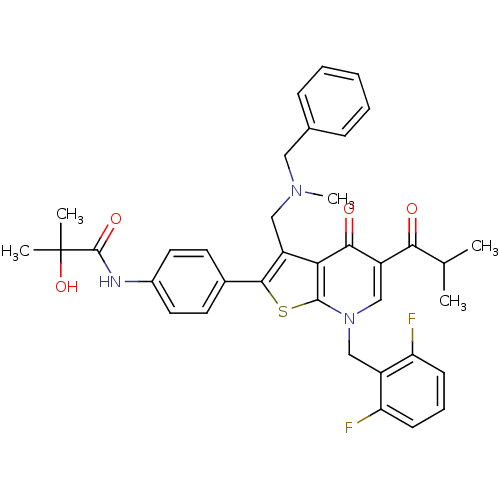 (3-(N-benzyl-N-methylaminomethyl)-7-(2,6-difluorobe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189727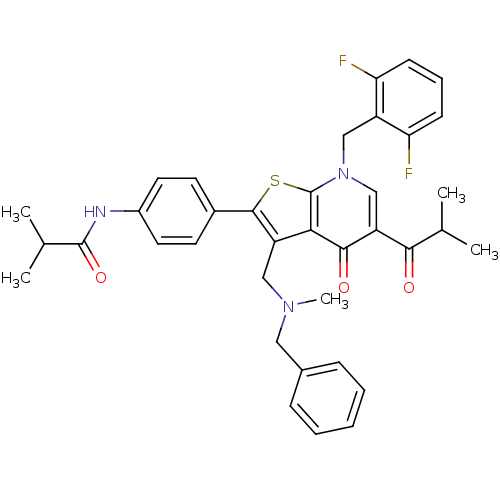 (CHEMBL211503 | N-(4-(7-(2,6-difluorobenzyl)-3-((be...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50189723 (CHEMBL540109 | ethyl 3-(N-benzyl-N-methylaminometh...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of [125I]leuprorelin binding to human recombinant LHRH receptor expressed in CHO cells | J Med Chem 49: 3809-25 (2006) Article DOI: 10.1021/jm0512894 BindingDB Entry DOI: 10.7270/Q2VQ33G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1925 total ) | Next | Last >> |