

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50278350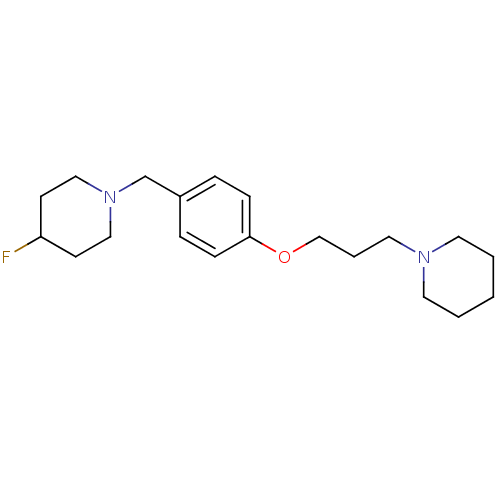 (4-fluoro-1-(4-(3-(piperidin-1-yl)propoxy)benzyl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a |
ETH Zurich (Swiss Federal Institute of Technology) Curated by ChEMBL | Assay Description Binding affinity to rat brain histamine H3 receptor after 1 hr by gamma counting | Bioorg Med Chem 20: 2889-96 (2012) Article DOI: 10.1016/j.bmc.2012.03.024 BindingDB Entry DOI: 10.7270/Q2JQ1258 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50346208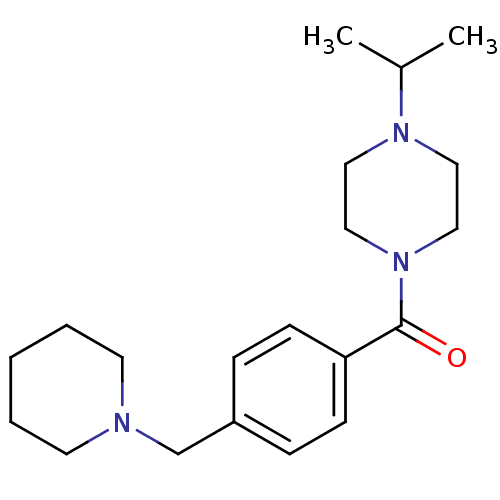 ((1-isopropylpiperidin-4-yl)(4-(piperidin-1-ylmethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.82 | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at histamine H3 receptor in rat cortical hemispheres assessed as inhibition of forskolin stimulated cAMP accumulation after 6 hrs | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50321467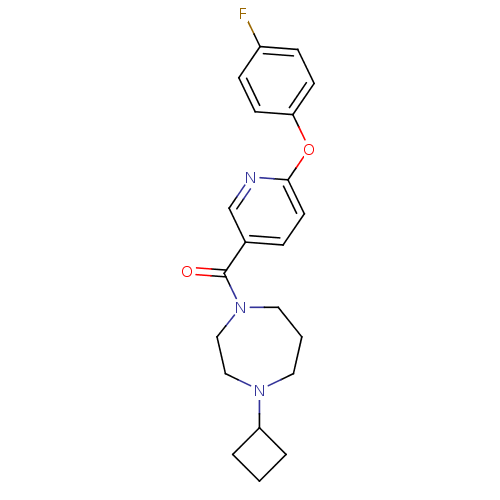 ((4-cyclobutyl-1,4-diazepan-1-yl)(6-(4-fluorophenox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 3.55 | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at rat histamine H3 receptor expressed in human SK-N-MC cells assessed as inhibition of forskolin-induced cAMP accumulation after... | Bioorg Med Chem Lett 20: 4210-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.041 BindingDB Entry DOI: 10.7270/Q29G5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50321472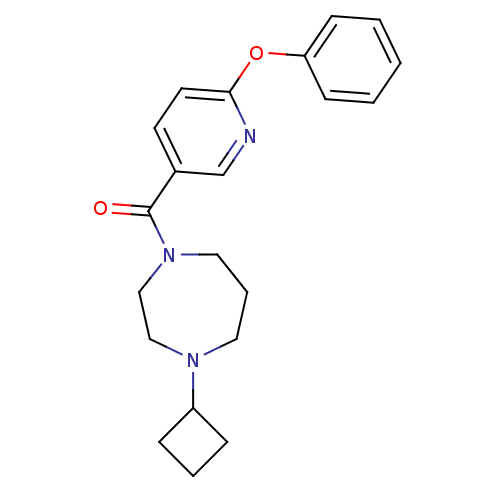 ((4-cyclobutyl-1,4-diazepan-1-yl)(6-phenoxypyridin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 3.72 | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at rat histamine H3 receptor expressed in human SK-N-MC cells assessed as inhibition of forskolin-induced cAMP accumulation after... | Bioorg Med Chem Lett 20: 4210-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.041 BindingDB Entry DOI: 10.7270/Q29G5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50321464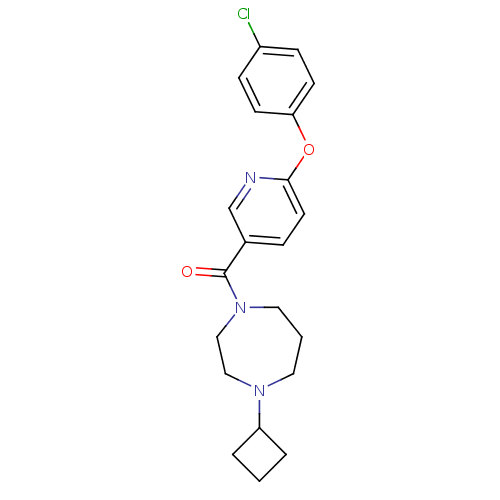 ((6-(4-chlorophenoxy)pyridin-3-yl)(4-cyclobutyl-1,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at rat histamine H3 receptor expressed in human SK-N-MC cells assessed as inhibition of forskolin-induced cAMP accumulation after... | Bioorg Med Chem Lett 20: 4210-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.041 BindingDB Entry DOI: 10.7270/Q29G5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50321468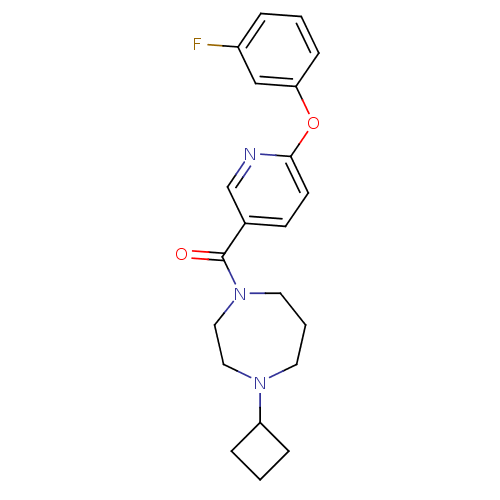 ((4-cyclobutyl-1,4-diazepan-1-yl)(6-(3-fluorophenox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 4.17 | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at rat histamine H3 receptor expressed in human SK-N-MC cells assessed as inhibition of forskolin-induced cAMP accumulation after... | Bioorg Med Chem Lett 20: 4210-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.041 BindingDB Entry DOI: 10.7270/Q29G5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50321465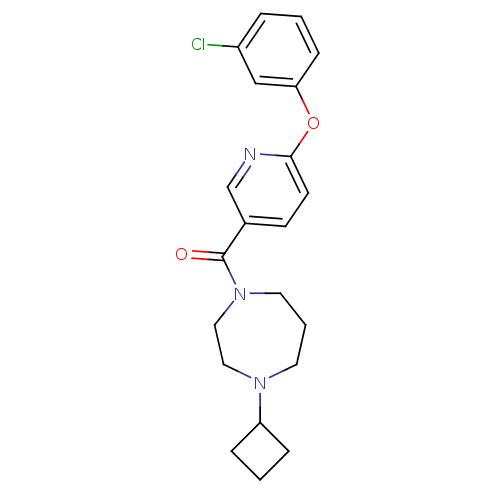 ((6-(3-chlorophenoxy)pyridin-3-yl)(4-cyclobutyl-1,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at rat histamine H3 receptor expressed in human SK-N-MC cells assessed as inhibition of forskolin-induced cAMP accumulation after... | Bioorg Med Chem Lett 20: 4210-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.041 BindingDB Entry DOI: 10.7270/Q29G5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50346201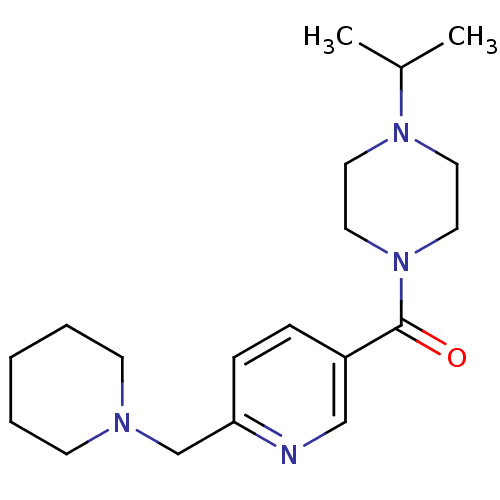 ((4-Isopropyl-piperazin-1-yl)-(6-piperidin-1-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at histamine H3 receptor in rat cortical hemispheres assessed as inhibition of forskolin stimulated cAMP accumulation after 6 hrs | Eur J Med Chem 44: 4413-25 (2009) Article DOI: 10.1016/j.ejmech.2009.06.007 BindingDB Entry DOI: 10.7270/Q28S4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50321471 ((4-cyclobutyl-1,4-diazepan-1-yl)(6-(3,4-dichloroph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 6.92 | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at rat histamine H3 receptor expressed in human SK-N-MC cells assessed as inhibition of forskolin-induced cAMP accumulation after... | Bioorg Med Chem Lett 20: 4210-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.041 BindingDB Entry DOI: 10.7270/Q29G5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50321466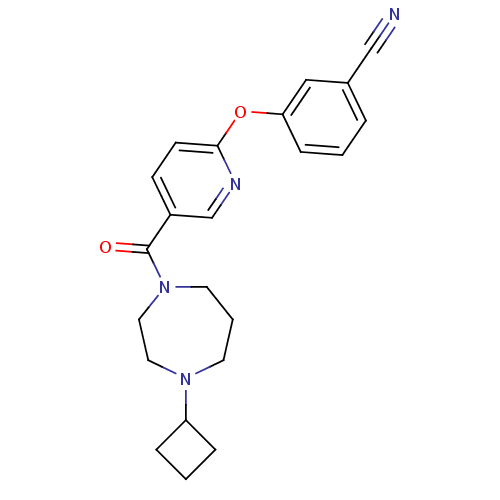 (3-(5-(4-cyclobutyl-1,4-diazepane-1-carbonyl)pyridi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 9.55 | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at rat histamine H3 receptor expressed in human SK-N-MC cells assessed as inhibition of forskolin-induced cAMP accumulation after... | Bioorg Med Chem Lett 20: 4210-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.041 BindingDB Entry DOI: 10.7270/Q29G5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50321470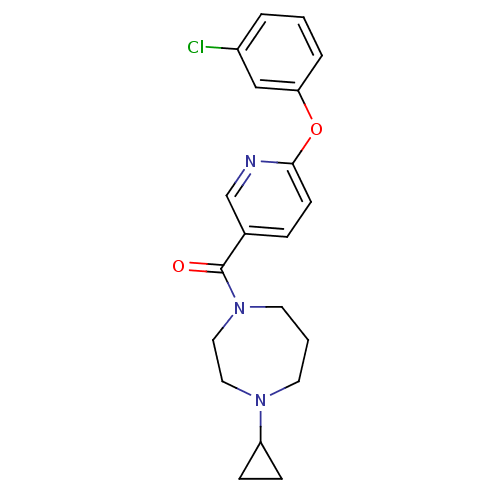 ((6-(3-chlorophenoxy)pyridin-3-yl)(4-cyclopropyl-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at rat histamine H3 receptor expressed in human SK-N-MC cells assessed as inhibition of forskolin-induced cAMP accumulation after... | Bioorg Med Chem Lett 20: 4210-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.041 BindingDB Entry DOI: 10.7270/Q29G5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50321463 ((4-cyclobutyl-1,4-diazepan-1-yl)(5-(4-fluorophenox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 26.3 | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at rat histamine H3 receptor expressed in human SK-N-MC cells assessed as inhibition of forskolin-induced cAMP accumulation after... | Bioorg Med Chem Lett 20: 4210-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.041 BindingDB Entry DOI: 10.7270/Q29G5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50278350 (4-fluoro-1-(4-(3-(piperidin-1-yl)propoxy)benzyl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a |
ETH Zurich (Swiss Federal Institute of Technology) Curated by ChEMBL | Assay Description Binding affinity to rat histamine H3 receptor low affinity site after 1 hr by Schard plot analysis | Bioorg Med Chem 20: 2889-96 (2012) Article DOI: 10.1016/j.bmc.2012.03.024 BindingDB Entry DOI: 10.7270/Q2JQ1258 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||