

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578446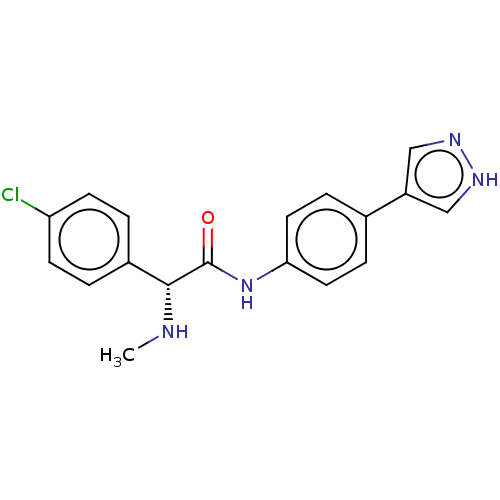 ((R)óN-(4-(1H-pyrazol-4-yl)phenyl)-2-(4-chloropheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578479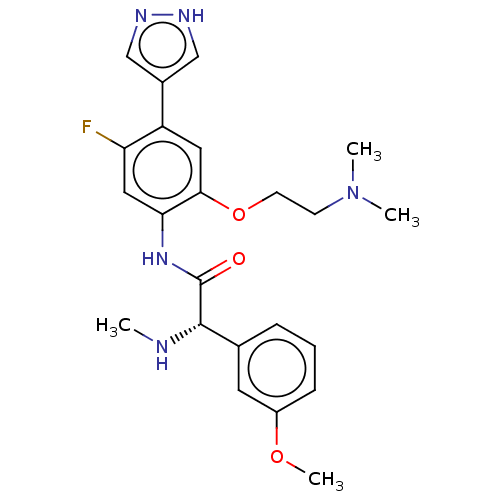 ((S)óN-(2-(2-(dimethylamino)ethoxy)-5-fluoro-4-(1H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578424 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(3-chlorophenyl)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578438 ((R)óN-(4-(1H-pyrazol-4-yl)phenyl)-2-(3-methoxyphen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578449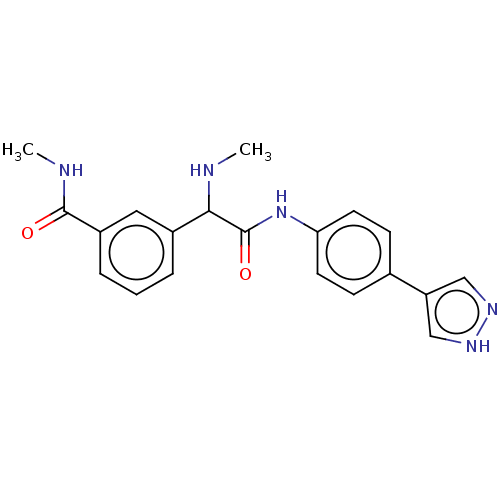 (3-(2-((4-(1H-pyrazol-4-yl)phenyl)amino)-1-(methyla...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578470 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(3-methoxyphenyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578435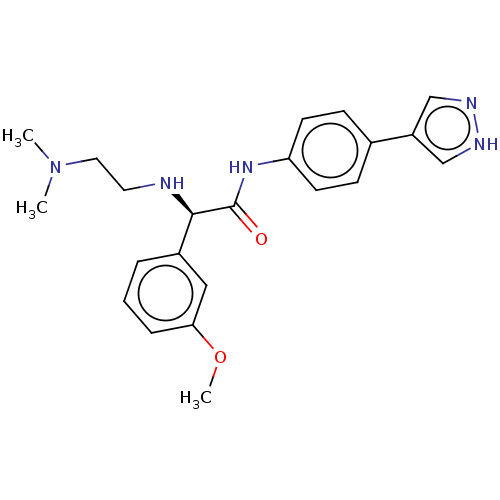 ((R)óN-(4-(1H-pyrazol-4-yl)phenyl)-2-((2-(dimethyla...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578454 (N-(2-(2-(dimethylamino)ethoxy)-5-fluoro-4-(1H-pyra...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578409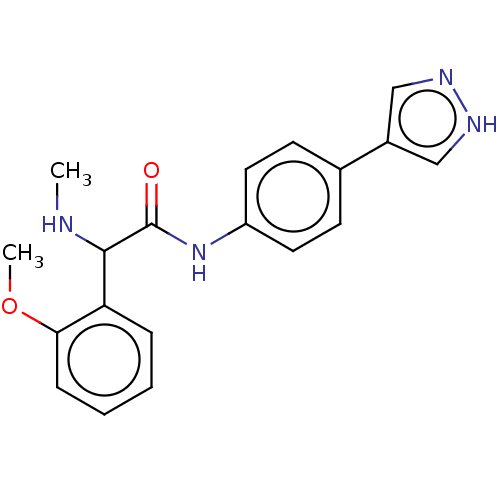 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(2-methoxyphenyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578410 ((S)óN-(4-(1H-pyrazol-4-yl)phenyl)-2-(3-methoxyphen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 [1-535] (Homo sapiens (Human)) | BDBM578438 ((R)óN-(4-(1H-pyrazol-4-yl)phenyl)-2-(3-methoxyphen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578415 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(ethylamino)-2-(p-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578420 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(3-chlorophenyl)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578415 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(ethylamino)-2-(p-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578411 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(3-methoxyphenyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578423 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(4-fluorophenyl)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578476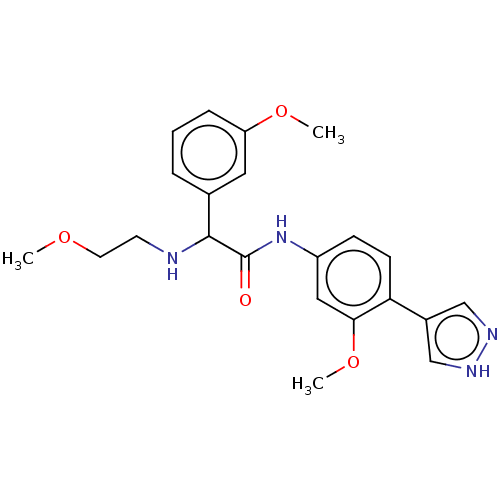 (N-(3-methoxy-4-(1H-pyrazol-4-yl)phenyl)-2-((2-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578430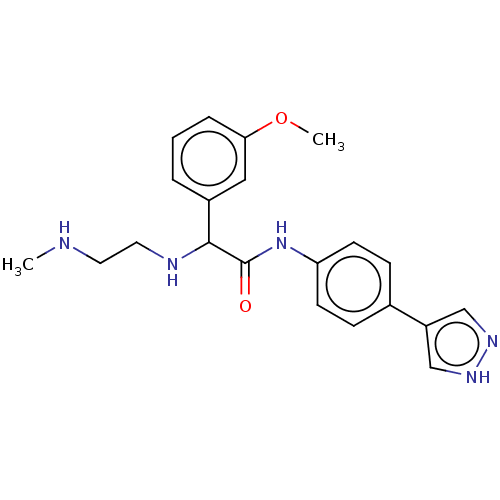 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(3-methoxyphenyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578417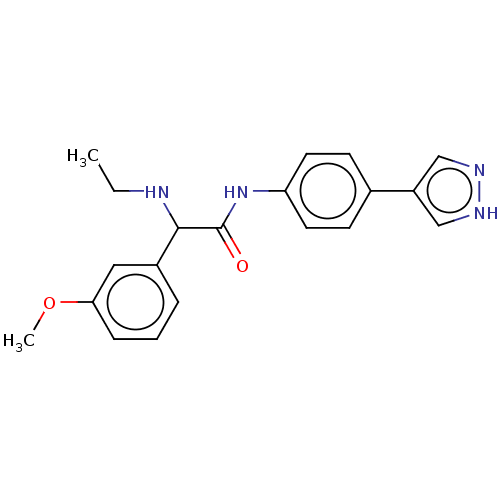 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(ethylamino)-2-(3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578421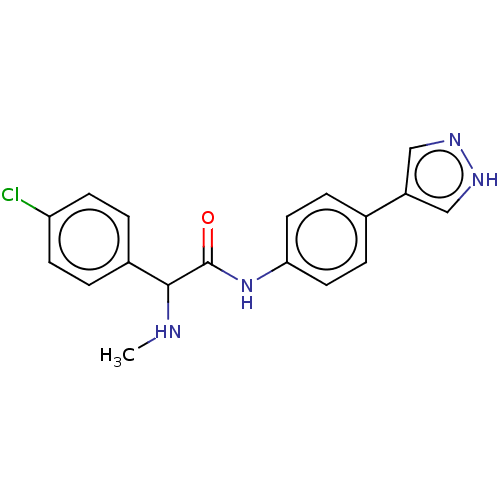 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(4-chlorophenyl)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578461 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(3-methoxyphenyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578418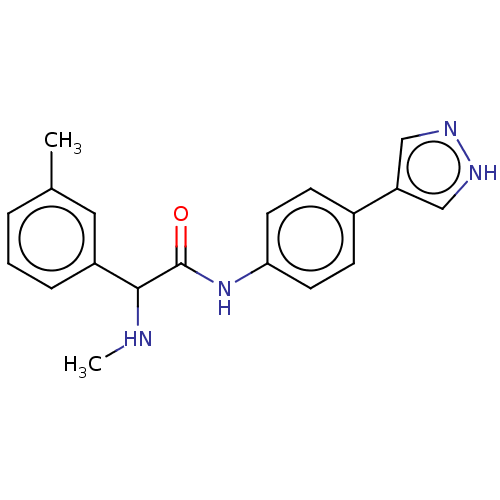 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(methylamino)-2-(m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578453 (N-(2-(3-(dimethylamino)propoxy)-5-fluoro-4-(1H-pyr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 [1-535] (Homo sapiens (Human)) | BDBM578435 ((R)óN-(4-(1H-pyrazol-4-yl)phenyl)-2-((2-(dimethyla...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578471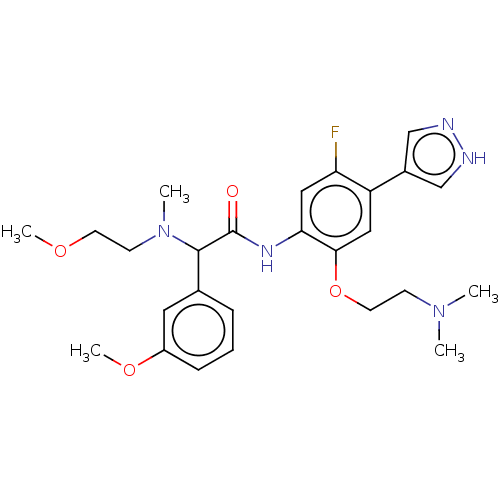 (N-(2-(2-(dimethylamino)ethoxy)-5-fluoro-4-(1H-pyra...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578457 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-((3-(dimethylamino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578426 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(4-chlorophenyl)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578431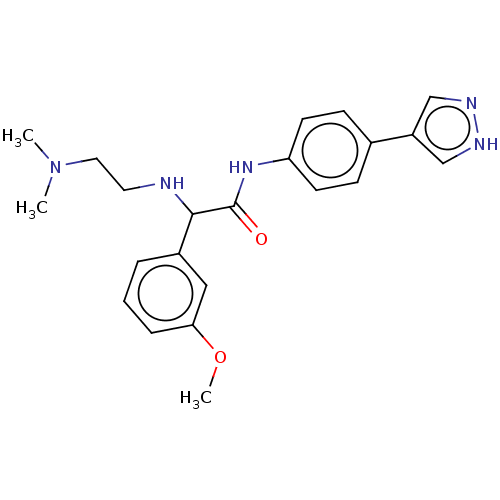 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-((2-(dimethylamino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578422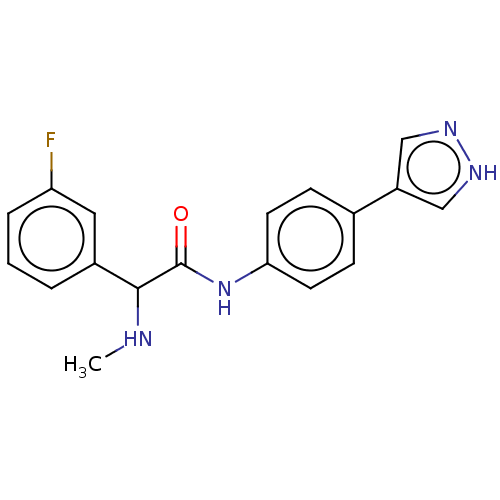 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(3-fluorophenyl)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 [1-535] (Homo sapiens (Human)) | BDBM578409 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(2-methoxyphenyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578412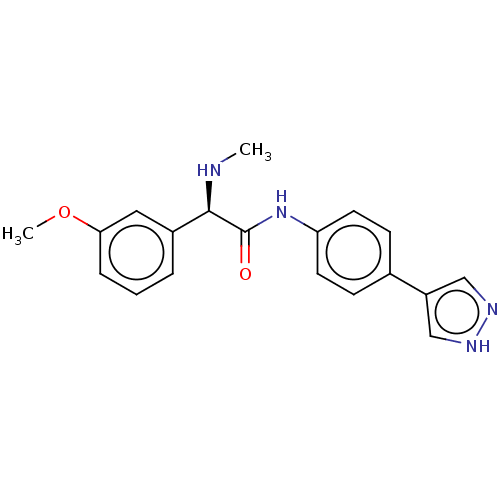 ((R)óN-(4-(1H-pyrazol-4-yl)phenyl)-2-(3-methoxyphen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578460 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-((2-amino-2-oxoeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578433 ((R)óN-(4-(1H-pyrazol-4-yl)phenyl)-2-((2-methoxyeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578440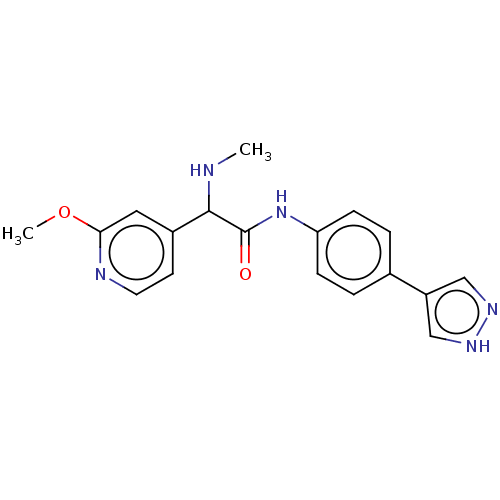 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(2-methoxypyridin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578413 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(4-methoxyphenyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15.4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578455 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(3-methoxyphenyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578458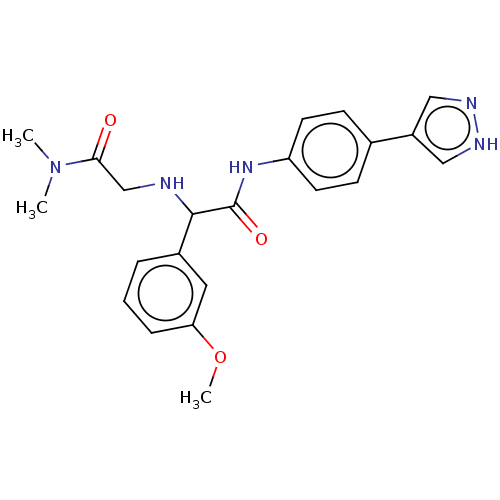 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-((2-(dimethylamino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578472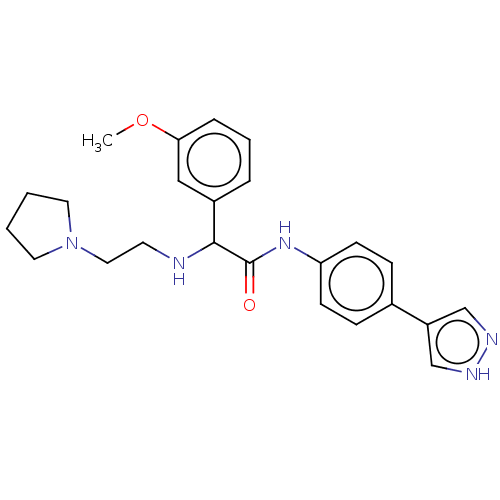 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(3-methoxyphenyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578475 (N-(3-fluoro-4-(1H-pyrazol-4-yl)phenyl)-2-((2-metho...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 [1-535] (Homo sapiens (Human)) | BDBM578411 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(3-methoxyphenyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578447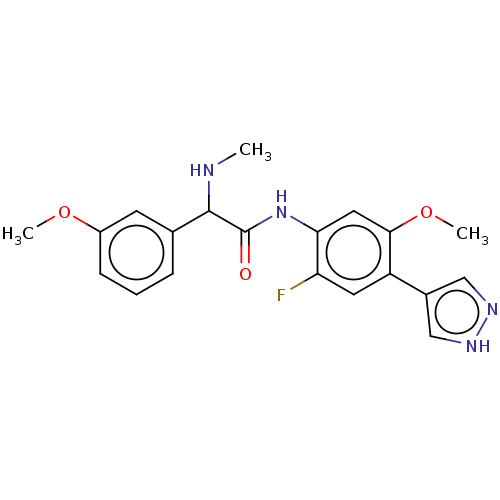 (N-(2-fluoro-5-methoxy-4-(1H-pyrazol-4-yl)phenyl)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578441 (Methyl 3-(2-((4-(1H-pyrazol-4-yl)phenyl)amino)-1-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578414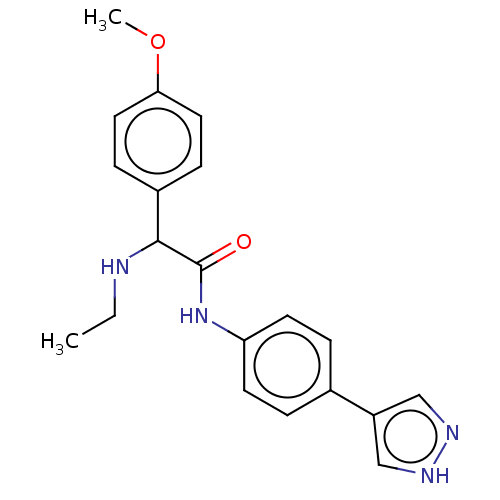 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(ethylamino)-2-(4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578427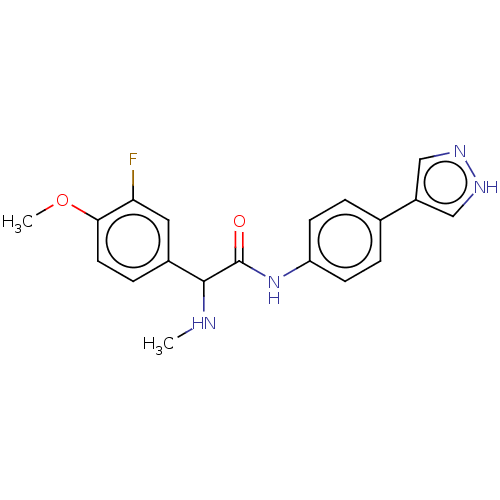 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(3-fluoro-4-methox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578439 ((S)óN-(4-(1H-pyrazol-4-yl)phenyl)-2-(3-methoxyphen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578428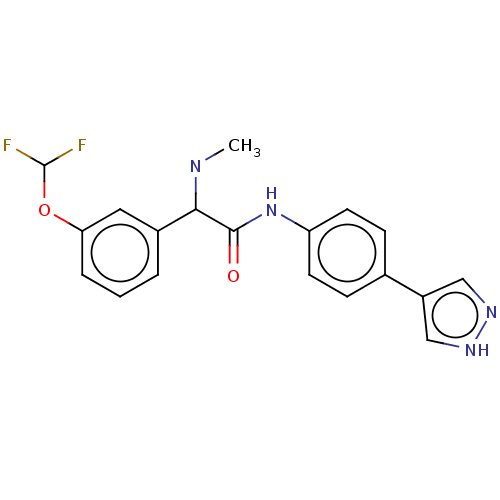 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(3-(difluoromethox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578445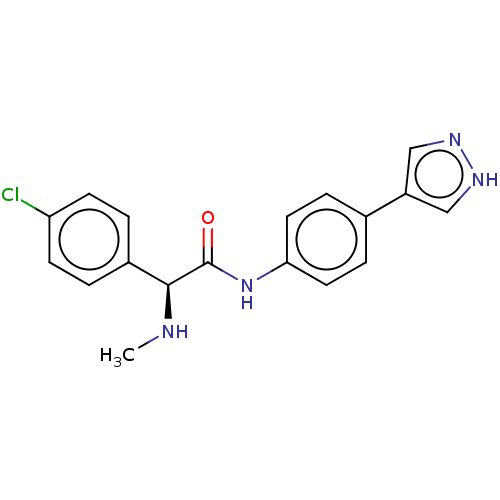 ((S)óN-(4-(1H-pyrazol-4-yl)phenyl)-2-(4-chloropheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578419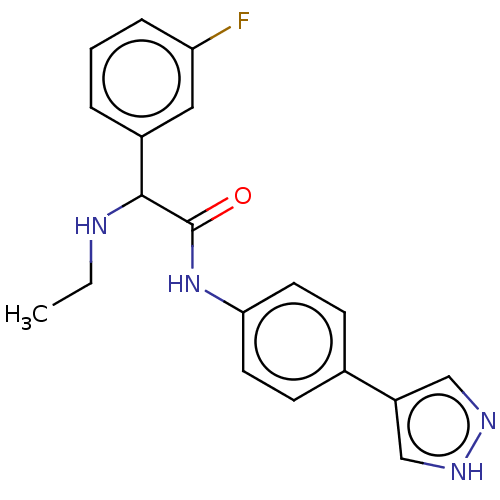 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(ethylamino)-2-(3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578444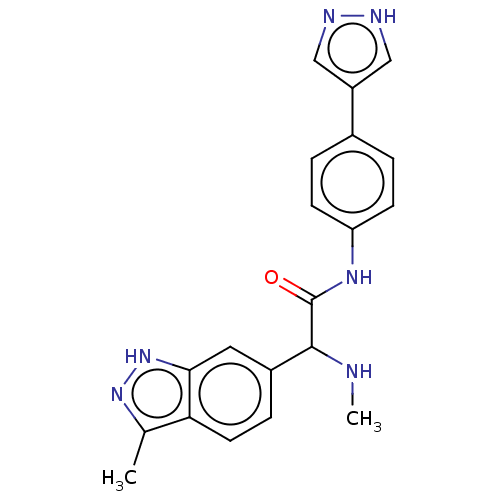 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(3-methyl-1H-indaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [1-552] (Homo sapiens (Human)) | BDBM578416 (N-(4-(1H-pyrazol-4-yl)phenyl)-2-(ethylamino)-2-(4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds activities were measured by Z'-lyte kinase kit (ThermoFisher Scientific). The percent inhibition rate was calculated by normalizing the... | Citation and Details BindingDB Entry DOI: 10.7270/Q2R78JGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 83 total ) | Next | Last >> |