

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26503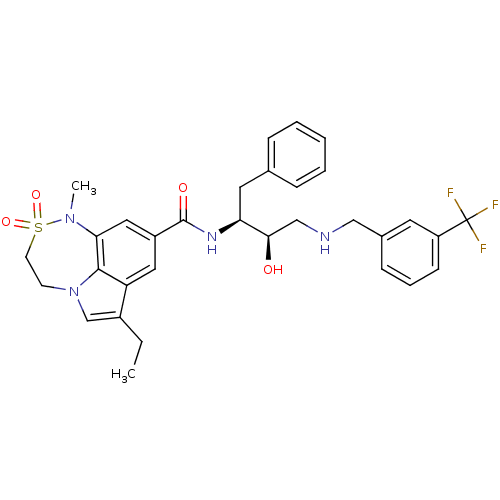 (3-ethyl-N-[(2S,3R)-3-hydroxy-1-phenyl-4-({[3-(trif...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29796 (sulfonamide tricyclic analogue, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29794 (sulfonamide tricyclic analogue, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29815 (sulfonamide tricyclic analogue, 28) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29777 (7,6,5 tricyclic sulfonamide, 15 | BMCL193674 Compo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29809 (sulfonamide tricyclic analogue, 22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29805 (sulfone tricyclic analogue, 18) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29804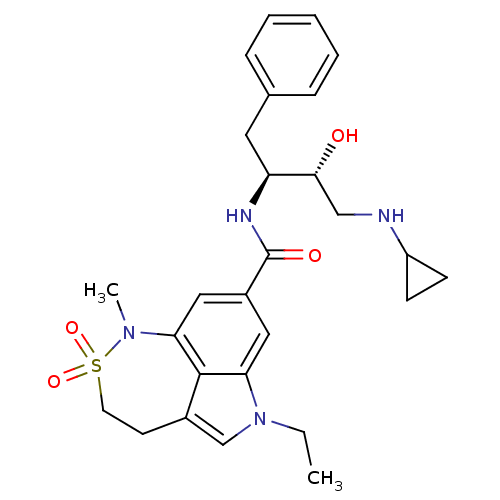 (sulfonamide tricyclic analogue, 15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM10145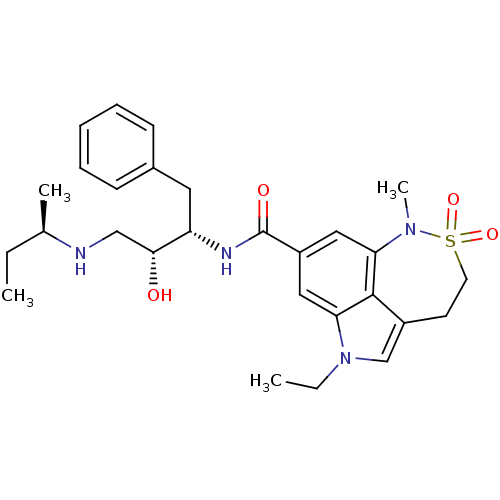 (lysine sulfonamide analogue 9 | methyl N-[(1S)-1-{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29800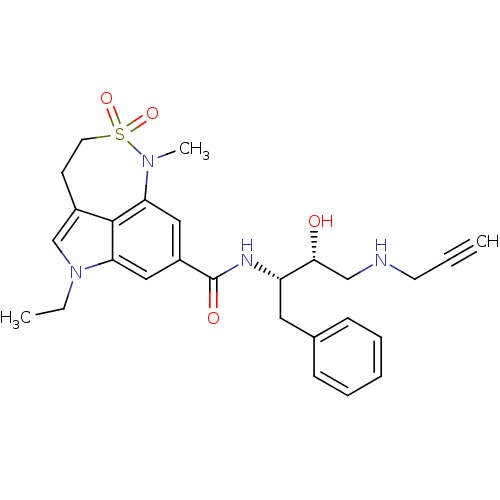 (sulfonamide tricyclic analogue, 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29775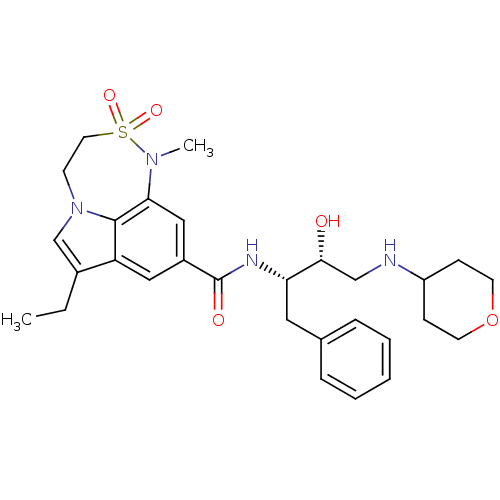 (7,6,5 tricyclic sulfonamide, 13 | BMCL193674 Compo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26510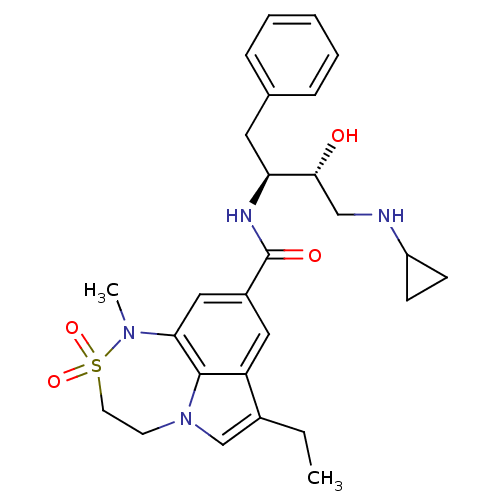 (BMCL193669 Compound 18 | N-[(2S,3R)-4-(cyclopropyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29812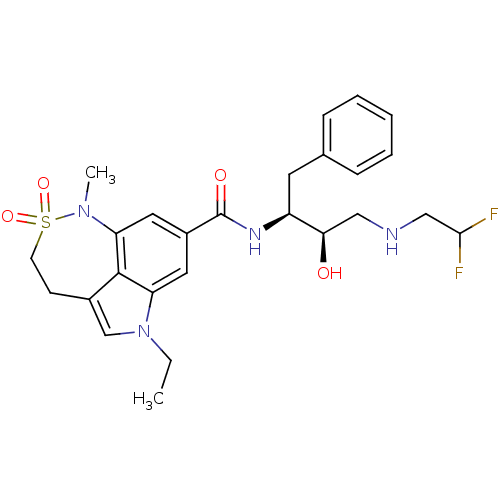 (sulfonamide tricyclic analogue, 25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29799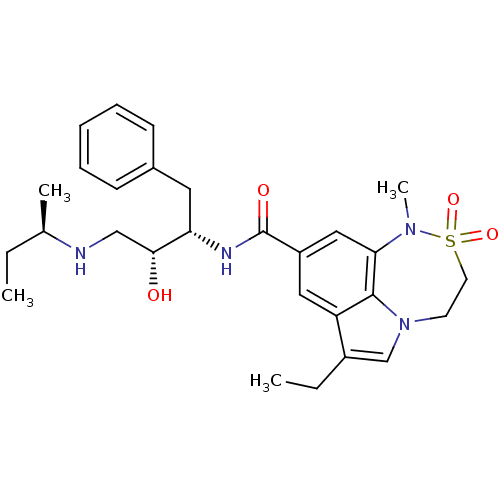 (sulfonamide tricyclic analogue, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29802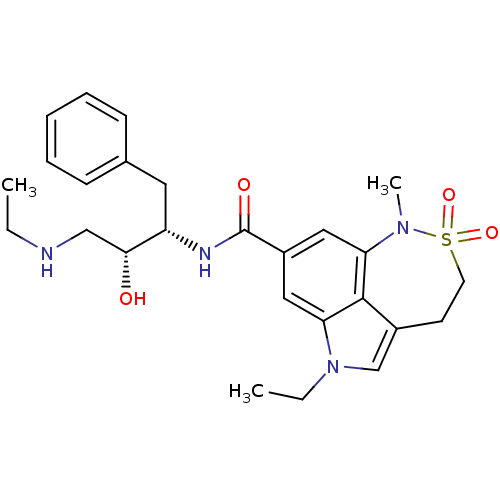 (sulfonamide tricyclic analogue, 13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM29777 (7,6,5 tricyclic sulfonamide, 15 | BMCL193674 Compo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29813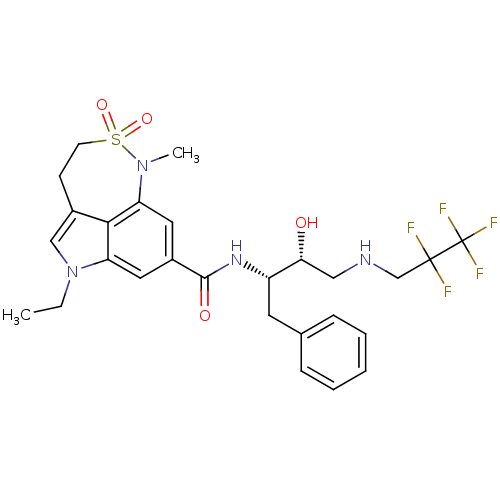 (sulfonamide tricyclic analogue, 26) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29811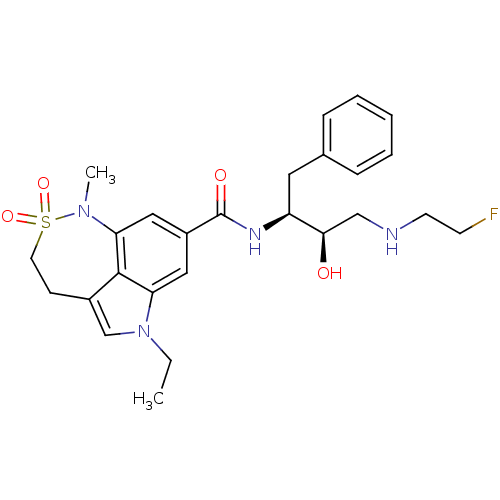 (sulfonamide tricyclic analogue, 24) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29803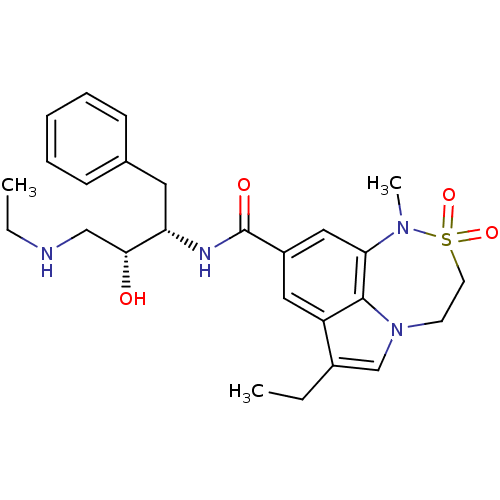 (sulfonamide tricyclic analogue, 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29810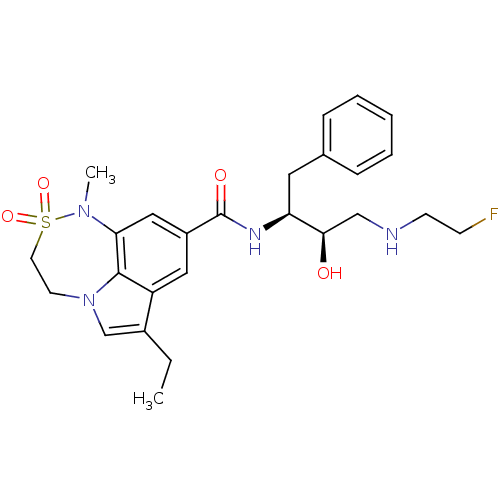 (sulfonamide tricyclic analogue, 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM26503 (3-ethyl-N-[(2S,3R)-3-hydroxy-1-phenyl-4-({[3-(trif...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29801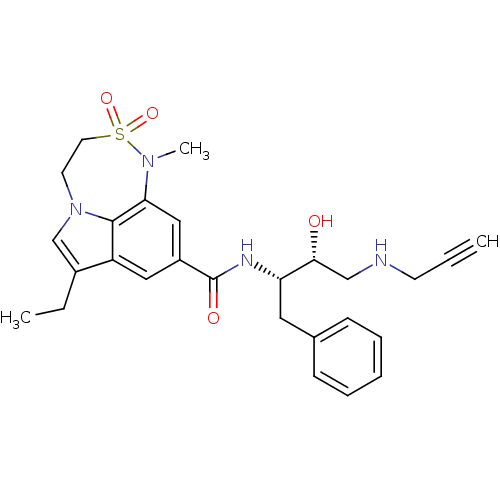 (sulfonamide tricyclic analogue, 12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM29796 (sulfonamide tricyclic analogue, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29814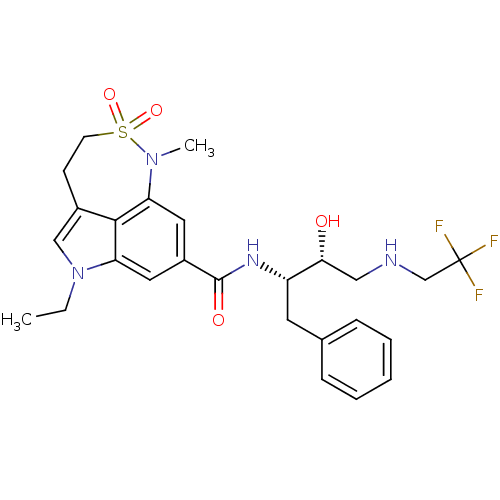 (sulfonamide tricyclic analogue, 27) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29806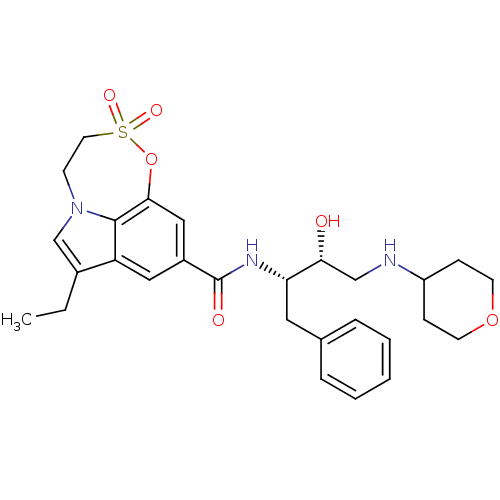 (sulfone tricyclic analogue, 19) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29806 (sulfone tricyclic analogue, 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29808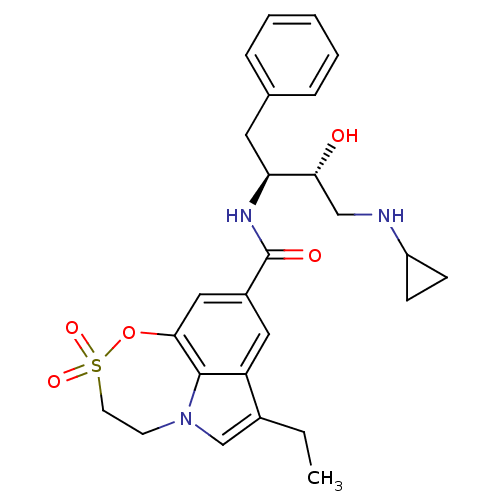 (sulfone tricyclic analogue, 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM29805 (sulfone tricyclic analogue, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM26510 (BMCL193669 Compound 18 | N-[(2S,3R)-4-(cyclopropyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 263 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29807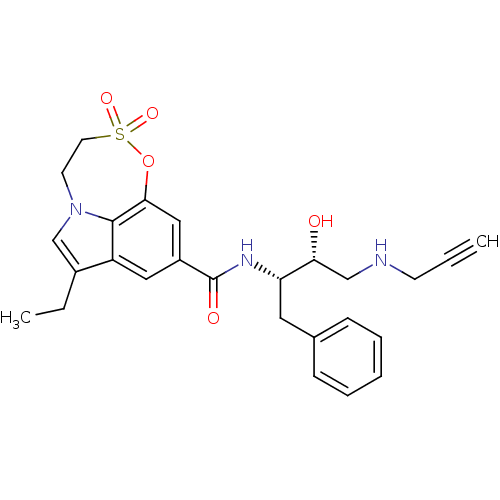 (sulfone tricyclic analogue, 20) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM29804 (sulfonamide tricyclic analogue, 15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM10145 (lysine sulfonamide analogue 9 | methyl N-[(1S)-1-{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM29799 (sulfonamide tricyclic analogue, 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM29806 (sulfone tricyclic analogue, 19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM29775 (7,6,5 tricyclic sulfonamide, 13 | BMCL193674 Compo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29796 (sulfonamide tricyclic analogue, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM29794 (sulfonamide tricyclic analogue, 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29777 (7,6,5 tricyclic sulfonamide, 15 | BMCL193674 Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM29803 (sulfonamide tricyclic analogue, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM29808 (sulfone tricyclic analogue, 21) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM10145 (lysine sulfonamide analogue 9 | methyl N-[(1S)-1-{...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM26503 (3-ethyl-N-[(2S,3R)-3-hydroxy-1-phenyl-4-({[3-(trif...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM29800 (sulfonamide tricyclic analogue, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29794 (sulfonamide tricyclic analogue, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29805 (sulfone tricyclic analogue, 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.16E+3 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM29802 (sulfonamide tricyclic analogue, 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29808 (sulfone tricyclic analogue, 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.97E+3 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM29801 (sulfonamide tricyclic analogue, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM29807 (sulfone tricyclic analogue, 20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM29799 (sulfonamide tricyclic analogue, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 58 total ) | Next | Last >> |