 Found 74 hits Enz. Inhib. hit(s) with all data for entry = 50000144
Found 74 hits Enz. Inhib. hit(s) with all data for entry = 50000144 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241539

(CHEMBL4084845)Show SMILES Cc1ccc(cc1)-c1ncc(OC[C@H]2CCNC2)cc1-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C24H23N3O/c1-17-2-6-21(7-3-17)24-23(20-8-4-18(13-25)5-9-20)12-22(15-27-24)28-16-19-10-11-26-14-19/h2-9,12,15,19,26H,10-11,14,16H2,1H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Kinetic constant was measured for PMII of Leishmania donovani promastigotes using supernatant (S12) fraction |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241533

(CHEMBL4102775)Show SMILES COc1ccc(CN(CC(=O)N2CCC3(CNC3)CC2)c2ccc(cc2)C#N)cc1F Show InChI InChI=1S/C24H27FN4O2/c1-31-22-7-4-19(12-21(22)25)14-29(20-5-2-18(13-26)3-6-20)15-23(30)28-10-8-24(9-11-28)16-27-17-24/h2-7,12,27H,8-11,14-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241526
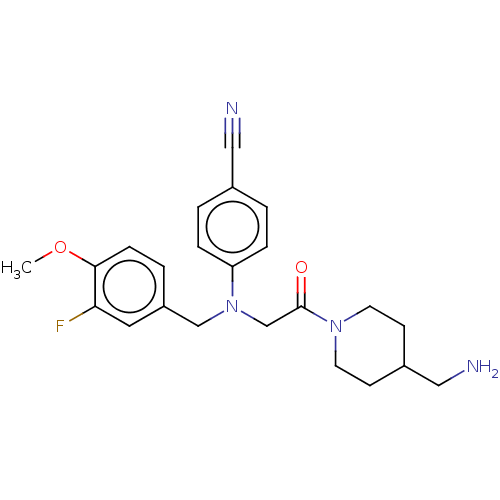
(CHEMBL4095018)Show SMILES COc1ccc(CN(CC(=O)N2CCC(CN)CC2)c2ccc(cc2)C#N)cc1F Show InChI InChI=1S/C23H27FN4O2/c1-30-22-7-4-19(12-21(22)24)15-28(20-5-2-17(13-25)3-6-20)16-23(29)27-10-8-18(14-26)9-11-27/h2-7,12,18H,8-11,14-16,26H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241535

(CHEMBL4068129)Show SMILES Cc1ccc(CN(CC(=O)N2CCC(CN)CC2)c2ccc(cc2)C#N)cc1 Show InChI InChI=1S/C23H28N4O/c1-18-2-4-21(5-3-18)16-27(22-8-6-19(14-24)7-9-22)17-23(28)26-12-10-20(15-25)11-13-26/h2-9,20H,10-13,15-17,25H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory activity against human immunodeficiency virus-1 reverse transcriptase(HIV-1 RT). |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241531
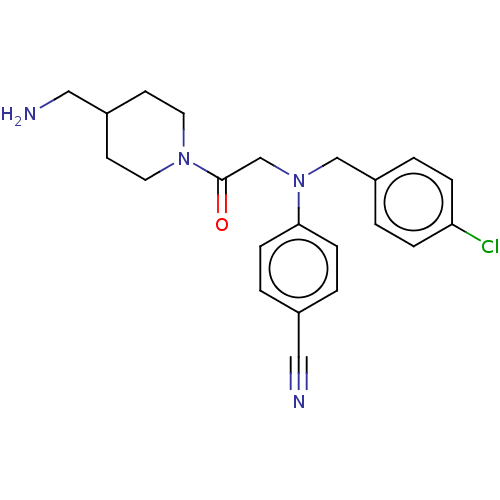
(CHEMBL4095517)Show SMILES NCC1CCN(CC1)C(=O)CN(Cc1ccc(Cl)cc1)c1ccc(cc1)C#N Show InChI InChI=1S/C22H25ClN4O/c23-20-5-1-19(2-6-20)15-27(21-7-3-17(13-24)4-8-21)16-22(28)26-11-9-18(14-25)10-12-26/h1-8,18H,9-12,14-16,25H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241520

(CHEMBL4064882)Show SMILES COc1cc(NS(=O)(=O)c2ccc3n(C)c(=O)c(C)cc3c2)ccc1-n1nc(C)cc1C Show InChI InChI=1S/C23H24N4O4S/c1-14-10-17-12-19(7-9-20(17)26(4)23(14)28)32(29,30)25-18-6-8-21(22(13-18)31-5)27-16(3)11-15(2)24-27/h6-13,25H,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241493

(CHEMBL4103264)Show SMILES Cn1ncc2cc(CN(CC(=O)N3CCC(N)CC3)c3ccc(cc3)C#N)ccc12 Show InChI InChI=1S/C23H26N6O/c1-27-22-7-4-18(12-19(22)14-26-27)15-29(21-5-2-17(13-24)3-6-21)16-23(30)28-10-8-20(25)9-11-28/h2-7,12,14,20H,8-11,15-16,25H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241495

(CHEMBL4079933)Show SMILES COc1ccc(CN(CC(=O)N2CCC(N)CC2)c2ccc(cc2)C#N)cc1F Show InChI InChI=1S/C22H25FN4O2/c1-29-21-7-4-17(12-20(21)23)14-27(19-5-2-16(13-24)3-6-19)15-22(28)26-10-8-18(25)9-11-26/h2-7,12,18H,8-11,14-15,25H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241534
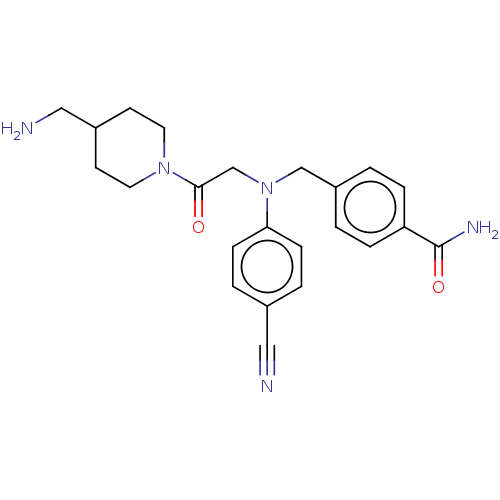
(CHEMBL4074550)Show SMILES NCC1CCN(CC1)C(=O)CN(Cc1ccc(cc1)C(N)=O)c1ccc(cc1)C#N Show InChI InChI=1S/C23H27N5O2/c24-13-17-3-7-21(8-4-17)28(15-19-1-5-20(6-2-19)23(26)30)16-22(29)27-11-9-18(14-25)10-12-27/h1-8,18H,9-12,14-16,25H2,(H2,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241523

(CHEMBL4084823)Show SMILES COc1ccc(CN(CC(=O)N2CCC(F)(CN)CC2)c2ccc(cc2)C#N)cc1F Show InChI InChI=1S/C23H26F2N4O2/c1-31-21-7-4-18(12-20(21)24)14-29(19-5-2-17(13-26)3-6-19)15-22(30)28-10-8-23(25,16-27)9-11-28/h2-7,12H,8-11,14-16,27H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241527

(CHEMBL4098894)Show SMILES NCC1CCN(CC1)C(=O)CN(Cc1cccc(c1)C(N)=O)c1ccc(cc1)C#N Show InChI InChI=1S/C23H27N5O2/c24-13-17-4-6-21(7-5-17)28(15-19-2-1-3-20(12-19)23(26)30)16-22(29)27-10-8-18(14-25)9-11-27/h1-7,12,18H,8-11,14-16,25H2,(H2,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241528
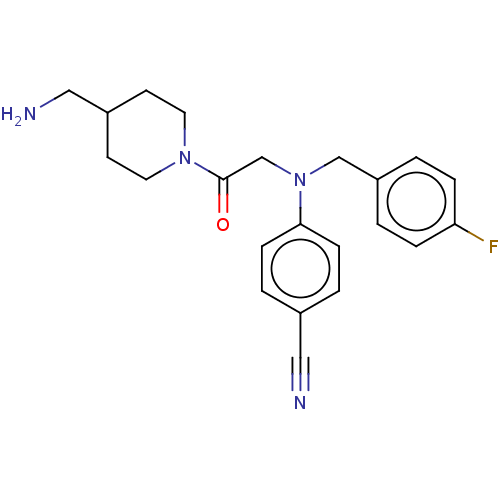
(CHEMBL4071901)Show SMILES NCC1CCN(CC1)C(=O)CN(Cc1ccc(F)cc1)c1ccc(cc1)C#N Show InChI InChI=1S/C22H25FN4O/c23-20-5-1-19(2-6-20)15-27(21-7-3-17(13-24)4-8-21)16-22(28)26-11-9-18(14-25)10-12-26/h1-8,18H,9-12,14-16,25H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241497

(CHEMBL4087810)Show SMILES Cc1ccc(CN(CC(=O)N2CCC(N)CC2)c2ccc(cc2)C#N)cc1 Show InChI InChI=1S/C22H26N4O/c1-17-2-4-19(5-3-17)15-26(21-8-6-18(14-23)7-9-21)16-22(27)25-12-10-20(24)11-13-25/h2-9,20H,10-13,15-16,24H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241519
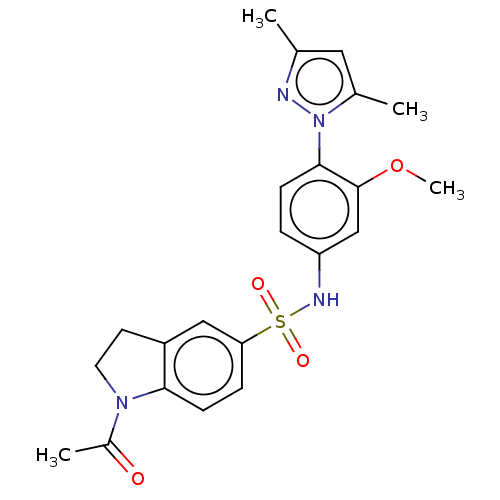
(CHEMBL4073481)Show SMILES COc1cc(NS(=O)(=O)c2ccc3N(CCc3c2)C(C)=O)ccc1-n1nc(C)cc1C Show InChI InChI=1S/C22H24N4O4S/c1-14-11-15(2)26(23-14)21-7-5-18(13-22(21)30-4)24-31(28,29)19-6-8-20-17(12-19)9-10-25(20)16(3)27/h5-8,11-13,24H,9-10H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50241539

(CHEMBL4084845)Show SMILES Cc1ccc(cc1)-c1ncc(OC[C@H]2CCNC2)cc1-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C24H23N3O/c1-17-2-6-21(7-3-17)24-23(20-8-4-18(13-25)5-9-20)12-22(15-27-24)28-16-19-10-11-26-14-19/h2-9,12,15,19,26H,10-11,14,16H2,1H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human hERG by patch clamp method |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241494

(CHEMBL4079000)Show InChI InChI=1S/C21H24N4O/c22-14-17-6-8-20(9-7-17)25(15-18-4-2-1-3-5-18)16-21(26)24-12-10-19(23)11-13-24/h1-9,19H,10-13,15-16,23H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241492
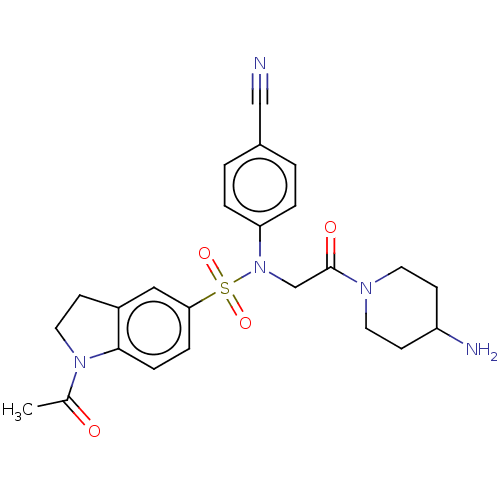
(CHEMBL4063384)Show SMILES CC(=O)N1CCc2cc(ccc12)S(=O)(=O)N(CC(=O)N1CCC(N)CC1)c1ccc(cc1)C#N Show InChI InChI=1S/C24H27N5O4S/c1-17(30)28-13-8-19-14-22(6-7-23(19)28)34(32,33)29(21-4-2-18(15-25)3-5-21)16-24(31)27-11-9-20(26)10-12-27/h2-7,14,20H,8-13,16,26H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of [3H]3-beta-(p-fluorophenyl)tropane-2beta-carboxylic acid methyl ester binding to Dopamine transporter in rat striatal membranes. |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241502

(CHEMBL4093998)Show SMILES CC(=O)N1CCc2cc(ccc12)S(=O)(=O)N(CC(=O)N1CCC[C@@H](N)C1)c1ccc(cc1)C#N |r| Show InChI InChI=1S/C24H27N5O4S/c1-17(30)28-12-10-19-13-22(8-9-23(19)28)34(32,33)29(21-6-4-18(14-25)5-7-21)16-24(31)27-11-2-3-20(26)15-27/h4-9,13,20H,2-3,10-12,15-16,26H2,1H3/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of [3H]3-beta-(p-fluorophenyl)tropane-2beta-carboxylic acid methyl ester [3H]7a binding to Dopamine transporter of rat striatal membranes |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241507

(CHEMBL4083732)Show SMILES Cc1ccc(cc1)S(=O)(=O)N(CC(=O)N1CCC(C)(N)CC1)c1ccc(cc1)C#N Show InChI InChI=1S/C22H26N4O3S/c1-17-3-9-20(10-4-17)30(28,29)26(19-7-5-18(15-23)6-8-19)16-21(27)25-13-11-22(2,24)12-14-25/h3-10H,11-14,16,24H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241486
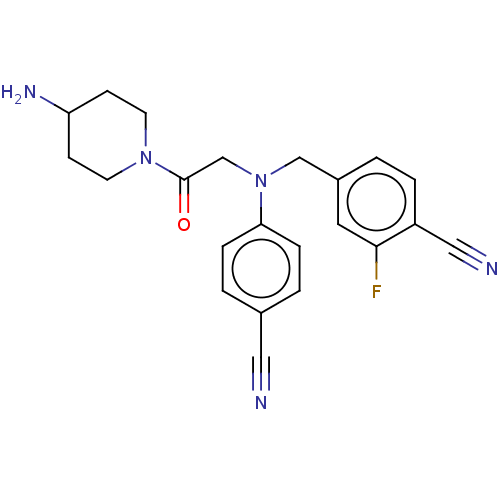
(CHEMBL4097953)Show SMILES NC1CCN(CC1)C(=O)CN(Cc1ccc(C#N)c(F)c1)c1ccc(cc1)C#N Show InChI InChI=1S/C22H22FN5O/c23-21-11-17(1-4-18(21)13-25)14-28(20-5-2-16(12-24)3-6-20)15-22(29)27-9-7-19(26)8-10-27/h1-6,11,19H,7-10,14-15,26H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241506
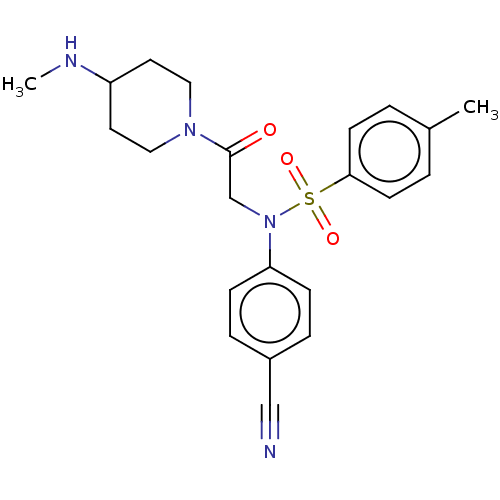
(CHEMBL4101729)Show SMILES CNC1CCN(CC1)C(=O)CN(c1ccc(cc1)C#N)S(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C22H26N4O3S/c1-17-3-9-21(10-4-17)30(28,29)26(20-7-5-18(15-23)6-8-20)16-22(27)25-13-11-19(24-2)12-14-25/h3-10,19,24H,11-14,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of [3H]3-beta-(p-fluorophenyl)tropane-2beta-carboxylic acid methyl ester [3H]7a binding to Dopamine transporter of rat striatal membranes |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241537
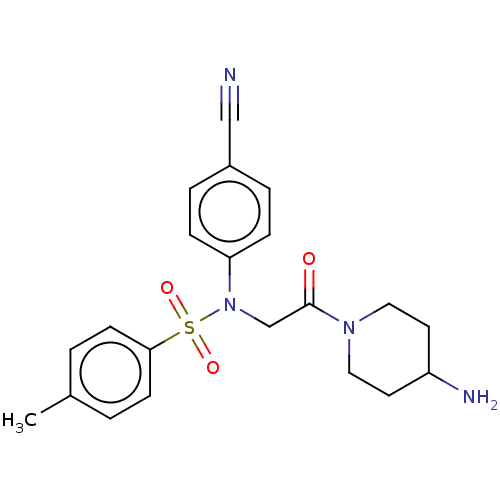
(CHEMBL4064746)Show SMILES Cc1ccc(cc1)S(=O)(=O)N(CC(=O)N1CCC(N)CC1)c1ccc(cc1)C#N Show InChI InChI=1S/C21H24N4O3S/c1-16-2-8-20(9-3-16)29(27,28)25(19-6-4-17(14-22)5-7-19)15-21(26)24-12-10-18(23)11-13-24/h2-9,18H,10-13,15,23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241517
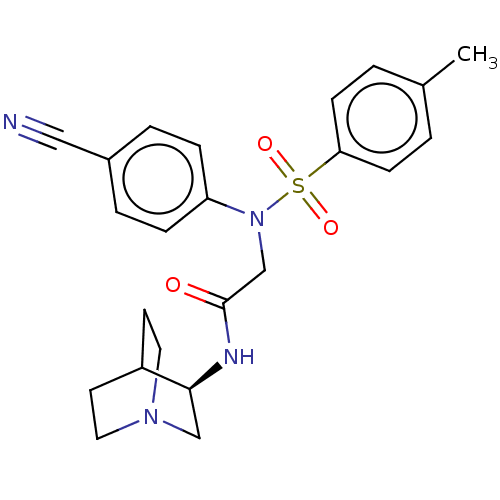
(CHEMBL4072551)Show SMILES Cc1ccc(cc1)S(=O)(=O)N(CC(=O)N[C@H]1CN2CCC1CC2)c1ccc(cc1)C#N |r,wD:15.15,(17.25,-37.07,;15.92,-37.85,;15.92,-39.39,;14.6,-40.16,;13.27,-39.39,;13.25,-37.86,;14.58,-37.08,;11.93,-40.16,;12.69,-41.49,;11.16,-41.48,;10.6,-39.4,;10.59,-37.86,;9.25,-37.09,;7.92,-37.87,;9.25,-35.55,;10.58,-34.78,;11.92,-35.54,;13.25,-34.77,;13.25,-33.23,;11.91,-32.46,;10.57,-33.24,;12.1,-33.23,;11.7,-34.72,;9.26,-40.17,;7.93,-39.41,;6.6,-40.18,;6.6,-41.72,;7.93,-42.5,;9.27,-41.72,;5.27,-42.5,;3.93,-43.27,)| Show InChI InChI=1S/C23H26N4O3S/c1-17-2-8-21(9-3-17)31(29,30)27(20-6-4-18(14-24)5-7-20)16-23(28)25-22-15-26-12-10-19(22)11-13-26/h2-9,19,22H,10-13,15-16H2,1H3,(H,25,28)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50241526

(CHEMBL4095018)Show SMILES COc1ccc(CN(CC(=O)N2CCC(CN)CC2)c2ccc(cc2)C#N)cc1F Show InChI InChI=1S/C23H27FN4O2/c1-30-22-7-4-19(12-21(22)24)15-28(20-5-2-17(13-25)3-6-20)16-23(29)27-10-8-18(14-26)9-11-27/h2-7,12,18H,8-11,14-16,26H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human hERG by patch clamp method |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241505
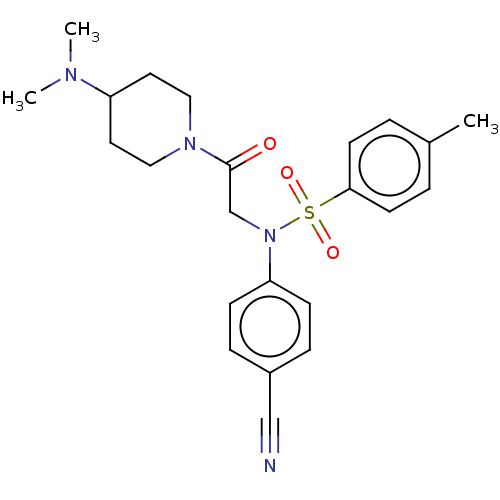
(CHEMBL4073334)Show SMILES CN(C)C1CCN(CC1)C(=O)CN(c1ccc(cc1)C#N)S(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C23H28N4O3S/c1-18-4-10-22(11-5-18)31(29,30)27(21-8-6-19(16-24)7-9-21)17-23(28)26-14-12-20(13-15-26)25(2)3/h4-11,20H,12-15,17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241516

(CHEMBL4083030)Show SMILES [H][C@]12CN(C(=O)CN(c3ccc(cc3)C#N)S(=O)(=O)c3ccc(C)cc3)[C@]([H])(CN1)C2 |r| Show InChI InChI=1S/C21H22N4O3S/c1-15-2-8-20(9-3-15)29(27,28)25(18-6-4-16(11-22)5-7-18)14-21(26)24-13-17-10-19(24)12-23-17/h2-9,17,19,23H,10,12-14H2,1H3/t17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241511
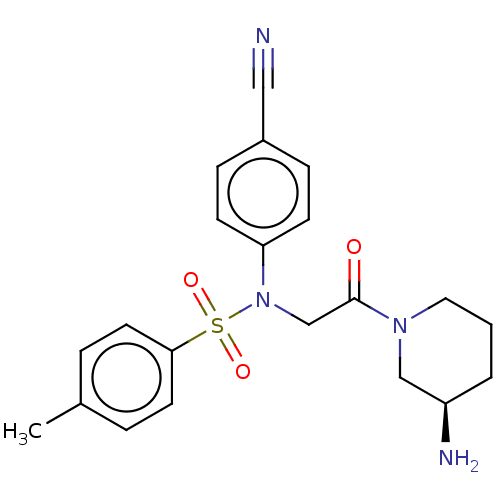
(CHEMBL4097636)Show SMILES Cc1ccc(cc1)S(=O)(=O)N(CC(=O)N1CCC[C@@H](N)C1)c1ccc(cc1)C#N |r| Show InChI InChI=1S/C21H24N4O3S/c1-16-4-10-20(11-5-16)29(27,28)25(19-8-6-17(13-22)7-9-19)15-21(26)24-12-2-3-18(23)14-24/h4-11,18H,2-3,12,14-15,23H2,1H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241538
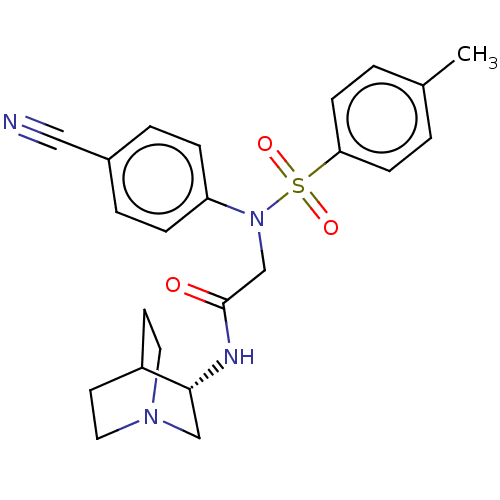
(CHEMBL4101011)Show SMILES Cc1ccc(cc1)S(=O)(=O)N(CC(=O)N[C@@H]1CN2CCC1CC2)c1ccc(cc1)C#N |r,wU:15.15,(36.24,-5.68,;34.91,-6.46,;34.91,-8,;33.59,-8.77,;32.26,-8,;32.25,-6.47,;33.57,-5.69,;30.92,-8.77,;31.68,-10.1,;30.15,-10.09,;29.59,-8.01,;29.58,-6.47,;28.24,-5.7,;26.91,-6.48,;28.24,-4.16,;29.57,-3.39,;30.91,-4.15,;32.24,-3.38,;32.24,-1.84,;30.9,-1.07,;29.56,-1.85,;31.09,-1.84,;30.69,-3.33,;28.26,-8.78,;26.92,-8.02,;25.59,-8.79,;25.59,-10.34,;26.92,-11.11,;28.26,-10.33,;24.26,-11.11,;22.93,-11.88,)| Show InChI InChI=1S/C23H26N4O3S/c1-17-2-8-21(9-3-17)31(29,30)27(20-6-4-18(14-24)5-7-20)16-23(28)25-22-15-26-12-10-19(22)11-13-26/h2-9,19,22H,10-13,15-16H2,1H3,(H,25,28)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241509

(CHEMBL4086227)Show SMILES Cc1ccc(cc1)S(=O)(=O)N(CC(=O)NC1CCNCC1)c1ccc(cc1)C#N Show InChI InChI=1S/C21H24N4O3S/c1-16-2-8-20(9-3-16)29(27,28)25(19-6-4-17(14-22)5-7-19)15-21(26)24-18-10-12-23-13-11-18/h2-9,18,23H,10-13,15H2,1H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241536
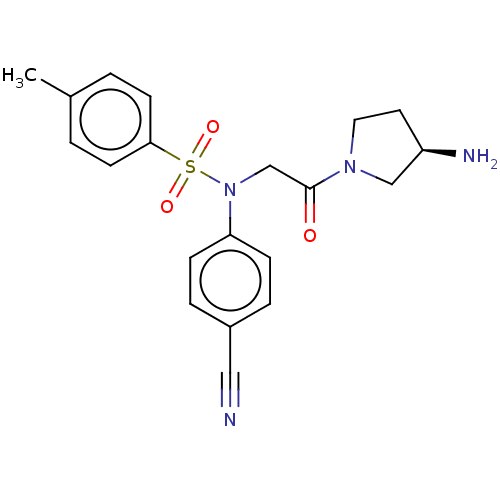
(CHEMBL4078362)Show SMILES Cc1ccc(cc1)S(=O)(=O)N(CC(=O)N1CC[C@@H](N)C1)c1ccc(cc1)C#N |r| Show InChI InChI=1S/C20H22N4O3S/c1-15-2-8-19(9-3-15)28(26,27)24(18-6-4-16(12-21)5-7-18)14-20(25)23-11-10-17(22)13-23/h2-9,17H,10-11,13-14,22H2,1H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50241533

(CHEMBL4102775)Show SMILES COc1ccc(CN(CC(=O)N2CCC3(CNC3)CC2)c2ccc(cc2)C#N)cc1F Show InChI InChI=1S/C24H27FN4O2/c1-31-22-7-4-19(12-21(22)25)14-29(20-5-2-18(13-26)3-6-20)15-23(30)28-10-8-24(9-11-28)16-27-17-24/h2-7,12,27H,8-11,14-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human hERG by patch clamp method |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241508

(CHEMBL4102632)Show SMILES Cc1ccc(cc1)S(=O)(=O)N(CC(=O)N1CC[C@H](N)C1)c1ccc(cc1)C#N |r| Show InChI InChI=1S/C20H22N4O3S/c1-15-2-8-19(9-3-15)28(26,27)24(18-6-4-16(12-21)5-7-18)14-20(25)23-11-10-17(22)13-23/h2-9,17H,10-11,13-14,22H2,1H3/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241510
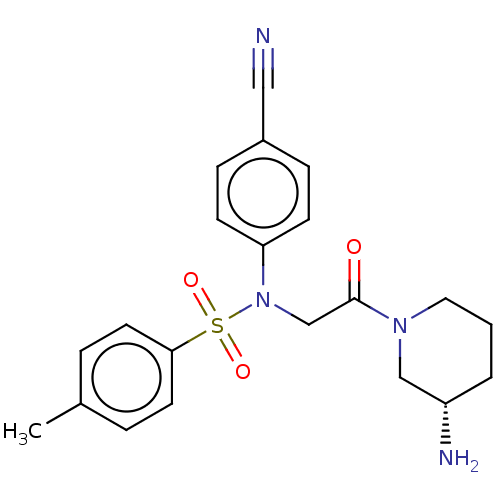
(CHEMBL4079604)Show SMILES Cc1ccc(cc1)S(=O)(=O)N(CC(=O)N1CCC[C@H](N)C1)c1ccc(cc1)C#N |r| Show InChI InChI=1S/C21H24N4O3S/c1-16-4-10-20(11-5-16)29(27,28)25(19-8-6-17(13-22)7-9-19)15-21(26)24-12-2-3-18(23)14-24/h4-11,18H,2-3,12,14-15,23H2,1H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50241495

(CHEMBL4079933)Show SMILES COc1ccc(CN(CC(=O)N2CCC(N)CC2)c2ccc(cc2)C#N)cc1F Show InChI InChI=1S/C22H25FN4O2/c1-29-21-7-4-17(12-20(21)23)14-27(19-5-2-16(13-24)3-6-19)15-22(28)26-10-8-18(25)9-11-26/h2-7,12,18H,8-11,14-15,25H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human hERG by patch clamp method |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241498
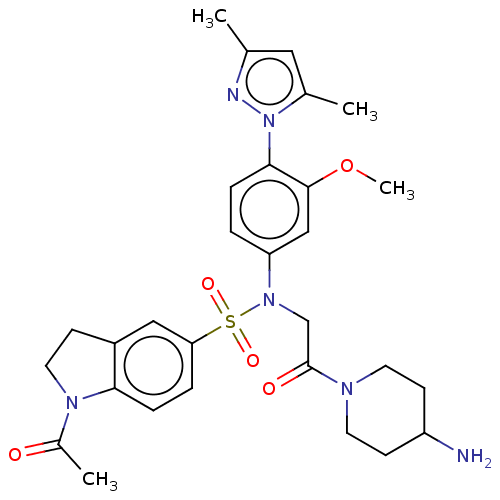
(CHEMBL4059468)Show SMILES COc1cc(ccc1-n1nc(C)cc1C)N(CC(=O)N1CCC(N)CC1)S(=O)(=O)c1ccc2N(CCc2c1)C(C)=O Show InChI InChI=1S/C29H36N6O5S/c1-19-15-20(2)35(31-19)27-7-5-24(17-28(27)40-4)34(18-29(37)32-12-10-23(30)11-13-32)41(38,39)25-6-8-26-22(16-25)9-14-33(26)21(3)36/h5-8,15-17,23H,9-14,18,30H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241500

(CHEMBL4077134)Show SMILES Cn1cc2cc(ccc2n1)N(CC(=O)N1CCC[C@@H](N)C1)S(=O)(=O)c1ccc(cc1)C#N |r| Show InChI InChI=1S/C22H24N6O3S/c1-26-13-17-11-19(6-9-21(17)25-26)28(15-22(29)27-10-2-3-18(24)14-27)32(30,31)20-7-4-16(12-23)5-8-20/h4-9,11,13,18H,2-3,10,14-15,24H2,1H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241522
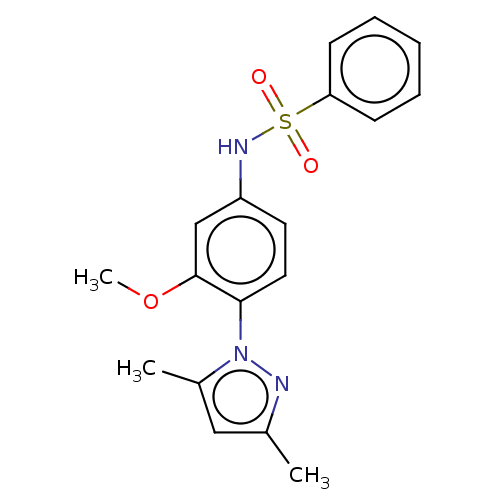
(CHEMBL1607608)Show InChI InChI=1S/C18H19N3O3S/c1-13-11-14(2)21(19-13)17-10-9-15(12-18(17)24-3)20-25(22,23)16-7-5-4-6-8-16/h4-12,20H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241518
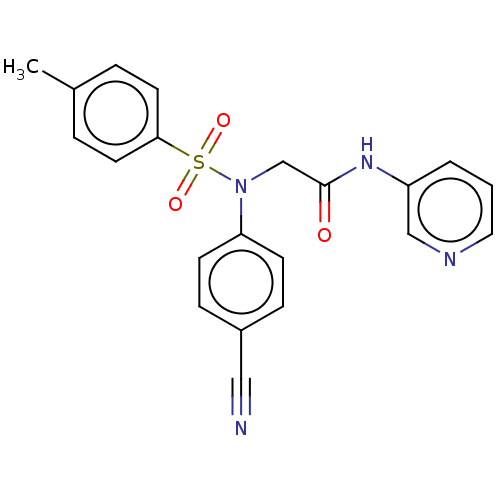
(CHEMBL4093280)Show SMILES Cc1ccc(cc1)S(=O)(=O)N(CC(=O)Nc1cccnc1)c1ccc(cc1)C#N Show InChI InChI=1S/C21H18N4O3S/c1-16-4-10-20(11-5-16)29(27,28)25(19-8-6-17(13-22)7-9-19)15-21(26)24-18-3-2-12-23-14-18/h2-12,14H,15H2,1H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal truncated human LSD1 (151 to 852 residues) expressed in Escherichia coli after 30 mins using histone H3(1-21)K4(Me1) biotin ... |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241486

(CHEMBL4097953)Show SMILES NC1CCN(CC1)C(=O)CN(Cc1ccc(C#N)c(F)c1)c1ccc(cc1)C#N Show InChI InChI=1S/C22H22FN5O/c23-21-11-17(1-4-18(21)13-25)14-28(20-5-2-16(12-24)3-6-20)15-22(29)27-9-7-19(26)8-10-27/h1-6,11,19H,7-10,14-15,26H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241492

(CHEMBL4063384)Show SMILES CC(=O)N1CCc2cc(ccc12)S(=O)(=O)N(CC(=O)N1CCC(N)CC1)c1ccc(cc1)C#N Show InChI InChI=1S/C24H27N5O4S/c1-17(30)28-13-8-19-14-22(6-7-23(19)28)34(32,33)29(21-4-2-18(15-25)3-5-21)16-24(31)27-11-9-20(26)10-12-27/h2-7,14,20H,8-13,16,26H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241505

(CHEMBL4073334)Show SMILES CN(C)C1CCN(CC1)C(=O)CN(c1ccc(cc1)C#N)S(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C23H28N4O3S/c1-18-4-10-22(11-5-18)31(29,30)27(21-8-6-19(16-24)7-9-21)17-23(28)26-14-12-20(13-15-26)25(2)3/h4-11,20H,12-15,17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241506

(CHEMBL4101729)Show SMILES CNC1CCN(CC1)C(=O)CN(c1ccc(cc1)C#N)S(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C22H26N4O3S/c1-17-3-9-21(10-4-17)30(28,29)26(20-7-5-18(15-23)6-8-20)16-22(27)25-13-11-19(24-2)12-14-25/h3-10,19,24H,11-14,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241507

(CHEMBL4083732)Show SMILES Cc1ccc(cc1)S(=O)(=O)N(CC(=O)N1CCC(C)(N)CC1)c1ccc(cc1)C#N Show InChI InChI=1S/C22H26N4O3S/c1-17-3-9-20(10-4-17)30(28,29)26(19-7-5-18(15-23)6-8-19)16-21(27)25-13-11-22(2,24)12-14-25/h3-10H,11-14,16,24H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241508

(CHEMBL4102632)Show SMILES Cc1ccc(cc1)S(=O)(=O)N(CC(=O)N1CC[C@H](N)C1)c1ccc(cc1)C#N |r| Show InChI InChI=1S/C20H22N4O3S/c1-15-2-8-19(9-3-15)28(26,27)24(18-6-4-16(12-21)5-7-18)14-20(25)23-11-10-17(22)13-23/h2-9,17H,10-11,13-14,22H2,1H3/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241509

(CHEMBL4086227)Show SMILES Cc1ccc(cc1)S(=O)(=O)N(CC(=O)NC1CCNCC1)c1ccc(cc1)C#N Show InChI InChI=1S/C21H24N4O3S/c1-16-2-8-20(9-3-16)29(27,28)25(19-6-4-17(14-22)5-7-19)15-21(26)24-18-10-12-23-13-11-18/h2-9,18,23H,10-13,15H2,1H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241510

(CHEMBL4079604)Show SMILES Cc1ccc(cc1)S(=O)(=O)N(CC(=O)N1CCC[C@H](N)C1)c1ccc(cc1)C#N |r| Show InChI InChI=1S/C21H24N4O3S/c1-16-4-10-20(11-5-16)29(27,28)25(19-8-6-17(13-22)7-9-19)15-21(26)24-12-2-3-18(23)14-24/h4-11,18H,2-3,12,14-15,23H2,1H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241511

(CHEMBL4097636)Show SMILES Cc1ccc(cc1)S(=O)(=O)N(CC(=O)N1CCC[C@@H](N)C1)c1ccc(cc1)C#N |r| Show InChI InChI=1S/C21H24N4O3S/c1-16-4-10-20(11-5-16)29(27,28)25(19-8-6-17(13-22)7-9-19)15-21(26)24-12-2-3-18(23)14-24/h4-11,18H,2-3,12,14-15,23H2,1H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241516

(CHEMBL4083030)Show SMILES [H][C@]12CN(C(=O)CN(c3ccc(cc3)C#N)S(=O)(=O)c3ccc(C)cc3)[C@]([H])(CN1)C2 |r| Show InChI InChI=1S/C21H22N4O3S/c1-15-2-8-20(9-3-15)29(27,28)25(18-6-4-16(11-22)5-7-18)14-21(26)24-13-17-10-19(24)12-23-17/h2-9,17,19,23H,10,12-14H2,1H3/t17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding potency against Vasopressin V1 receptor in rat liver cells. |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241517

(CHEMBL4072551)Show SMILES Cc1ccc(cc1)S(=O)(=O)N(CC(=O)N[C@H]1CN2CCC1CC2)c1ccc(cc1)C#N |r,wD:15.15,(17.25,-37.07,;15.92,-37.85,;15.92,-39.39,;14.6,-40.16,;13.27,-39.39,;13.25,-37.86,;14.58,-37.08,;11.93,-40.16,;12.69,-41.49,;11.16,-41.48,;10.6,-39.4,;10.59,-37.86,;9.25,-37.09,;7.92,-37.87,;9.25,-35.55,;10.58,-34.78,;11.92,-35.54,;13.25,-34.77,;13.25,-33.23,;11.91,-32.46,;10.57,-33.24,;12.1,-33.23,;11.7,-34.72,;9.26,-40.17,;7.93,-39.41,;6.6,-40.18,;6.6,-41.72,;7.93,-42.5,;9.27,-41.72,;5.27,-42.5,;3.93,-43.27,)| Show InChI InChI=1S/C23H26N4O3S/c1-17-2-8-21(9-3-17)31(29,30)27(20-6-4-18(14-24)5-7-20)16-23(28)25-22-15-26-12-10-19(22)11-13-26/h2-9,19,22H,10-13,15-16H2,1H3,(H,25,28)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50241518

(CHEMBL4093280)Show SMILES Cc1ccc(cc1)S(=O)(=O)N(CC(=O)Nc1cccnc1)c1ccc(cc1)C#N Show InChI InChI=1S/C21H18N4O3S/c1-16-4-10-20(11-5-16)29(27,28)25(19-8-6-17(13-22)7-9-19)15-21(26)24-18-3-2-12-23-14-18/h2-12,14H,15H2,1H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Binding affinity to LSD1 (unknown origin) by SPR analysis |
J Med Chem 60: 7984-7999 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00462
BindingDB Entry DOI: 10.7270/Q2M32XX8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data