

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257135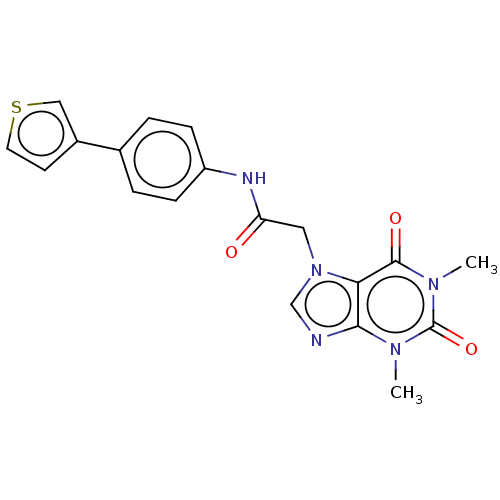 (CHEMBL4080208) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50133870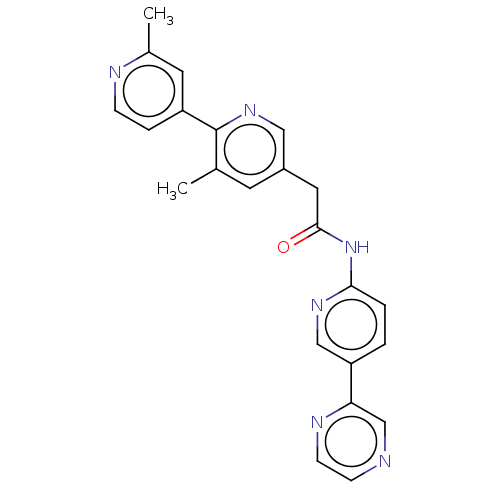 (CHEBI:78030 | CHEMBL3188386 | US10251893, Compound...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257160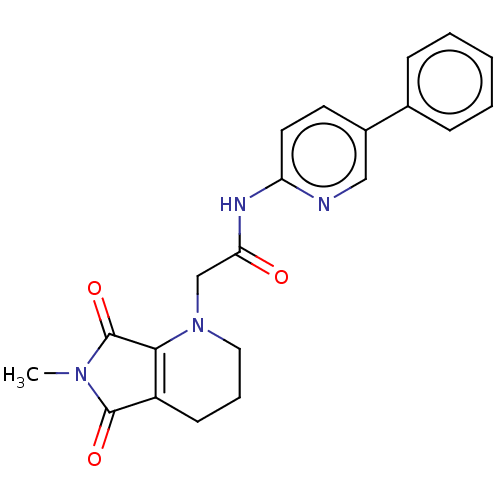 (CHEMBL4096728) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257143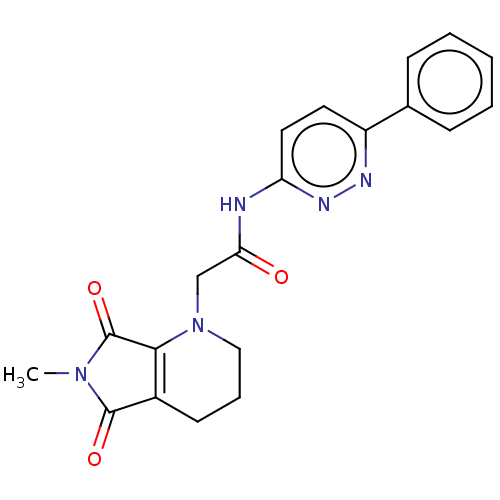 (CHEMBL4080021) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50133866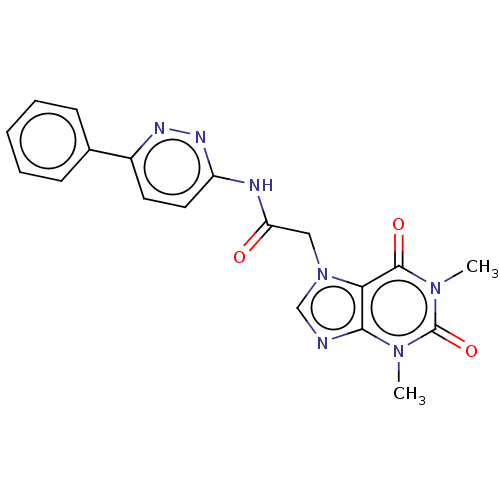 (CHEMBL3633802) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257141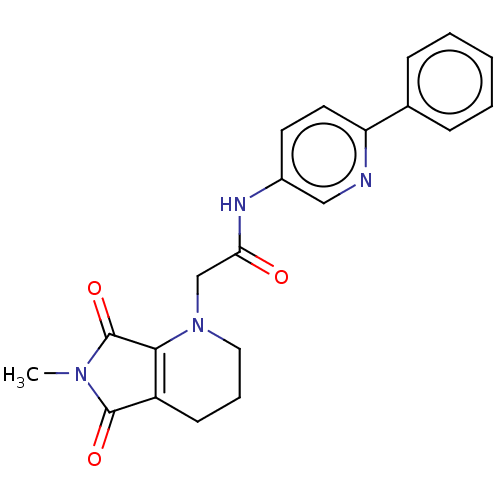 (CHEMBL4069295) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257139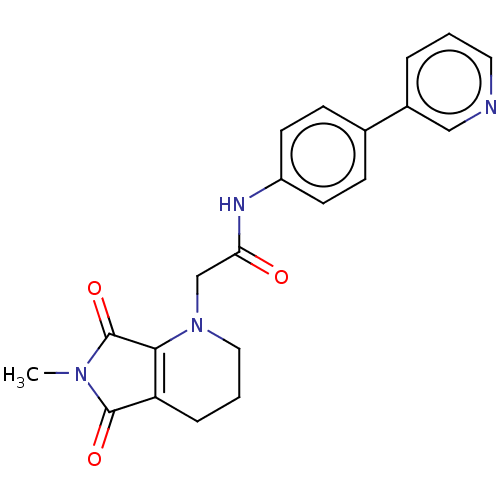 (CHEMBL4104447) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257144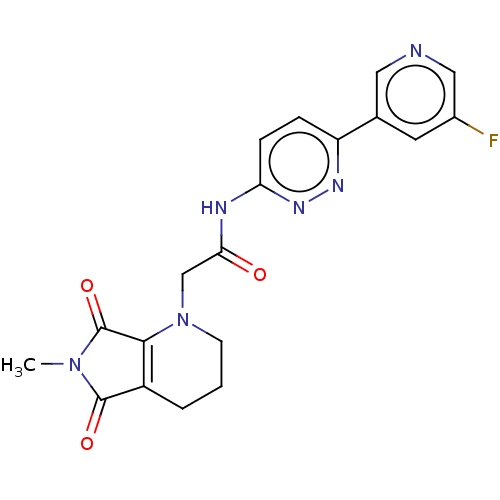 (CHEMBL4066589) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257142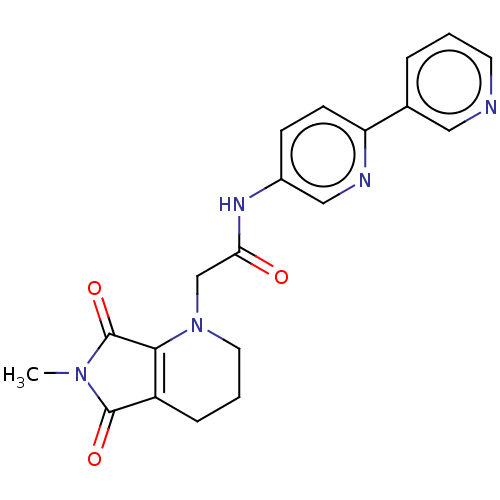 (CHEMBL4096529) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257145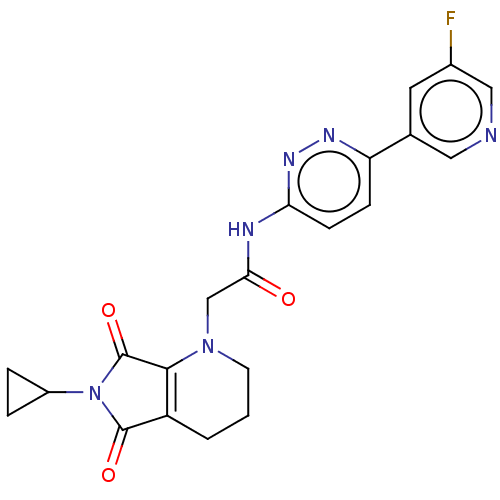 (CHEMBL4095609) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257157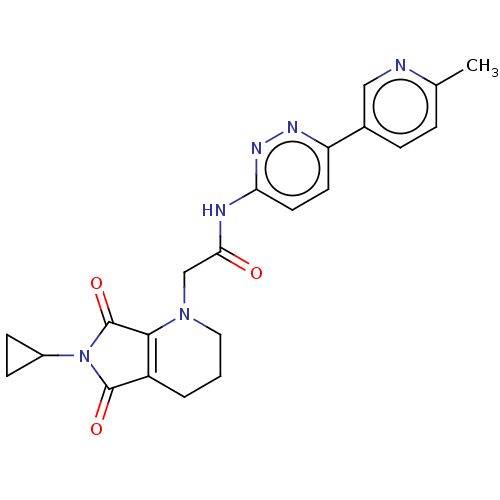 (CHEMBL4103356) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257138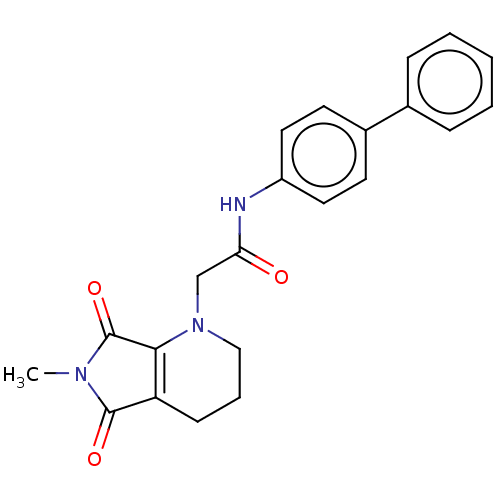 (CHEMBL4066796) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257128 (CHEMBL4087906) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257136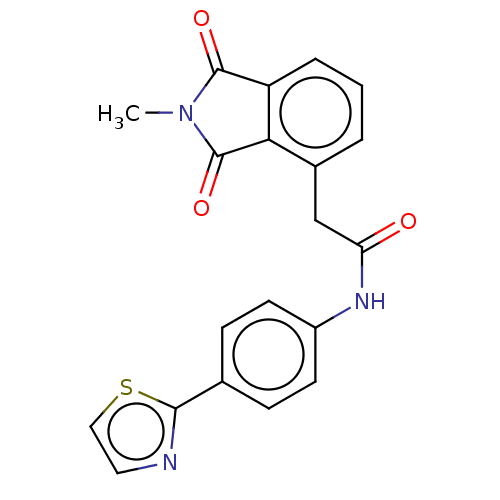 (CHEMBL4061069) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257137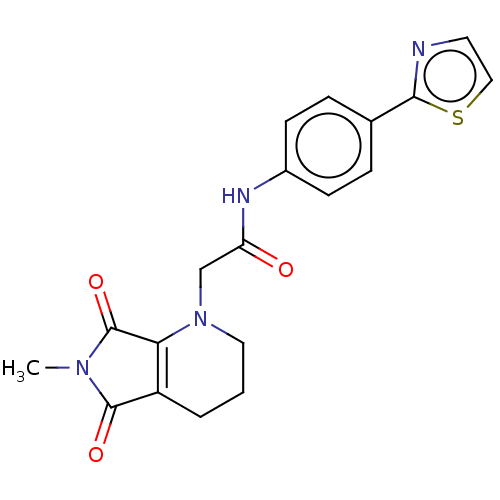 (CHEMBL4094309) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257134 (CHEMBL4066798) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257158 (CHEMBL4104257) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257161 (CHEMBL4075204) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257162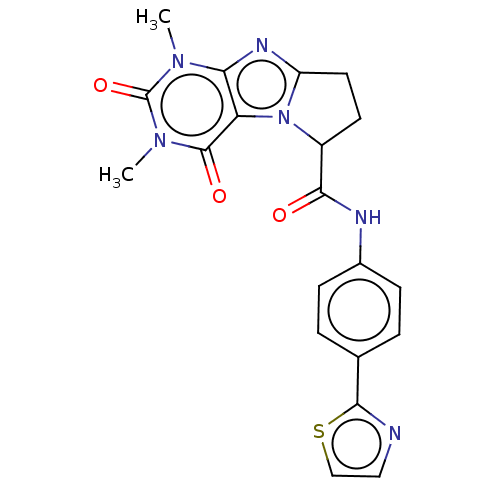 (CHEMBL4102043) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257129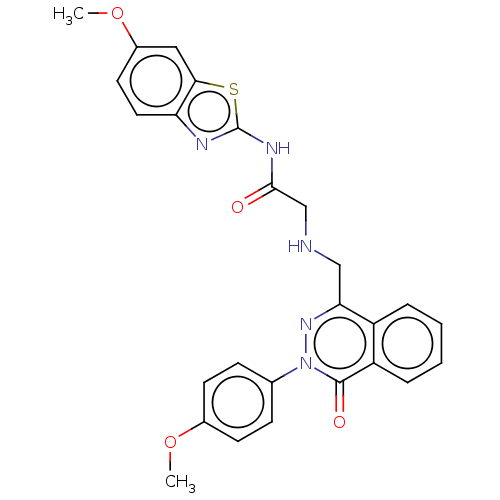 (CHEMBL4088085) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257159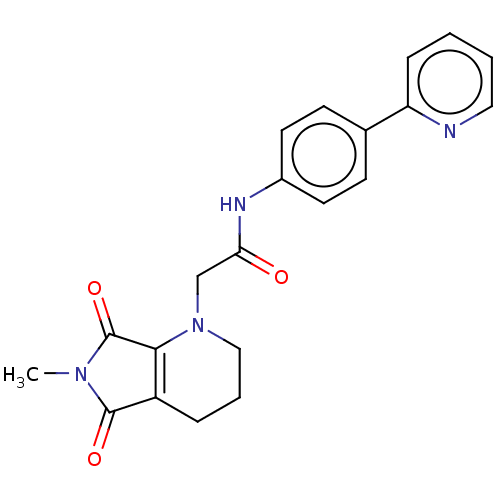 (CHEMBL4086549) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257140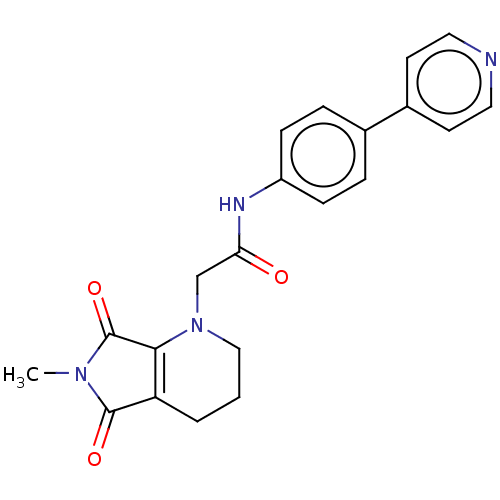 (CHEMBL4078705) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50257128 (CHEMBL4087906) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50257137 (CHEMBL4094309) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50257158 (CHEMBL4104257) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50257144 (CHEMBL4066589) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50257157 (CHEMBL4103356) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50257128 (CHEMBL4087906) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50257128 (CHEMBL4087906) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50257145 (CHEMBL4095609) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50257145 (CHEMBL4095609) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50257158 (CHEMBL4104257) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50257137 (CHEMBL4094309) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50257144 (CHEMBL4066589) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50257161 (CHEMBL4075204) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50257157 (CHEMBL4103356) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50257161 (CHEMBL4075204) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50257128 (CHEMBL4087906) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of human ERG by patch clamp assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity mitogen-activated protein kinase kinase 5 (Homo sapiens (Human)) | BDBM50257128 (CHEMBL4087906) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of partial length human MEK5 expressed in mammalian expression system by KINOMEscan assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase RIO2 (Homo sapiens (Human)) | BDBM50257128 (CHEMBL4087906) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of partial length human RIOK2 expressed in mammalian expression system by KINOMEscan assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||