 Found 36 hits Enz. Inhib. hit(s) with all data for entry = 50000768
Found 36 hits Enz. Inhib. hit(s) with all data for entry = 50000768 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50260267
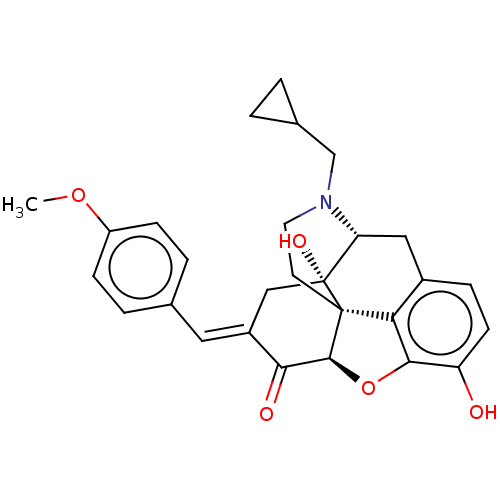
(CHEMBL2059382)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(OC)cc1)C2=O)ccc3O |r,TLB:32:5:9.14.15:17,THB:3:4:9.14.15:17| Show InChI InChI=1S/C28H29NO5/c1-33-20-7-4-16(5-8-20)12-19-14-28(32)22-13-18-6-9-21(30)25-23(18)27(28,26(34-25)24(19)31)10-11-29(22)15-17-2-3-17/h4-9,12,17,22,26,30,32H,2-3,10-11,13-15H2,1H3/b19-12+/t22-,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50260248
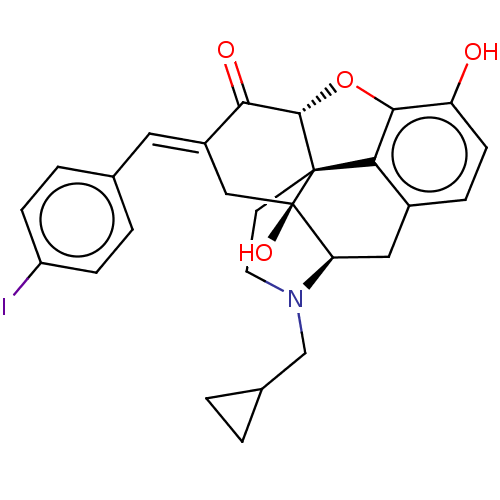
(CHEMBL4092119)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(I)cc1)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26INO4/c28-19-6-3-15(4-7-19)11-18-13-27(32)21-12-17-5-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-16-1-2-16/h3-8,11,16,21,25,30,32H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50454798
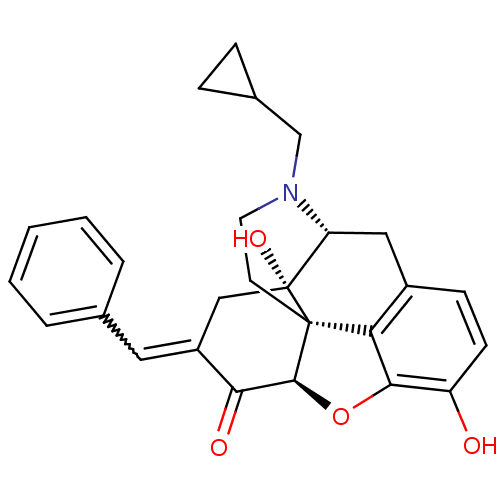
(7-Benzylidenenaltrexone | BNTX)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccccc1)C2=O)ccc3O |r,THB:10:9:17:6.5.4| Show InChI InChI=1S/C27H27NO4/c29-20-9-8-18-13-21-27(31)14-19(12-16-4-2-1-3-5-16)23(30)25-26(27,22(18)24(20)32-25)10-11-28(21)15-17-6-7-17/h1-5,8-9,12,17,21,25,29,31H,6-7,10-11,13-15H2/b19-12+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of Fibrinogen to thrombocyte alpha IIb beta-3 integrin |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50260257
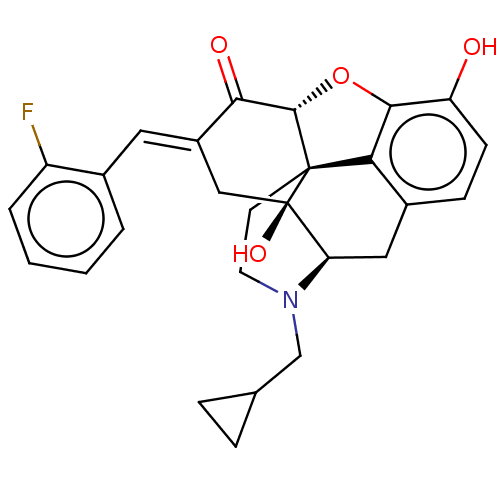
(CHEMBL4105306)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccccc1F)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26FNO4/c28-19-4-2-1-3-16(19)11-18-13-27(32)21-12-17-7-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-15-5-6-15/h1-4,7-8,11,15,21,25,30,32H,5-6,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50260244
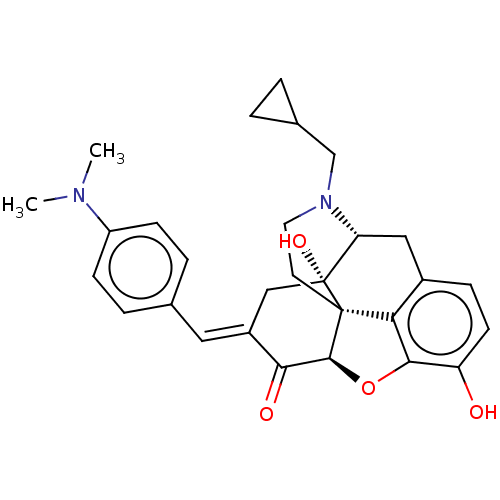
(CHEMBL3632690)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(cc1)N(C)C)C2=O)ccc3O |r,TLB:10:9:17:6.4.5| Show InChI InChI=1S/C29H32N2O4/c1-30(2)21-8-5-17(6-9-21)13-20-15-29(34)23-14-19-7-10-22(32)26-24(19)28(29,27(35-26)25(20)33)11-12-31(23)16-18-3-4-18/h5-10,13,18,23,27,32,34H,3-4,11-12,14-16H2,1-2H3/b20-13+/t23-,27+,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50260242
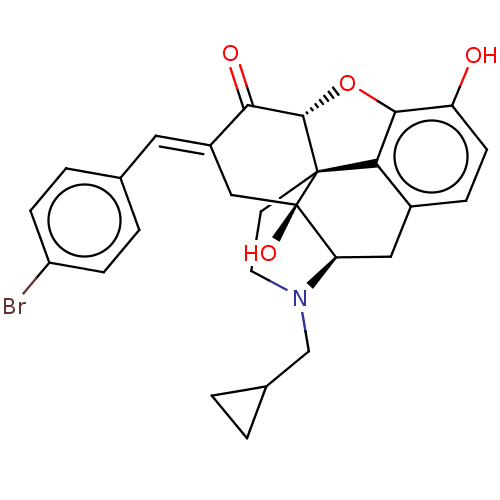
(CHEMBL4071324)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(Br)cc1)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26BrNO4/c28-19-6-3-15(4-7-19)11-18-13-27(32)21-12-17-5-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-16-1-2-16/h3-8,11,16,21,25,30,32H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50260242

(CHEMBL4071324)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(Br)cc1)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26BrNO4/c28-19-6-3-15(4-7-19)11-18-13-27(32)21-12-17-5-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-16-1-2-16/h3-8,11,16,21,25,30,32H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50260267

(CHEMBL2059382)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(OC)cc1)C2=O)ccc3O |r,TLB:32:5:9.14.15:17,THB:3:4:9.14.15:17| Show InChI InChI=1S/C28H29NO5/c1-33-20-7-4-16(5-8-20)12-19-14-28(32)22-13-18-6-9-21(30)25-23(18)27(28,26(34-25)24(19)31)10-11-29(22)15-17-2-3-17/h4-9,12,17,22,26,30,32H,2-3,10-11,13-15H2,1H3/b19-12+/t22-,26+,27+,28-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50260243

(CHEMBL4070277)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(Cl)cc1)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26ClNO4/c28-19-6-3-15(4-7-19)11-18-13-27(32)21-12-17-5-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-16-1-2-16/h3-8,11,16,21,25,30,32H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50260243

(CHEMBL4070277)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(Cl)cc1)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26ClNO4/c28-19-6-3-15(4-7-19)11-18-13-27(32)21-12-17-5-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-16-1-2-16/h3-8,11,16,21,25,30,32H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50260249

(CHEMBL4081053)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1cccc(F)c1)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26FNO4/c28-19-3-1-2-16(11-19)10-18-13-27(32)21-12-17-6-7-20(30)24-22(17)26(27,25(33-24)23(18)31)8-9-29(21)14-15-4-5-15/h1-3,6-7,10-11,15,21,25,30,32H,4-5,8-9,12-14H2/b18-10+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50454798

(7-Benzylidenenaltrexone | BNTX)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccccc1)C2=O)ccc3O |r,THB:10:9:17:6.5.4| Show InChI InChI=1S/C27H27NO4/c29-20-9-8-18-13-21-27(31)14-19(12-16-4-2-1-3-5-16)23(30)25-26(27,22(18)24(20)32-25)10-11-28(21)15-17-6-7-17/h1-5,8-9,12,17,21,25,29,31H,6-7,10-11,13-15H2/b19-12+/t21-,25+,26+,27-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50260249

(CHEMBL4081053)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1cccc(F)c1)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26FNO4/c28-19-3-1-2-16(11-19)10-18-13-27(32)21-12-17-6-7-20(30)24-22(17)26(27,25(33-24)23(18)31)8-9-29(21)14-15-4-5-15/h1-3,6-7,10-11,15,21,25,30,32H,4-5,8-9,12-14H2/b18-10+/t21-,25+,26+,27-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of Fibrinogen to thrombocyte alpha IIb beta-3 integrin |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50260257

(CHEMBL4105306)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccccc1F)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26FNO4/c28-19-4-2-1-3-16(19)11-18-13-27(32)21-12-17-7-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-15-5-6-15/h1-4,7-8,11,15,21,25,30,32H,5-6,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of Fibrinogen to thrombocyte alpha IIb beta-3 integrin |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50260244

(CHEMBL3632690)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(cc1)N(C)C)C2=O)ccc3O |r,TLB:10:9:17:6.4.5| Show InChI InChI=1S/C29H32N2O4/c1-30(2)21-8-5-17(6-9-21)13-20-15-29(34)23-14-19-7-10-22(32)26-24(19)28(29,27(35-26)25(20)33)11-12-31(23)16-18-3-4-18/h5-10,13,18,23,27,32,34H,3-4,11-12,14-16H2,1-2H3/b20-13+/t23-,27+,28+,29-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50260258

(CHEMBL4089193)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(cc1)C#N)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C28H26N2O4/c29-14-17-3-1-16(2-4-17)11-20-13-28(33)22-12-19-7-8-21(31)25-23(19)27(28,26(34-25)24(20)32)9-10-30(22)15-18-5-6-18/h1-4,7-8,11,18,22,26,31,33H,5-6,9-10,12-13,15H2/b20-11+/t22-,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50260248

(CHEMBL4092119)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(I)cc1)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26INO4/c28-19-6-3-15(4-7-19)11-18-13-27(32)21-12-17-5-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-16-1-2-16/h3-8,11,16,21,25,30,32H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50260247
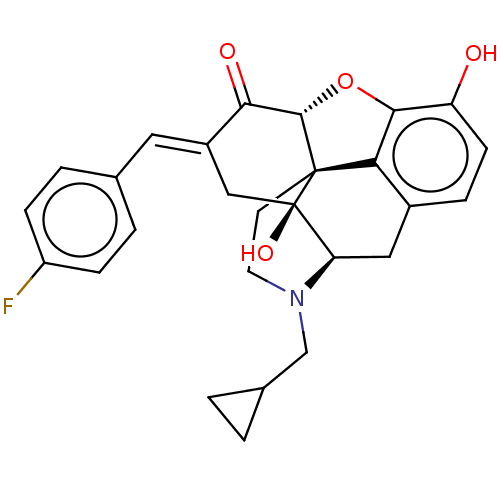
(CHEMBL2059381)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(F)cc1)C2=O)ccc3O |r,TLB:31:5:9.14.15:17,THB:3:4:9.14.15:17| Show InChI InChI=1S/C27H26FNO4/c28-19-6-3-15(4-7-19)11-18-13-27(32)21-12-17-5-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-16-1-2-16/h3-8,11,16,21,25,30,32H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of Fibrinogen to thrombocyte alpha IIb beta-3 integrin |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50260267

(CHEMBL2059382)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(OC)cc1)C2=O)ccc3O |r,TLB:32:5:9.14.15:17,THB:3:4:9.14.15:17| Show InChI InChI=1S/C28H29NO5/c1-33-20-7-4-16(5-8-20)12-19-14-28(32)22-13-18-6-9-21(30)25-23(18)27(28,26(34-25)24(19)31)10-11-29(22)15-17-2-3-17/h4-9,12,17,22,26,30,32H,2-3,10-11,13-15H2,1H3/b19-12+/t22-,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50260244

(CHEMBL3632690)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(cc1)N(C)C)C2=O)ccc3O |r,TLB:10:9:17:6.4.5| Show InChI InChI=1S/C29H32N2O4/c1-30(2)21-8-5-17(6-9-21)13-20-15-29(34)23-14-19-7-10-22(32)26-24(19)28(29,27(35-26)25(20)33)11-12-31(23)16-18-3-4-18/h5-10,13,18,23,27,32,34H,3-4,11-12,14-16H2,1-2H3/b20-13+/t23-,27+,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50260258

(CHEMBL4089193)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(cc1)C#N)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C28H26N2O4/c29-14-17-3-1-16(2-4-17)11-20-13-28(33)22-12-19-7-8-21(31)25-23(19)27(28,26(34-25)24(20)32)9-10-30(22)15-18-5-6-18/h1-4,7-8,11,18,22,26,31,33H,5-6,9-10,12-13,15H2/b20-11+/t22-,26+,27+,28-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50454798

(7-Benzylidenenaltrexone | BNTX)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccccc1)C2=O)ccc3O |r,THB:10:9:17:6.5.4| Show InChI InChI=1S/C27H27NO4/c29-20-9-8-18-13-21-27(31)14-19(12-16-4-2-1-3-5-16)23(30)25-26(27,22(18)24(20)32-25)10-11-28(21)15-17-6-7-17/h1-5,8-9,12,17,21,25,29,31H,6-7,10-11,13-15H2/b19-12+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50260257

(CHEMBL4105306)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccccc1F)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26FNO4/c28-19-4-2-1-3-16(19)11-18-13-27(32)21-12-17-7-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-15-5-6-15/h1-4,7-8,11,15,21,25,30,32H,5-6,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69,593 from human kappa opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50260247

(CHEMBL2059381)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(F)cc1)C2=O)ccc3O |r,TLB:31:5:9.14.15:17,THB:3:4:9.14.15:17| Show InChI InChI=1S/C27H26FNO4/c28-19-6-3-15(4-7-19)11-18-13-27(32)21-12-17-5-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-16-1-2-16/h3-8,11,16,21,25,30,32H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50260249

(CHEMBL4081053)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1cccc(F)c1)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26FNO4/c28-19-3-1-2-16(11-19)10-18-13-27(32)21-12-17-6-7-20(30)24-22(17)26(27,25(33-24)23(18)31)8-9-29(21)14-15-4-5-15/h1-3,6-7,10-11,15,21,25,30,32H,4-5,8-9,12-14H2/b18-10+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69,593 from human kappa opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50260242

(CHEMBL4071324)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(Br)cc1)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26BrNO4/c28-19-6-3-15(4-7-19)11-18-13-27(32)21-12-17-5-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-16-1-2-16/h3-8,11,16,21,25,30,32H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50260243

(CHEMBL4070277)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(Cl)cc1)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26ClNO4/c28-19-6-3-15(4-7-19)11-18-13-27(32)21-12-17-5-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-16-1-2-16/h3-8,11,16,21,25,30,32H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50260262
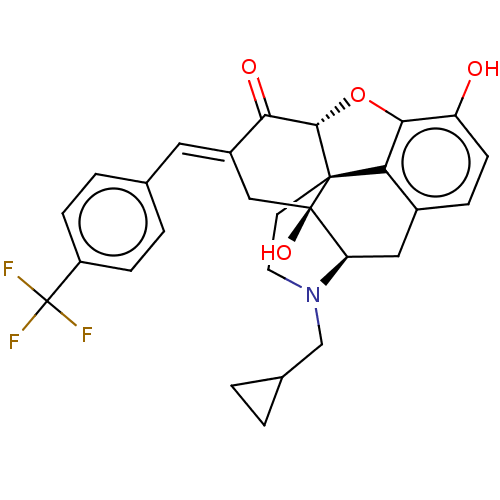
(CHEMBL4061882)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(cc1)C(F)(F)F)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C28H26F3NO4/c29-28(30,31)19-6-3-15(4-7-19)11-18-13-27(35)21-12-17-5-8-20(33)24-22(17)26(27,25(36-24)23(18)34)9-10-32(21)14-16-1-2-16/h3-8,11,16,21,25,33,35H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50260263

(CHEMBL4090365)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccccc1)C2=O)ccc3OC |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C28H29NO4/c1-32-21-10-9-19-14-22-28(31)15-20(13-17-5-3-2-4-6-17)24(30)26-27(28,23(19)25(21)33-26)11-12-29(22)16-18-7-8-18/h2-6,9-10,13,18,22,26,31H,7-8,11-12,14-16H2,1H3/b20-13+/t22-,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50260247

(CHEMBL2059381)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(F)cc1)C2=O)ccc3O |r,TLB:31:5:9.14.15:17,THB:3:4:9.14.15:17| Show InChI InChI=1S/C27H26FNO4/c28-19-6-3-15(4-7-19)11-18-13-27(32)21-12-17-5-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-16-1-2-16/h3-8,11,16,21,25,30,32H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 108 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69,593 from human kappa opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50260248

(CHEMBL4092119)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(I)cc1)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26INO4/c28-19-6-3-15(4-7-19)11-18-13-27(32)21-12-17-5-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-16-1-2-16/h3-8,11,16,21,25,30,32H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69,593 from human kappa opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50260262

(CHEMBL4061882)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(cc1)C(F)(F)F)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C28H26F3NO4/c29-28(30,31)19-6-3-15(4-7-19)11-18-13-27(35)21-12-17-5-8-20(33)24-22(17)26(27,25(36-24)23(18)34)9-10-32(21)14-16-1-2-16/h3-8,11,16,21,25,33,35H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 176 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69,593 from human kappa opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50260262

(CHEMBL4061882)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(cc1)C(F)(F)F)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C28H26F3NO4/c29-28(30,31)19-6-3-15(4-7-19)11-18-13-27(35)21-12-17-5-8-20(33)24-22(17)26(27,25(36-24)23(18)34)9-10-32(21)14-16-1-2-16/h3-8,11,16,21,25,33,35H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 192 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50260258

(CHEMBL4089193)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(cc1)C#N)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C28H26N2O4/c29-14-17-3-1-16(2-4-17)11-20-13-28(33)22-12-19-7-8-21(31)25-23(19)27(28,26(34-25)24(20)32)9-10-30(22)15-18-5-6-18/h1-4,7-8,11,18,22,26,31,33H,5-6,9-10,12-13,15H2/b20-11+/t22-,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69,593 from human kappa opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50260263

(CHEMBL4090365)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccccc1)C2=O)ccc3OC |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C28H29NO4/c1-32-21-10-9-19-14-22-28(31)15-20(13-17-5-3-2-4-6-17)24(30)26-27(28,23(19)25(21)33-26)11-12-29(22)16-18-7-8-18/h2-6,9-10,13,18,22,26,31H,7-8,11-12,14-16H2,1H3/b20-13+/t22-,26+,27+,28-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 499 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50260263

(CHEMBL4090365)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccccc1)C2=O)ccc3OC |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C28H29NO4/c1-32-21-10-9-19-14-22-28(31)15-20(13-17-5-3-2-4-6-17)24(30)26-27(28,23(19)25(21)33-26)11-12-29(22)16-18-7-8-18/h2-6,9-10,13,18,22,26,31H,7-8,11-12,14-16H2,1H3/b20-13+/t22-,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69,593 from human kappa opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data