 Found 46 hits Enz. Inhib. hit(s) with all data for entry = 50001503
Found 46 hits Enz. Inhib. hit(s) with all data for entry = 50001503 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50241662
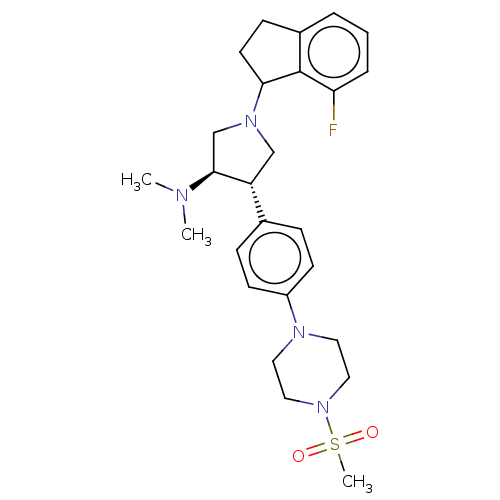
(CHEMBL4104741)Show SMILES CN(C)[C@H]1CN(C[C@@H]1c1ccc(cc1)N1CCN(CC1)S(C)(=O)=O)C1CCc2cccc(F)c12 |r| Show InChI InChI=1S/C26H35FN4O2S/c1-28(2)25-18-30(24-12-9-20-5-4-6-23(27)26(20)24)17-22(25)19-7-10-21(11-8-19)29-13-15-31(16-14-29)34(3,32)33/h4-8,10-11,22,24-25H,9,12-18H2,1-3H3/t22-,24?,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged EED (unknown origin) after 1 hr by OG(488) as probe-based TR-FRET analysis |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Lethal(3)malignant brain tumor-like protein 1
(Homo sapiens) | BDBM50282169
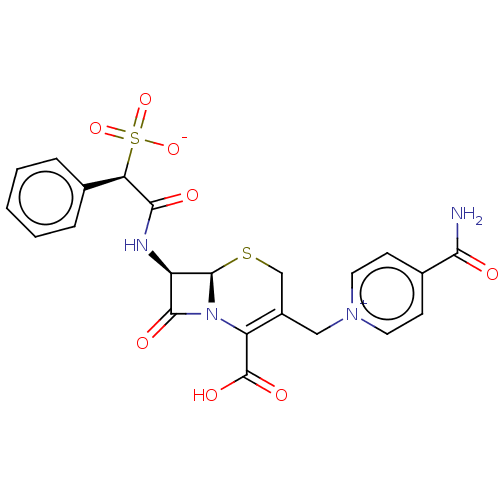
(CHEBI:3507 | Cefsulodin)Show SMILES [H][C@]12SCC(C[n+]3ccc(cc3)C(N)=O)=C(N1C(=O)[C@H]2NC(=O)[C@@H](c1ccccc1)S([O-])(=O)=O)C(O)=O |c:15| Show InChI InChI=1S/C22H20N4O8S2/c23-18(27)13-6-8-25(9-7-13)10-14-11-35-21-15(20(29)26(21)16(14)22(30)31)24-19(28)17(36(32,33)34)12-4-2-1-3-5-12/h1-9,15,17,21H,10-11H2,(H4-,23,24,27,28,30,31,32,33,34)/t15-,17-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of L3MBTL1 (unknown origin) by AlphaScreen assay |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Lethal(3)malignant brain tumor-like protein 1
(Homo sapiens) | BDBM60983

((E)-2-(3,4-dihydroxybenzoyl)-3-(4-hydroxy-3-iodo-5...)Show SMILES COc1cc(\C=C(/C#N)C(=O)c2ccc(O)c(O)c2)cc(I)c1O Show InChI InChI=1S/C17H12INO5/c1-24-15-6-9(5-12(18)17(15)23)4-11(8-19)16(22)10-2-3-13(20)14(21)7-10/h2-7,20-21,23H,1H3/b11-4+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 282 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of L3MBTL1 (unknown origin) by AlphaScreen assay |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50164787
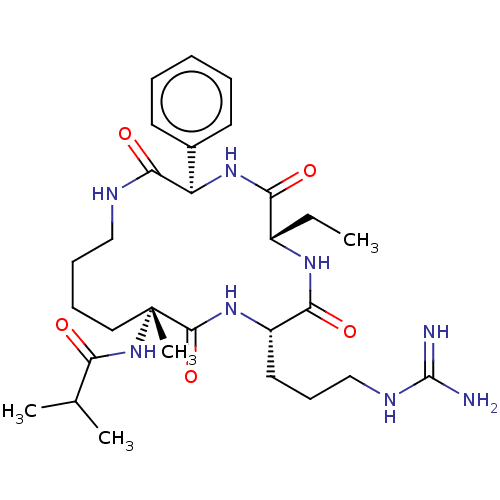
(CHEMBL3798088)Show SMILES CC[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@](C)(CCCCNC(=O)[C@H](NC1=O)c1ccccc1)NC(=O)C(C)C |r| Show InChI InChI=1S/C29H46N8O5/c1-5-20-24(39)36-22(19-12-7-6-8-13-19)26(41)32-16-10-9-15-29(4,37-23(38)18(2)3)27(42)35-21(25(40)34-20)14-11-17-33-28(30)31/h6-8,12-13,18,20-22H,5,9-11,14-17H2,1-4H3,(H,32,41)(H,34,40)(H,35,42)(H,36,39)(H,37,38)(H4,30,31,33)/t20-,21-,22+,29+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of recombinant SUMO-tagged MLL1 (unknown origin) by histone methyl transferase assay |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50282161
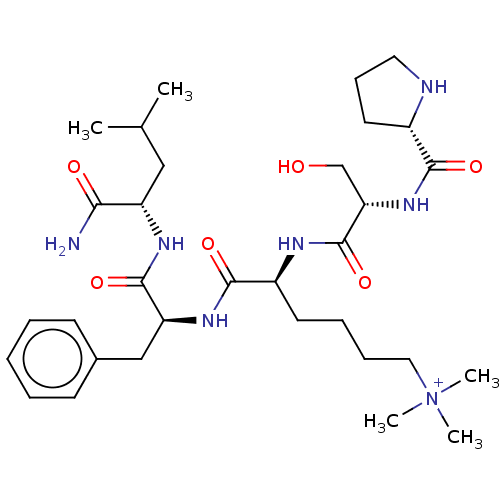
(CHEMBL4175336)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCC[N+](C)(C)C)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1)C(N)=O |r| Show InChI InChI=1S/C32H53N7O6/c1-21(2)18-25(28(33)41)36-31(44)26(19-22-12-7-6-8-13-22)37-30(43)24(14-9-10-17-39(3,4)5)35-32(45)27(20-40)38-29(42)23-15-11-16-34-23/h6-8,12-13,21,23-27,34,40H,9-11,14-20H2,1-5H3,(H5-,33,35,36,37,38,41,42,43,44,45)/p+1/t23-,24-,25-,26-,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of full length N-terminal His-tagged EED (unknown origin) expressed in Rosetta2 BL21(DE3)pLysS cells after 30 mins by 3-FAM labelled fluor... |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50282163
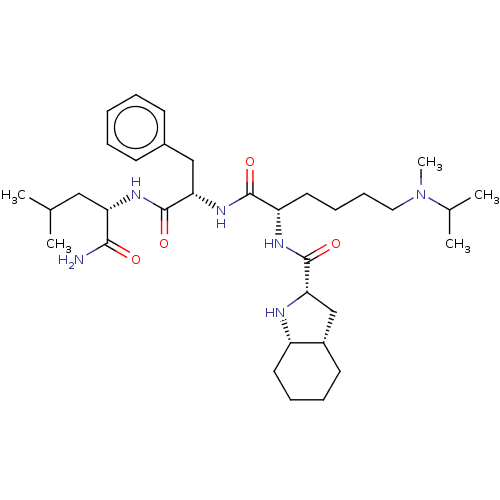
(CHEMBL4171889)Show SMILES [H][C@]12C[C@H](N[C@@]1([H])CCCC2)C(=O)N[C@@H](CCCCN(C)C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C34H56N6O4/c1-22(2)19-28(31(35)41)38-33(43)29(20-24-13-7-6-8-14-24)39-32(42)27(17-11-12-18-40(5)23(3)4)37-34(44)30-21-25-15-9-10-16-26(25)36-30/h6-8,13-14,22-23,25-30,36H,9-12,15-21H2,1-5H3,(H2,35,41)(H,37,44)(H,38,43)(H,39,42)/t25-,26-,27-,28-,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of full length N-terminal His-tagged EED (unknown origin) expressed in Rosetta2 BL21(DE3)pLysS cells after 30 mins by 3-FAM labelled fluor... |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50282157

(CHEMBL4161291)Show SMILES [H][C@]12C[C@H](N[C@@]1([H])CCCC2)C(=O)N[C@@H](CCCCN(C)C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCCCC1 |r| Show InChI InChI=1S/C33H53N5O3/c1-24(2)37(3)19-13-10-18-28(35-32(40)29-23-26-16-8-9-17-27(26)34-29)31(39)36-30(22-25-14-6-4-7-15-25)33(41)38-20-11-5-12-21-38/h4,6-7,14-15,24,26-30,34H,5,8-13,16-23H2,1-3H3,(H,35,40)(H,36,39)/t26-,27-,28-,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of full length N-terminal His-tagged EED (unknown origin) expressed in Rosetta2 BL21(DE3)pLysS cells after 30 mins by 3-FAM labelled fluor... |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Chromobox protein homolog 7
(Homo sapiens (Human)) | BDBM50179360

(CHEMBL3040216)Show SMILES CC1=CCC(=C\C1=N\C(=O)C1=CC=C\C(C1)=N/C(=O)/N=C1/CC(=CC=C1)C(=O)\N=C1\CC(=CC=C1C)C(=O)\N=C1/CC=C(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)C(=O)\N=C1/CC=C(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O |c:4,13,24,26,34,36,45,72,t:1,11| Show InChI InChI=1S/C51H40N6O23S6/c1-25-9-11-29(49(60)54-37-13-15-41(83(69,70)71)35-21-33(81(63,64)65)23-43(45(35)37)85(75,76)77)19-39(25)56-47(58)27-5-3-7-31(17-27)52-51(62)53-32-8-4-6-28(18-32)48(59)57-40-20-30(12-10-26(40)2)50(61)55-38-14-16-42(84(72,73)74)36-22-34(82(66,67)68)24-44(46(36)38)86(78,79)80/h3-11,15-16,20-24H,12-14,17-19H2,1-2H3,(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)/b52-31+,53-32+,54-37+,55-38+,56-39-,57-40- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of Cbx7 (unknown origin) using FITC-labeled SETDB1-K1170me3 peptide as probe by fluorescence polarization assay |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM50058655

(1,1',1'',1'''-[disulfanediylbis(carbonothioylnitri...)Show InChI InChI=1S/C10H20N2S4/c1-5-11(6-2)9(13)15-16-10(14)12(7-3)8-4/h5-8H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of JARID1A PHD finger domain (unknown origin) |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Chromobox protein homolog 7
(Homo sapiens (Human)) | BDBM50009454
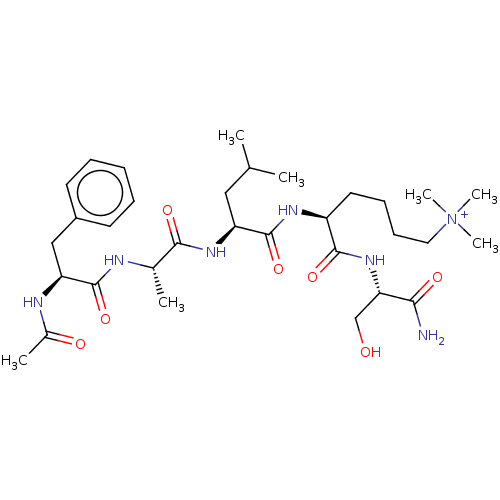
(CHEMBL3234136)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N[C@@H](CCCC[N+](C)(C)C)C(=O)N[C@@H](CO)C(N)=O |r| Show InChI InChI=1S/C32H53N7O7/c1-20(2)17-25(32(46)36-24(15-11-12-16-39(5,6)7)30(44)38-27(19-40)28(33)42)37-29(43)21(3)34-31(45)26(35-22(4)41)18-23-13-9-8-10-14-23/h8-10,13-14,20-21,24-27,40H,11-12,15-19H2,1-7H3,(H6-,33,34,35,36,37,38,41,42,43,44,45,46)/p+1/t21-,24-,25-,26-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of N terminally fluorescein-tagged H3K27me3 binding to N terminallyHis6-tagged human Cbx7 expressed in Escherichia coli BL21 (DE3) by fluo... |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
TP53-binding protein 1
(Homo sapiens (Human)) | BDBM154563
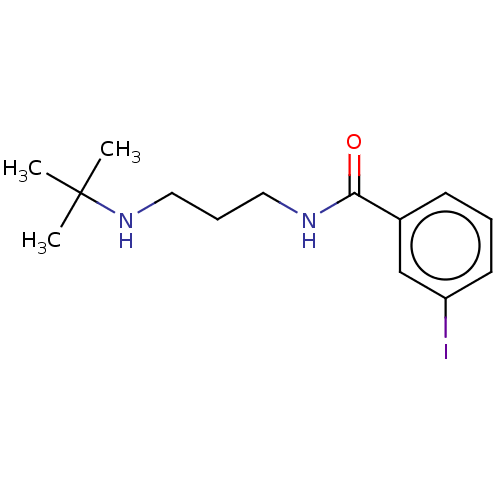
(N-(3-(Tert-butylamino)propyl)-3-iodobenzamide (19))Show InChI InChI=1S/C14H21IN2O/c1-14(2,3)17-9-5-8-16-13(18)11-6-4-7-12(15)10-11/h4,6-7,10,17H,5,8-9H2,1-3H3,(H,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of 53BP1 methyllysine binding domain (unknown origin) after 30 mins by AlphaScreen assay |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
TP53-binding protein 1
(Homo sapiens (Human)) | BDBM154564
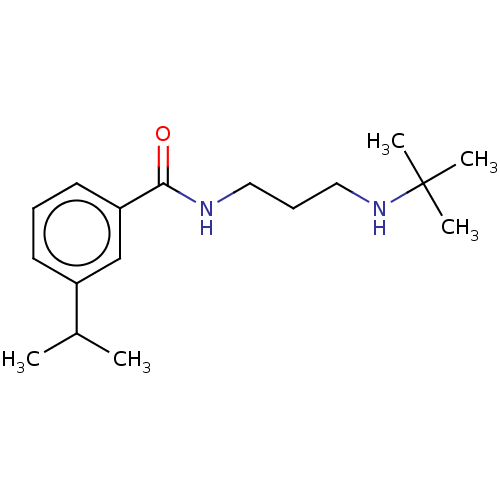
(N-(3-(Tert-butylamino)propyl)-3-isopropylbenzamide...)Show InChI InChI=1S/C17H28N2O/c1-13(2)14-8-6-9-15(12-14)16(20)18-10-7-11-19-17(3,4)5/h6,8-9,12-13,19H,7,10-11H2,1-5H3,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of 53BP1 methyllysine binding domain (unknown origin) after 30 mins by AlphaScreen assay |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
TP53-binding protein 1
(Homo sapiens (Human)) | BDBM154565
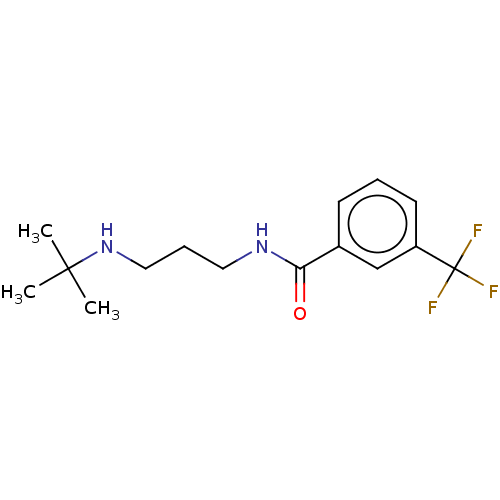
(N-(3-(Tert-butylamino)propyl)-3-(trifluoromethyl)b...)Show InChI InChI=1S/C15H21F3N2O/c1-14(2,3)20-9-5-8-19-13(21)11-6-4-7-12(10-11)15(16,17)18/h4,6-7,10,20H,5,8-9H2,1-3H3,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Potency at neuronal 5-hydroxytryptamine 3 receptor in the rabbit heart |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM92787

(WAG-003)Show SMILES CCCCc1oc2ccccc2c1C(=O)c1cc(I)c(OCC[N+](C)(C)C)c(I)c1 Show InChI InChI=1S/C24H28I2NO3/c1-5-6-10-21-22(17-9-7-8-11-20(17)30-21)23(28)16-14-18(25)24(19(26)15-16)29-13-12-27(2,3)4/h7-9,11,14-15H,5-6,10,12-13H2,1-4H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HQ5-Halo-tagged JARID1A PHD3 finger domain (unknown origin) expressed in BL21(DE3)pLysS cells after 1 hr by chemiluminescen... |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM92788

(WAG-005)Show SMILES CCCCc1oc2ccccc2c1C(=O)c1cc(I)c(OCCC[N+](C)(C)C)c(I)c1 Show InChI InChI=1S/C25H30I2NO3/c1-5-6-11-22-23(18-10-7-8-12-21(18)31-22)24(29)17-15-19(26)25(20(27)16-17)30-14-9-13-28(2,3)4/h7-8,10,12,15-16H,5-6,9,11,13-14H2,1-4H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HQ5-Halo-tagged JARID1A PHD3 finger domain (unknown origin) expressed in BL21(DE3)pLysS cells after 1 hr by chemiluminescen... |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
TP53-binding protein 1
(Homo sapiens (Human)) | BDBM154545
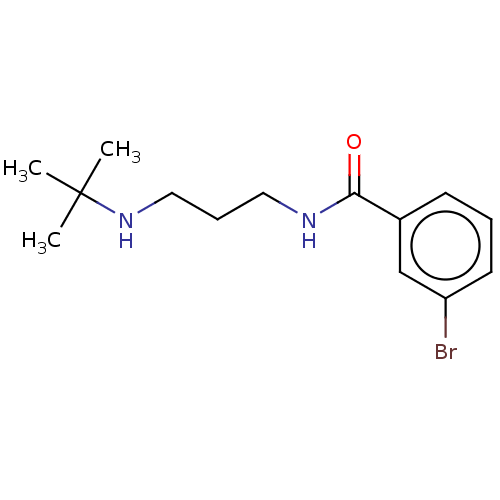
(3-Bromo-N-(3-(tert-butylamino)propyl)benzamide (UN...)Show InChI InChI=1S/C14H21BrN2O/c1-14(2,3)17-9-5-8-16-13(18)11-6-4-7-12(15)10-11/h4,6-7,10,17H,5,8-9H2,1-3H3,(H,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Competitive displacement of p53K382me2 from His-tagged 53BP1 tandem Tudor domain (unknown origin) |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Chromobox protein homolog 7
(Homo sapiens (Human)) | BDBM50189780
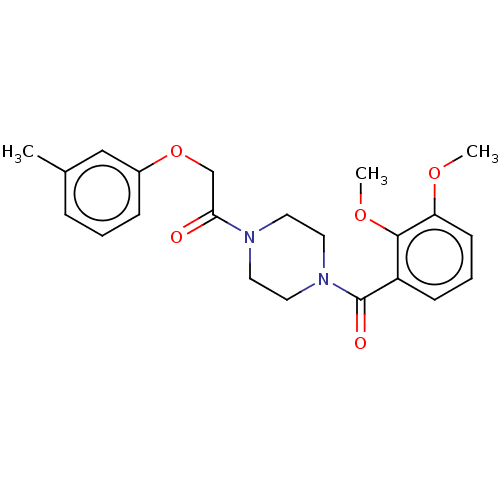
(CHEMBL3827954)Show SMILES COc1cccc(C(=O)N2CCN(CC2)C(=O)COc2cccc(C)c2)c1OC Show InChI InChI=1S/C22H26N2O5/c1-16-6-4-7-17(14-16)29-15-20(25)23-10-12-24(13-11-23)22(26)18-8-5-9-19(27-2)21(18)28-3/h4-9,14H,10-13,15H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of Cbx7 (unknown origin) using FITC-labeled SETDB1-K1170me3 peptide as probe by fluorescence polarization assay |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM50282162

(CHEMBL4173016)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN(C)C)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=S)Nc1ccc(C2=C3C=CC(O)=CC3Oc3cc(O)ccc23)c(c1)C(O)=O)[C@@H](C)O)[C@@H](C)O)C(=O)NCC(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O |r,c:87,89,92| Show InChI InChI=1S/C86H134N26O26S/c1-41(70(122)103-57(20-15-32-94-84(90)91)74(126)105-55(18-10-13-31-88)75(127)108-60(40-113)78(130)109-67(44(4)114)79(131)97-38-64(120)96-39-65(121)102-54(17-9-12-30-87)72(124)99-43(3)82(134)135)98-80(132)68(45(5)115)110-77(129)59(28-29-63(89)119)106-73(125)56(19-11-14-34-112(7)8)107-81(133)69(46(6)116)111-76(128)58(21-16-33-95-85(92)93)104-71(123)42(2)100-86(139)101-47-22-25-50(53(35-47)83(136)137)66-51-26-23-48(117)36-61(51)138-62-37-49(118)24-27-52(62)66/h22-27,35-37,41-46,54-61,67-69,113-118H,9-21,28-34,38-40,87-88H2,1-8H3,(H2,89,119)(H,96,120)(H,97,131)(H,98,132)(H,99,124)(H,102,121)(H,103,122)(H,104,123)(H,105,126)(H,106,125)(H,107,133)(H,108,127)(H,109,130)(H,110,129)(H,111,128)(H,134,135)(H,136,137)(H4,90,91,94)(H4,92,93,95)(H2,100,101,139)/t41-,42-,43-,44+,45+,46+,54-,55-,56-,57-,58-,59-,60-,61?,67-,68-,69-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of JARID1A (unknown origin) |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Chromodomain Y-like protein 2
(Homo sapiens (Human)) | BDBM50282159
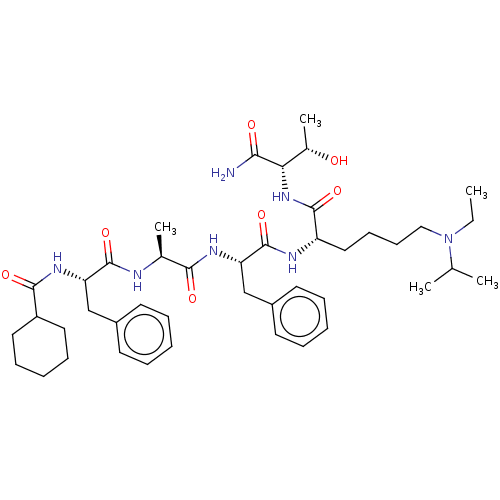
(CHEMBL4168117)Show SMILES CCN(CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)C1CCCCC1)C(=O)N[C@@H]([C@H](C)O)C(N)=O)C(C)C |r| Show InChI InChI=1S/C43H65N7O7/c1-6-50(28(2)3)25-17-16-24-34(41(55)49-37(30(5)51)38(44)52)46-43(57)36(27-32-20-12-8-13-21-32)47-39(53)29(4)45-42(56)35(26-31-18-10-7-11-19-31)48-40(54)33-22-14-9-15-23-33/h7-8,10-13,18-21,28-30,33-37,51H,6,9,14-17,22-27H2,1-5H3,(H2,44,52)(H,45,56)(H,46,57)(H,47,53)(H,48,54)(H,49,55)/t29-,30-,34-,35-,36-,37-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to C-terminal His-tagged CDYL2 (1 to 75 residues) (unknown origin) expressed in Rosetta2 BL21(DE3)pLysS cells by ITC method |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT1
(Homo sapiens (Human)) | BDBM50282160
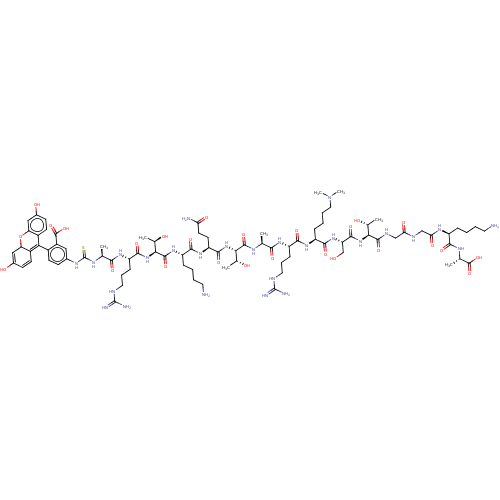
(CHEMBL4176399)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=S)Nc1ccc(C2=C3C=CC(O)=CC3Oc3cc(O)ccc23)c(c1)C(O)=O)[C@@H](C)O)[C@@H](C)O)C(=O)NCC(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O |r,c:87,89,92| Show InChI InChI=1S/C86H134N26O26S/c1-41(70(122)103-57(20-15-32-94-84(90)91)74(126)105-55(19-11-14-34-112(7)8)75(127)108-60(40-113)78(130)109-67(44(4)114)79(131)97-38-64(120)96-39-65(121)102-54(17-9-12-30-87)72(124)99-43(3)82(134)135)98-80(132)68(45(5)115)110-77(129)59(28-29-63(89)119)106-73(125)56(18-10-13-31-88)107-81(133)69(46(6)116)111-76(128)58(21-16-33-95-85(92)93)104-71(123)42(2)100-86(139)101-47-22-25-50(53(35-47)83(136)137)66-51-26-23-48(117)36-61(51)138-62-37-49(118)24-27-52(62)66/h22-27,35-37,41-46,54-61,67-69,113-118H,9-21,28-34,38-40,87-88H2,1-8H3,(H2,89,119)(H,96,120)(H,97,131)(H,98,132)(H,99,124)(H,102,121)(H,103,122)(H,104,123)(H,105,126)(H,106,125)(H,107,133)(H,108,127)(H,109,130)(H,110,129)(H,111,128)(H,134,135)(H,136,137)(H4,90,91,94)(H4,92,93,95)(H2,100,101,139)/t41-,42-,43-,44+,45+,46+,54-,55-,56-,57-,58-,59-,60-,61?,67-,68-,69-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to GLP (unknown origin) after 30 mins by fluorescence polarization assay |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT1
(Homo sapiens (Human)) | BDBM50282113
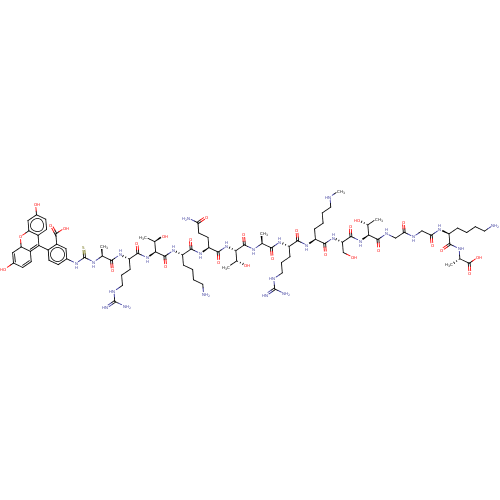
(CHEMBL4168535)Show SMILES CNCCCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=S)Nc1ccc(C2=C3C=CC(O)=CC3Oc3cc(O)ccc23)c(c1)C(O)=O)[C@@H](C)O)[C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O |r,c:73,75,78| Show InChI InChI=1S/C85H132N26O26S/c1-40(69(121)103-56(19-14-32-94-83(89)90)73(125)105-54(18-10-13-31-93-7)74(126)108-59(39-112)77(129)109-66(43(4)113)78(130)97-37-63(119)96-38-64(120)102-53(16-8-11-29-86)71(123)99-42(3)81(133)134)98-79(131)67(44(5)114)110-76(128)58(27-28-62(88)118)106-72(124)55(17-9-12-30-87)107-80(132)68(45(6)115)111-75(127)57(20-15-33-95-84(91)92)104-70(122)41(2)100-85(138)101-46-21-24-49(52(34-46)82(135)136)65-50-25-22-47(116)35-60(50)137-61-36-48(117)23-26-51(61)65/h21-26,34-36,40-45,53-60,66-68,93,112-117H,8-20,27-33,37-39,86-87H2,1-7H3,(H2,88,118)(H,96,119)(H,97,130)(H,98,131)(H,99,123)(H,102,120)(H,103,121)(H,104,122)(H,105,125)(H,106,124)(H,107,132)(H,108,126)(H,109,129)(H,110,128)(H,111,127)(H,133,134)(H,135,136)(H4,89,90,94)(H4,91,92,95)(H2,100,101,138)/t40-,41-,42-,43+,44+,45+,53-,54-,55-,56-,57-,58-,59-,60?,66-,67-,68-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to GLP (unknown origin) after 30 mins by fluorescence polarization assay |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50282163

(CHEMBL4171889)Show SMILES [H][C@]12C[C@H](N[C@@]1([H])CCCC2)C(=O)N[C@@H](CCCCN(C)C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C34H56N6O4/c1-22(2)19-28(31(35)41)38-33(43)29(20-24-13-7-6-8-14-24)39-32(42)27(17-11-12-18-40(5)23(3)4)37-34(44)30-21-25-15-9-10-16-26(25)36-30/h6-8,13-14,22-23,25-30,36H,9-12,15-21H2,1-5H3,(H2,35,41)(H,37,44)(H,38,43)(H,39,42)/t25-,26-,27-,28-,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to full length N-terminal His-tagged EED (unknown origin) expressed in Rosetta2 BL21(DE3)pLysS cells by ITC method |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50282164
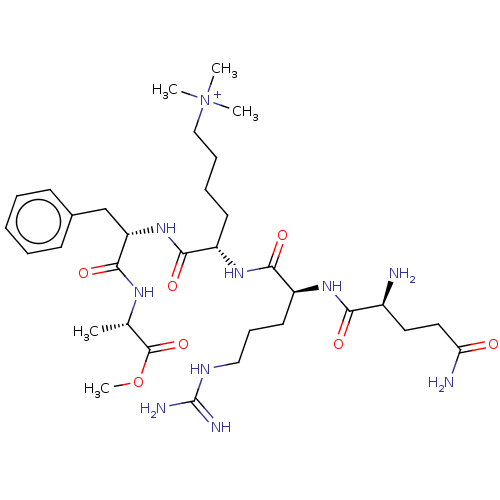
(CHEMBL4164708)Show SMILES COC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCC[N+](C)(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCC(N)=O |r| Show InChI InChI=1S/C33H56N10O7/c1-21(32(49)50-5)39-31(48)26(20-22-12-7-6-8-13-22)42-30(47)24(14-9-10-19-43(2,3)4)41-29(46)25(15-11-18-38-33(36)37)40-28(45)23(34)16-17-27(35)44/h6-8,12-13,21,23-26H,9-11,14-20,34H2,1-5H3,(H9-,35,36,37,38,39,40,41,42,44,45,46,47,48)/p+1/t21-,23-,24-,25-,26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to full length N-terminal His-tagged EED (unknown origin) expressed in Rosetta2 BL21(DE3)pLysS cells by ITC method |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50282165
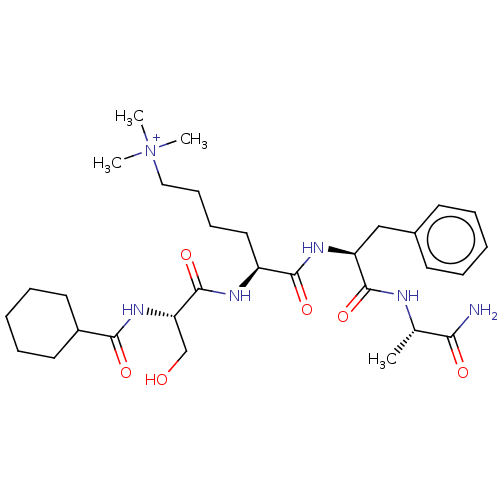
(CHEMBL4168151)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCC[N+](C)(C)C)NC(=O)[C@H](CO)NC(=O)C1CCCCC1)C(N)=O |r| Show InChI InChI=1S/C31H50N6O6/c1-21(27(32)39)33-30(42)25(19-22-13-7-5-8-14-22)35-29(41)24(17-11-12-18-37(2,3)4)34-31(43)26(20-38)36-28(40)23-15-9-6-10-16-23/h5,7-8,13-14,21,23-26,38H,6,9-12,15-20H2,1-4H3,(H5-,32,33,34,35,36,39,40,41,42,43)/p+1/t21-,24-,25-,26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to full length N-terminal His-tagged EED (unknown origin) expressed in Rosetta2 BL21(DE3)pLysS cells by ITC method |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM225230

(EED226 | US11013745, Compound EED226)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cnc(NCc2ccco2)n2cnnc12 Show InChI InChI=1S/C17H15N5O3S/c1-26(23,24)14-6-4-12(5-7-14)15-10-19-17(22-11-20-21-16(15)22)18-9-13-3-2-8-25-13/h2-8,10-11H,9H2,1H3,(H,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to EED (unknown origin) by ITC method |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Inhibitor of growth protein 2
(Homo sapiens (Human)) | BDBM50282166
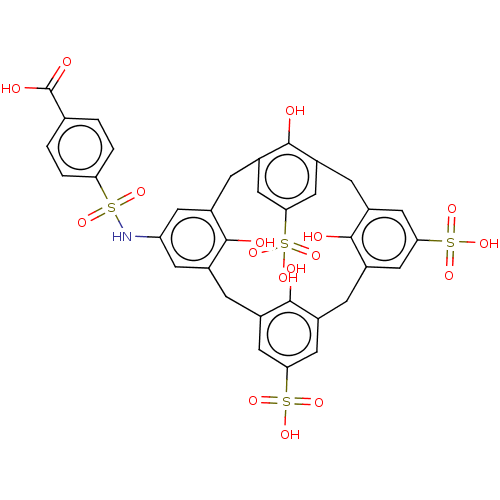
(CHEMBL4176148)Show SMILES OC(=O)c1ccc(cc1)S(=O)(=O)Nc1cc2Cc3cc(cc(Cc4cc(cc(Cc5cc(cc(Cc(c1)c2O)c5O)S(O)(=O)=O)c4O)S(O)(=O)=O)c3O)S(O)(=O)=O Show InChI InChI=1S/C35H29NO17S4/c37-31-18-5-20-11-28(55(45,46)47)13-22(32(20)38)7-24-15-30(57(51,52)53)16-25(34(24)40)8-23-14-29(56(48,49)50)12-21(33(23)39)6-19(31)10-26(9-18)36-54(43,44)27-3-1-17(2-4-27)35(41)42/h1-4,9-16,36-40H,5-8H2,(H,41,42)(H,45,46,47)(H,48,49,50)(H,51,52,53) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to 15N-labeled GST-tagged ING2 PHD finger domain (212 to 264 residues) (unknown origin) expressed in Escherichia coli Rosetta2 BL21(... |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Inhibitor of growth protein 2
(Homo sapiens (Human)) | BDBM50282167
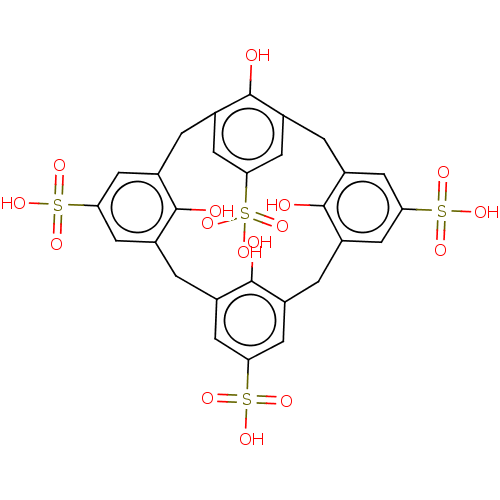
(CHEMBL1092710 | P-Sulfonatocalix[4]Arene)Show SMILES Oc1c2Cc3cc(cc(Cc4cc(cc(Cc5cc(cc(Cc1cc(c2)S(O)(=O)=O)c5O)S(O)(=O)=O)c4O)S(O)(=O)=O)c3O)S(O)(=O)=O Show InChI InChI=1S/C28H24O16S4/c29-25-13-1-14-6-22(46(36,37)38)8-16(26(14)30)3-18-10-24(48(42,43)44)12-20(28(18)32)4-19-11-23(47(39,40)41)9-17(27(19)31)2-15(25)7-21(5-13)45(33,34)35/h5-12,29-32H,1-4H2,(H,33,34,35)(H,36,37,38)(H,39,40,41)(H,42,43,44) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to 15N-labeled GST-tagged ING2 PHD finger domain (212 to 264 residues) (unknown origin) expressed in Escherichia coli Rosetta2 BL21(... |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Chromobox protein homolog 7
(Homo sapiens (Human)) | BDBM50189780

(CHEMBL3827954)Show SMILES COc1cccc(C(=O)N2CCN(CC2)C(=O)COc2cccc(C)c2)c1OC Show InChI InChI=1S/C22H26N2O5/c1-16-6-4-7-17(14-16)29-15-20(25)23-10-12-24(13-11-23)22(26)18-8-5-9-19(27-2)21(18)28-3/h4-9,14H,10-13,15H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to Cbx7 (unknown origin) |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50282161

(CHEMBL4175336)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCC[N+](C)(C)C)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1)C(N)=O |r| Show InChI InChI=1S/C32H53N7O6/c1-21(2)18-25(28(33)41)36-31(44)26(19-22-12-7-6-8-13-22)37-30(43)24(14-9-10-17-39(3,4)5)35-32(45)27(20-40)38-29(42)23-15-11-16-34-23/h6-8,12-13,21,23-27,34,40H,9-11,14-20H2,1-5H3,(H5-,33,35,36,37,38,41,42,43,44,45)/p+1/t23-,24-,25-,26-,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to full length N-terminal His-tagged EED (unknown origin) expressed in Rosetta2 BL21(DE3)pLysS cells by ITC method |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50282160

(CHEMBL4176399)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=S)Nc1ccc(C2=C3C=CC(O)=CC3Oc3cc(O)ccc23)c(c1)C(O)=O)[C@@H](C)O)[C@@H](C)O)C(=O)NCC(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O |r,c:87,89,92| Show InChI InChI=1S/C86H134N26O26S/c1-41(70(122)103-57(20-15-32-94-84(90)91)74(126)105-55(19-11-14-34-112(7)8)75(127)108-60(40-113)78(130)109-67(44(4)114)79(131)97-38-64(120)96-39-65(121)102-54(17-9-12-30-87)72(124)99-43(3)82(134)135)98-80(132)68(45(5)115)110-77(129)59(28-29-63(89)119)106-73(125)56(18-10-13-31-88)107-81(133)69(46(6)116)111-76(128)58(21-16-33-95-85(92)93)104-71(123)42(2)100-86(139)101-47-22-25-50(53(35-47)83(136)137)66-51-26-23-48(117)36-61(51)138-62-37-49(118)24-27-52(62)66/h22-27,35-37,41-46,54-61,67-69,113-118H,9-21,28-34,38-40,87-88H2,1-8H3,(H2,89,119)(H,96,120)(H,97,131)(H,98,132)(H,99,124)(H,102,121)(H,103,122)(H,104,123)(H,105,126)(H,106,125)(H,107,133)(H,108,127)(H,109,130)(H,110,129)(H,111,128)(H,134,135)(H,136,137)(H4,90,91,94)(H4,92,93,95)(H2,100,101,139)/t41-,42-,43-,44+,45+,46+,54-,55-,56-,57-,58-,59-,60-,61?,67-,68-,69-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to G9a (unknown origin) after 30 mins by fluorescence polarization assay |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
TP53-binding protein 1
(Homo sapiens (Human)) | BDBM154545

(3-Bromo-N-(3-(tert-butylamino)propyl)benzamide (UN...)Show InChI InChI=1S/C14H21BrN2O/c1-14(2,3)17-9-5-8-16-13(18)11-6-4-7-12(15)10-11/h4,6-7,10,17H,5,8-9H2,1-3H3,(H,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to 53BP1 methyllysine binding domain (unknown origin) by ITC method |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Chromobox protein homolog 7
(Homo sapiens (Human)) | BDBM50194259

(CHEMBL3939958)Show SMILES CCN(CC)CCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(cc1)C(C)(C)C)C(=O)N[C@@H](CO)C(=O)OC |r| Show InChI InChI=1S/C43H66N6O8/c1-10-49(11-2)24-16-15-19-33(39(53)48-36(27-50)42(56)57-9)45-41(55)34(25-28(3)4)46-37(51)29(5)44-40(54)35(26-30-17-13-12-14-18-30)47-38(52)31-20-22-32(23-21-31)43(6,7)8/h12-14,17-18,20-23,28-29,33-36,50H,10-11,15-16,19,24-27H2,1-9H3,(H,44,54)(H,45,55)(H,46,51)(H,47,52)(H,48,53)/t29-,33-,34-,35-,36-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to Cbx7 (unknown origin) by ITC method |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Chromodomain Y-like protein
(Homo sapiens (Human)) | BDBM50282159

(CHEMBL4168117)Show SMILES CCN(CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)C1CCCCC1)C(=O)N[C@@H]([C@H](C)O)C(N)=O)C(C)C |r| Show InChI InChI=1S/C43H65N7O7/c1-6-50(28(2)3)25-17-16-24-34(41(55)49-37(30(5)51)38(44)52)46-43(57)36(27-32-20-12-8-13-21-32)47-39(53)29(4)45-42(56)35(26-31-18-10-7-11-19-31)48-40(54)33-22-14-9-15-23-33/h7-8,10-13,18-21,28-30,33-37,51H,6,9,14-17,22-27H2,1-5H3,(H2,44,52)(H,45,56)(H,46,57)(H,47,53)(H,48,54)(H,49,55)/t29-,30-,34-,35-,36-,37-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminal GST-tagged CDYL (1 to 78 residues) (unknown origin) expressed in Rosetta2 BL21(DE3)pLysS cells after 30 mins by fluore... |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Inhibitor of growth protein 2
(Homo sapiens (Human)) | BDBM50282158
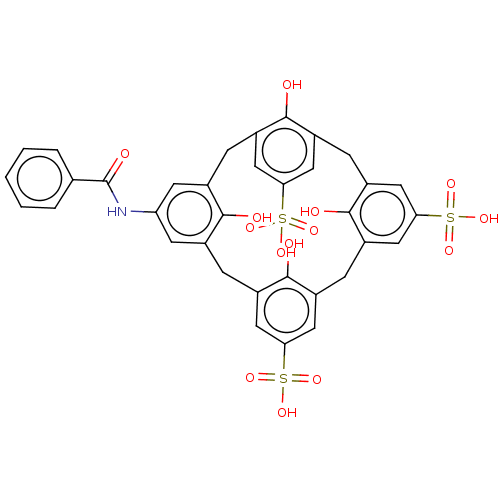
(CHEMBL4168964)Show SMILES Oc1c2Cc3cc(cc(Cc4cc(cc(Cc5cc(cc(Cc1cc(NC(=O)c1ccccc1)c2)c5O)S(O)(=O)=O)c4O)S(O)(=O)=O)c3O)S(O)(=O)=O Show InChI InChI=1S/C35H29NO14S3/c37-31-19-6-21-12-28(51(42,43)44)14-23(32(21)38)8-25-16-30(53(48,49)50)17-26(34(25)40)9-24-15-29(52(45,46)47)13-22(33(24)39)7-20(31)11-27(10-19)36-35(41)18-4-2-1-3-5-18/h1-5,10-17,37-40H,6-9H2,(H,36,41)(H,42,43,44)(H,45,46,47)(H,48,49,50) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to 15N-labeled GST-tagged ING2 PHD finger domain (212 to 264 residues) (unknown origin) expressed in Escherichia coli Rosetta2 BL21(... |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50282157

(CHEMBL4161291)Show SMILES [H][C@]12C[C@H](N[C@@]1([H])CCCC2)C(=O)N[C@@H](CCCCN(C)C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCCCC1 |r| Show InChI InChI=1S/C33H53N5O3/c1-24(2)37(3)19-13-10-18-28(35-32(40)29-23-26-16-8-9-17-27(26)34-29)31(39)36-30(22-25-14-6-4-7-15-25)33(41)38-20-11-5-12-21-38/h4,6-7,14-15,24,26-30,34H,5,8-13,16-23H2,1-3H3,(H,35,40)(H,36,39)/t26-,27-,28-,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to full length N-terminal His-tagged EED (unknown origin) expressed in Rosetta2 BL21(DE3)pLysS cells by ITC method |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5A
(Homo sapiens (Human)) | BDBM50282115
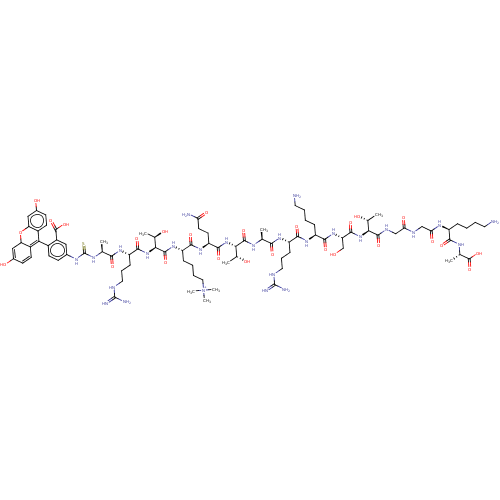
(CHEMBL4165111)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCC[N+](C)(C)C)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=S)Nc1ccc(C2=C3C=CC(O)=CC3Oc3cc(O)ccc23)c(c1)C(O)=O)[C@@H](C)O)[C@@H](C)O)C(=O)NCC(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O |r,c:88,90,93| Show InChI InChI=1S/C87H136N26O26S/c1-42(71(123)104-58(21-16-33-95-85(91)92)75(127)106-56(19-11-14-32-89)76(128)109-61(41-114)79(131)110-68(45(4)115)80(132)98-39-65(121)97-40-66(122)103-55(18-10-13-31-88)73(125)100-44(3)83(135)136)99-81(133)69(46(5)116)111-78(130)60(29-30-64(90)120)107-74(126)57(20-12-15-35-113(7,8)9)108-82(134)70(47(6)117)112-77(129)59(22-17-34-96-86(93)94)105-72(124)43(2)101-87(140)102-48-23-26-51(54(36-48)84(137)138)67-52-27-24-49(118)37-62(52)139-63-38-50(119)25-28-53(63)67/h23-28,36-38,42-47,55-62,68-70,114-117H,10-22,29-35,39-41,88-89H2,1-9H3,(H29-,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,140)/p+1/t42-,43-,44-,45+,46+,47+,55-,56-,57-,58-,59-,60-,61-,62?,68-,69-,70-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of JARID1A (unknown origin) |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase 2A
(Homo sapiens (Human)) | BDBM50282115

(CHEMBL4165111)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCC[N+](C)(C)C)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=S)Nc1ccc(C2=C3C=CC(O)=CC3Oc3cc(O)ccc23)c(c1)C(O)=O)[C@@H](C)O)[C@@H](C)O)C(=O)NCC(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O |r,c:88,90,93| Show InChI InChI=1S/C87H136N26O26S/c1-42(71(123)104-58(21-16-33-95-85(91)92)75(127)106-56(19-11-14-32-89)76(128)109-61(41-114)79(131)110-68(45(4)115)80(132)98-39-65(121)97-40-66(122)103-55(18-10-13-31-88)73(125)100-44(3)83(135)136)99-81(133)69(46(5)116)111-78(130)60(29-30-64(90)120)107-74(126)57(20-12-15-35-113(7,8)9)108-82(134)70(47(6)117)112-77(129)59(22-17-34-96-86(93)94)105-72(124)43(2)101-87(140)102-48-23-26-51(54(36-48)84(137)138)67-52-27-24-49(118)37-62(52)139-63-38-50(119)25-28-53(63)67/h23-28,36-38,42-47,55-62,68-70,114-117H,10-22,29-35,39-41,88-89H2,1-9H3,(H29-,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,140)/p+1/t42-,43-,44-,45+,46+,47+,55-,56-,57-,58-,59-,60-,61-,62?,68-,69-,70-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to 15N-labeled GST-tagged MLL1 PHD3 finger domain (1565 to 1627 residues) (unknown origin) expressed in Escherichia coli Rosetta2 BL... |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Chromobox protein homolog 7
(Homo sapiens (Human)) | BDBM50194463
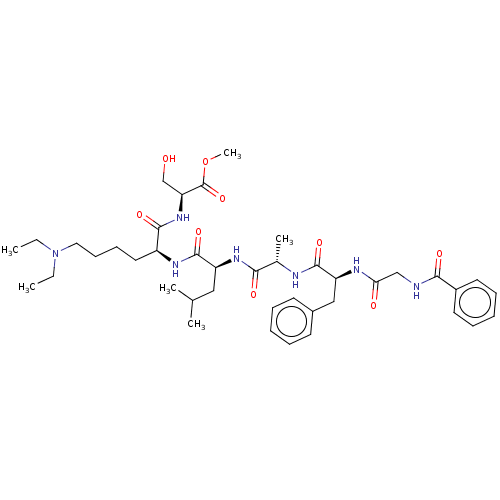
(CHEMBL3974828)Show SMILES CCN(CC)CCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)c1ccccc1)C(=O)N[C@@H](CO)C(=O)OC |r| Show InChI InChI=1S/C41H61N7O9/c1-7-48(8-2)22-16-15-21-31(38(53)47-34(26-49)41(56)57-6)45-40(55)32(23-27(3)4)46-36(51)28(5)43-39(54)33(24-29-17-11-9-12-18-29)44-35(50)25-42-37(52)30-19-13-10-14-20-30/h9-14,17-20,27-28,31-34,49H,7-8,15-16,21-26H2,1-6H3,(H,42,52)(H,43,54)(H,44,50)(H,45,55)(H,46,51)(H,47,53)/t28-,31-,32-,33-,34-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to Cbx7 (unknown origin) by ITC method |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50282114
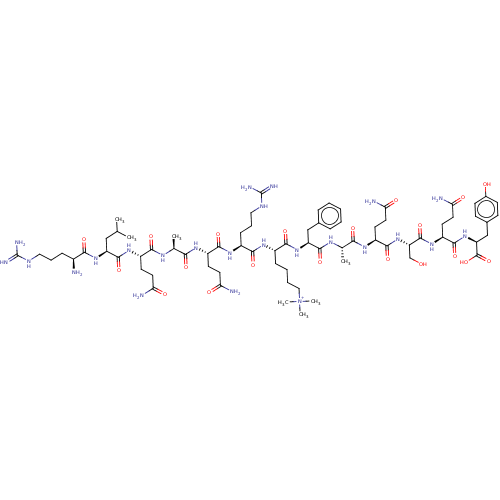
(CHEMBL4160235)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCC[N+](C)(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C74H120N24O20/c1-39(2)35-52(94-62(107)45(75)17-13-32-84-73(80)81)70(115)92-48(24-28-56(76)101)63(108)86-40(3)60(105)88-49(25-29-57(77)102)66(111)91-47(19-14-33-85-74(82)83)64(109)90-46(18-11-12-34-98(5,6)7)65(110)95-53(36-42-15-9-8-10-16-42)69(114)87-41(4)61(106)89-50(26-30-58(78)103)68(113)97-55(38-99)71(116)93-51(27-31-59(79)104)67(112)96-54(72(117)118)37-43-20-22-44(100)23-21-43/h8-10,15-16,20-23,39-41,45-55,99H,11-14,17-19,24-38,75H2,1-7H3,(H29-,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118)/p+1/t40-,41-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,55-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to full length N-terminal His-tagged EED (unknown origin) expressed in Rosetta2 BL21(DE3)pLysS cells by ITC method |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50282113

(CHEMBL4168535)Show SMILES CNCCCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=S)Nc1ccc(C2=C3C=CC(O)=CC3Oc3cc(O)ccc23)c(c1)C(O)=O)[C@@H](C)O)[C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O |r,c:73,75,78| Show InChI InChI=1S/C85H132N26O26S/c1-40(69(121)103-56(19-14-32-94-83(89)90)73(125)105-54(18-10-13-31-93-7)74(126)108-59(39-112)77(129)109-66(43(4)113)78(130)97-37-63(119)96-38-64(120)102-53(16-8-11-29-86)71(123)99-42(3)81(133)134)98-79(131)67(44(5)114)110-76(128)58(27-28-62(88)118)106-72(124)55(17-9-12-30-87)107-80(132)68(45(6)115)111-75(127)57(20-15-33-95-84(91)92)104-70(122)41(2)100-85(138)101-46-21-24-49(52(34-46)82(135)136)65-50-25-22-47(116)35-60(50)137-61-36-48(117)23-26-51(61)65/h21-26,34-36,40-45,53-60,66-68,93,112-117H,8-20,27-33,37-39,86-87H2,1-7H3,(H2,88,118)(H,96,119)(H,97,130)(H,98,131)(H,99,123)(H,102,120)(H,103,121)(H,104,122)(H,105,125)(H,106,124)(H,107,132)(H,108,126)(H,109,129)(H,110,128)(H,111,127)(H,133,134)(H,135,136)(H4,89,90,94)(H4,91,92,95)(H2,100,101,138)/t40-,41-,42-,43+,44+,45+,53-,54-,55-,56-,57-,58-,59-,60?,66-,67-,68-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to G9a (unknown origin) after 30 mins by fluorescence polarization assay |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
E3 SUMO-protein ligase CBX4
(Homo sapiens (Human)) | BDBM50194259

(CHEMBL3939958)Show SMILES CCN(CC)CCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(cc1)C(C)(C)C)C(=O)N[C@@H](CO)C(=O)OC |r| Show InChI InChI=1S/C43H66N6O8/c1-10-49(11-2)24-16-15-19-33(39(53)48-36(27-50)42(56)57-9)45-41(55)34(25-28(3)4)46-37(51)29(5)44-40(54)35(26-30-17-13-12-14-18-30)47-38(52)31-20-22-32(23-21-31)43(6,7)8/h12-14,17-18,20-23,28-29,33-36,50H,10-11,15-16,19,24-27H2,1-9H3,(H,44,54)(H,45,55)(H,46,51)(H,47,52)(H,48,53)/t29-,33-,34-,35-,36-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to Cbx4 (unknown origin) by ITC method |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Chromobox protein homolog 7
(Homo sapiens (Human)) | BDBM50282170
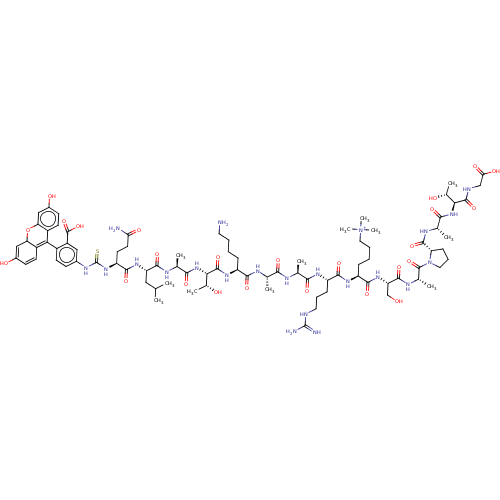
(CHEMBL4169589)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCC(N)=O)NC(=S)Nc1ccc(C2=C3C=CC(O)=CC3Oc3cc(O)ccc23)c(c1)C(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCC[N+](C)(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(O)=O |r,c:22,24,27| Show InChI InChI=1S/C86H130N22O25S/c1-41(2)35-60(102-77(124)59(29-30-65(88)114)104-86(134)98-49-23-26-52(55(36-49)84(131)132)67-53-27-24-50(112)37-63(53)133-64-38-51(113)25-28-54(64)67)78(125)95-44(5)72(119)106-69(48(9)111)82(129)101-56(19-13-15-31-87)74(121)94-42(3)70(117)93-43(4)71(118)99-58(21-17-32-91-85(89)90)75(122)100-57(20-14-16-34-108(10,11)12)76(123)103-61(40-109)79(126)97-46(7)83(130)107-33-18-22-62(107)80(127)96-45(6)73(120)105-68(47(8)110)81(128)92-39-66(115)116/h23-28,36-38,41-48,56-63,68-69,109-111H,13-22,29-35,39-40,87H2,1-12H3,(H24-,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,131,132,134)/p+1/t42-,43-,44-,45-,46-,47+,48+,56-,57-,58-,59-,60-,61-,62-,63?,68-,69-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to Cbx7 (unknown origin) by ITC method |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Chromobox protein homolog 7
(Homo sapiens (Human)) | BDBM50282168
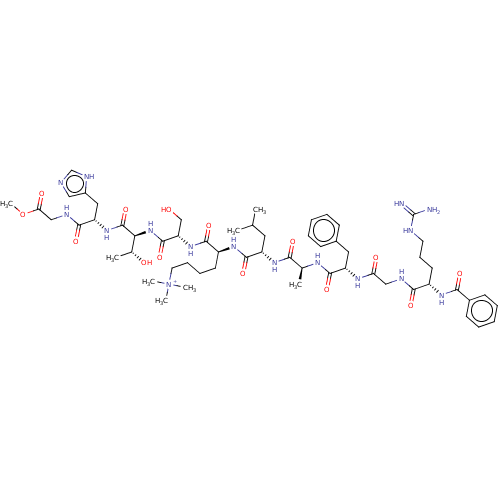
(CHEMBL4175216)Show SMILES COC(=O)CNC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CCCC[N+](C)(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@H](CCCNC(N)=N)NC(=O)c1ccccc1)[C@@H](C)O |r| Show InChI InChI=1S/C58H88N16O14/c1-34(2)26-42(70-49(79)35(3)66-54(84)43(27-37-18-11-9-12-19-37)67-46(77)30-63-51(81)40(23-17-24-62-58(59)60)68-50(80)38-20-13-10-14-21-38)55(85)69-41(22-15-16-25-74(5,6)7)53(83)72-45(32-75)56(86)73-48(36(4)76)57(87)71-44(28-39-29-61-33-65-39)52(82)64-31-47(78)88-8/h9-14,18-21,29,33-36,40-45,48,75-76H,15-17,22-28,30-32H2,1-8H3,(H14-,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,77,79,80,81,82,83,84,85,86,87)/p+1/t35-,36+,40-,41-,42-,43-,44-,45-,48-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to Cbx7 (unknown origin) by ITC method |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Chromodomain Y-like protein 2
(Homo sapiens (Human)) | BDBM50194259

(CHEMBL3939958)Show SMILES CCN(CC)CCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(cc1)C(C)(C)C)C(=O)N[C@@H](CO)C(=O)OC |r| Show InChI InChI=1S/C43H66N6O8/c1-10-49(11-2)24-16-15-19-33(39(53)48-36(27-50)42(56)57-9)45-41(55)34(25-28(3)4)46-37(51)29(5)44-40(54)35(26-30-17-13-12-14-18-30)47-38(52)31-20-22-32(23-21-31)43(6,7)8/h12-14,17-18,20-23,28-29,33-36,50H,10-11,15-16,19,24-27H2,1-9H3,(H,44,54)(H,45,55)(H,46,51)(H,47,52)(H,48,53)/t29-,33-,34-,35-,36-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to C-terminal His-tagged CDYL2 (1 to 75 residues) (unknown origin) expressed in Rosetta2 BL21(DE3)pLysS cells after 30 mins by fluor... |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Chromodomain Y-like protein
(Homo sapiens (Human)) | BDBM50194259

(CHEMBL3939958)Show SMILES CCN(CC)CCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(cc1)C(C)(C)C)C(=O)N[C@@H](CO)C(=O)OC |r| Show InChI InChI=1S/C43H66N6O8/c1-10-49(11-2)24-16-15-19-33(39(53)48-36(27-50)42(56)57-9)45-41(55)34(25-28(3)4)46-37(51)29(5)44-40(54)35(26-30-17-13-12-14-18-30)47-38(52)31-20-22-32(23-21-31)43(6,7)8/h12-14,17-18,20-23,28-29,33-36,50H,10-11,15-16,19,24-27H2,1-9H3,(H,44,54)(H,45,55)(H,46,51)(H,47,52)(H,48,53)/t29-,33-,34-,35-,36-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to CDYL1b (unknown origin) after 30 mins by fluorescence polarization assay |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |
Testis-specific chromodomain protein Y 1
(Homo sapiens (Human)) | BDBM50194259

(CHEMBL3939958)Show SMILES CCN(CC)CCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(cc1)C(C)(C)C)C(=O)N[C@@H](CO)C(=O)OC |r| Show InChI InChI=1S/C43H66N6O8/c1-10-49(11-2)24-16-15-19-33(39(53)48-36(27-50)42(56)57-9)45-41(55)34(25-28(3)4)46-37(51)29(5)44-40(54)35(26-30-17-13-12-14-18-30)47-38(52)31-20-22-32(23-21-31)43(6,7)8/h12-14,17-18,20-23,28-29,33-36,50H,10-11,15-16,19,24-27H2,1-9H3,(H,44,54)(H,45,55)(H,46,51)(H,47,52)(H,48,53)/t29-,33-,34-,35-,36-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminal GST-tagged CDY1 (1 to 101 residues) (unknown origin) expressed in Rosetta2 BL21(DE3)pLysS cells after 30 mins by fluor... |
Eur J Med Chem 136: 14-35 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.047
BindingDB Entry DOI: 10.7270/Q2F76G3G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data