

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50021529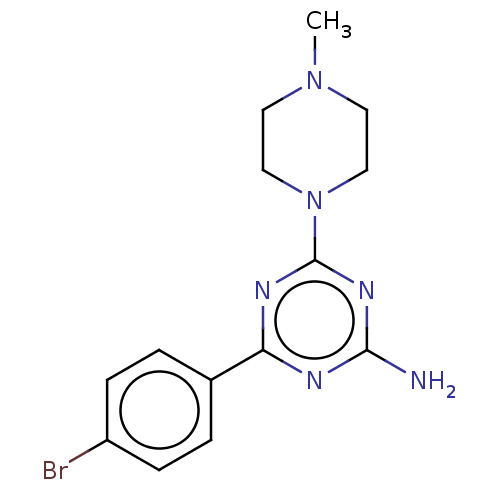 (CHEMBL3290578) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Palermo Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H4R expressed in Sf9 cell membranes co-expressed with G protein Gai2 and Gb1gamma2 incubated for 60 mins by ... | Eur J Med Chem 142: 523-549 (2017) Article DOI: 10.1016/j.ejmech.2017.09.035 BindingDB Entry DOI: 10.7270/Q2HH6NRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50021557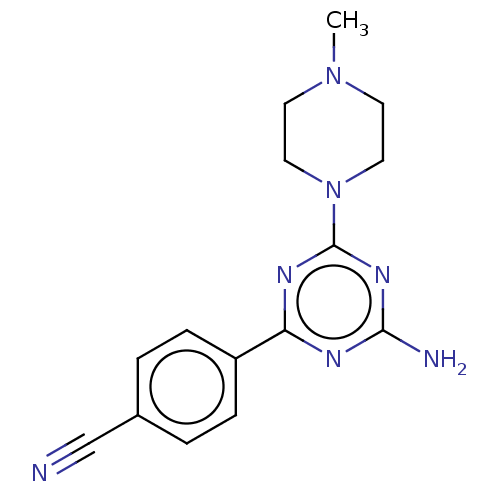 (CHEMBL3290582) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Palermo Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H4R expressed in Sf9 cell membranes co-expressed with G protein Gai2 and Gb1gamma2 incubated for 60 mins by ... | Eur J Med Chem 142: 523-549 (2017) Article DOI: 10.1016/j.ejmech.2017.09.035 BindingDB Entry DOI: 10.7270/Q2HH6NRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM189355 (4-(4-Methylpiperazin-1-yl)-6-(pyridin-4-yl)-1,3,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Palermo Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H4R expressed in Sf9 cell membranes co-expressed with G protein Gai2 and Gb1gamma2 incubated for 60 mins by ... | Eur J Med Chem 142: 523-549 (2017) Article DOI: 10.1016/j.ejmech.2017.09.035 BindingDB Entry DOI: 10.7270/Q2HH6NRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM189352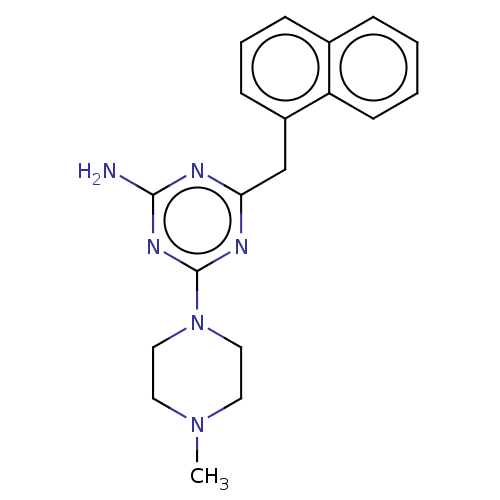 (4-(4-Methylpiperazin-1-yl)-6-(naphthalen-1-ylmethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Palermo Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H4R expressed in Sf9 cell membranes co-expressed with G protein Gai2 and Gb1gamma2 incubated for 60 mins by ... | Eur J Med Chem 142: 523-549 (2017) Article DOI: 10.1016/j.ejmech.2017.09.035 BindingDB Entry DOI: 10.7270/Q2HH6NRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM189353 (4-Benzyl-6-(4-methylpiperazin-1-yl)-1,3,5-triazin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Palermo Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H4R expressed in Sf9 cell membranes co-expressed with G protein Gai2 and Gb1gamma2 incubated for 60 mins by ... | Eur J Med Chem 142: 523-549 (2017) Article DOI: 10.1016/j.ejmech.2017.09.035 BindingDB Entry DOI: 10.7270/Q2HH6NRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM189351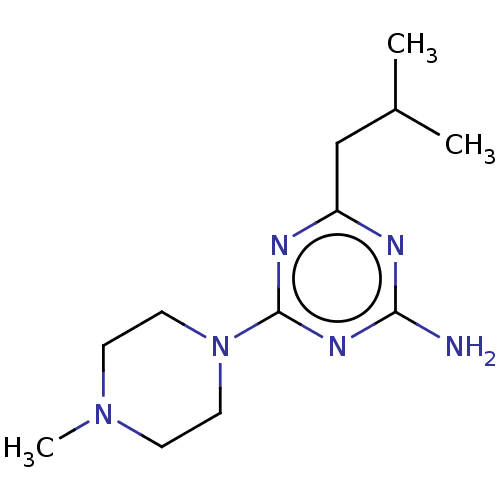 (4-(4-Methylpiperazin-1-yl)-6-(2-methylpropyl)-1,3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Palermo Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H4R expressed in Sf9 cell membranes co-expressed with G protein Gai2 and Gb1gamma2 incubated for 60 mins by ... | Eur J Med Chem 142: 523-549 (2017) Article DOI: 10.1016/j.ejmech.2017.09.035 BindingDB Entry DOI: 10.7270/Q2HH6NRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM189354 (4-((4-Chlorophenoxy)methyl)-6-(4-methylpiperazin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Palermo Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H4R expressed in Sf9 cell membranes co-expressed with G protein Gai2 and Gb1gamma2 incubated for 60 mins by ... | Eur J Med Chem 142: 523-549 (2017) Article DOI: 10.1016/j.ejmech.2017.09.035 BindingDB Entry DOI: 10.7270/Q2HH6NRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM189350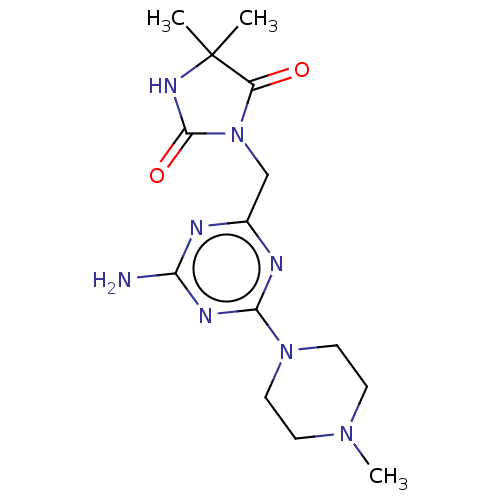 (3-((4-Amino-6-(4-methylpiperazin-1-yl)-1,3,5-triaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Palermo Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H4R expressed in Sf9 cell membranes co-expressed with G protein Gai2 and Gb1gamma2 incubated for 60 mins by ... | Eur J Med Chem 142: 523-549 (2017) Article DOI: 10.1016/j.ejmech.2017.09.035 BindingDB Entry DOI: 10.7270/Q2HH6NRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50315213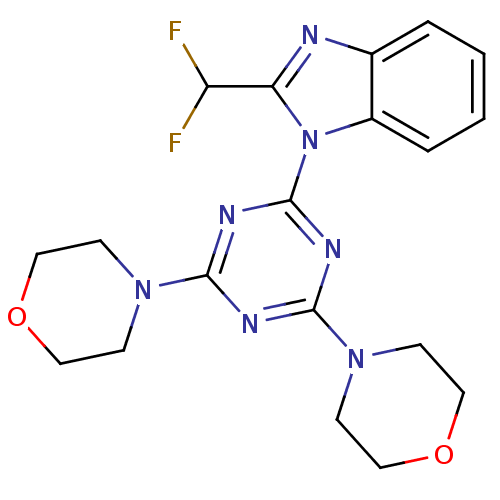 (2-(difluoromethyl)-1-(4,6-dimorpholin-4-yl-1,3,5-t...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Palermo Curated by ChEMBL | Assay Description Inhibition of PI3K p110gamma/p85alpha (unknown origin) assessed as reduction in PIP2 to PIP3 conversion by HTRF assay | Eur J Med Chem 142: 523-549 (2017) Article DOI: 10.1016/j.ejmech.2017.09.035 BindingDB Entry DOI: 10.7270/Q2HH6NRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin-conjugating enzyme E2 B (Homo sapiens) | BDBM50468268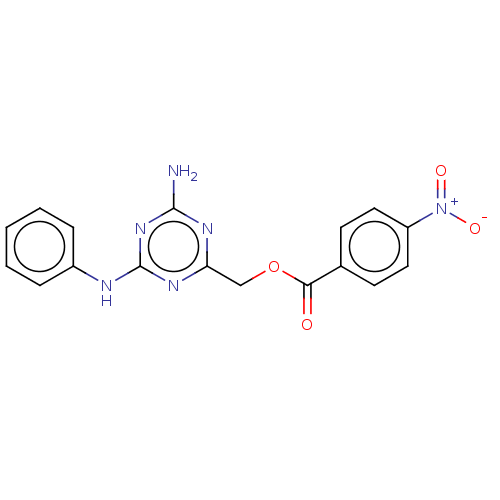 (CHEMBL3787660) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Palermo Curated by ChEMBL | Assay Description Inhibition of human recombinant Rad6B using ubiquitin and histone H2A and ubiquitin-activating enzyme E1 preincubated for 1 hr before ubiquitin and h... | Eur J Med Chem 142: 523-549 (2017) Article DOI: 10.1016/j.ejmech.2017.09.035 BindingDB Entry DOI: 10.7270/Q2HH6NRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 5 activator 1 (Homo sapiens (Human)) | BDBM27217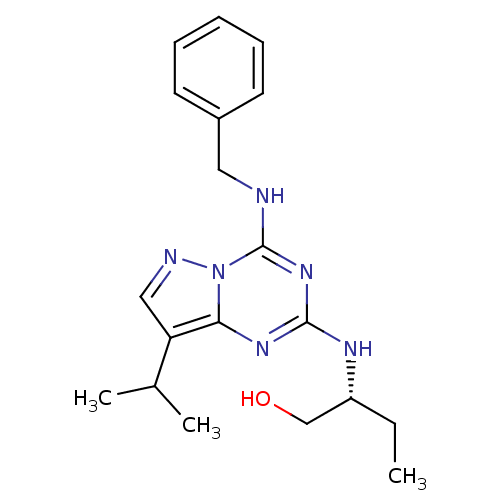 ((2R)-2-{[4-(benzylamino)-8-(propan-2-yl)pyrazolo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Palermo Curated by ChEMBL | Assay Description Inhibition of human recombinant CDK5/p25 using histone H1 and [gamma-33P]ATP incubated fro 30 mins by scintillation counting analysis | Eur J Med Chem 142: 523-549 (2017) Article DOI: 10.1016/j.ejmech.2017.09.035 BindingDB Entry DOI: 10.7270/Q2HH6NRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM27217 ((2R)-2-{[4-(benzylamino)-8-(propan-2-yl)pyrazolo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Palermo Curated by ChEMBL | Assay Description Inhibition of human recombinant CDK2/cyclin A using histone H1 and [gamma-33P]ATP incubated fro 30 mins by scintillation counting analysis | Eur J Med Chem 142: 523-549 (2017) Article DOI: 10.1016/j.ejmech.2017.09.035 BindingDB Entry DOI: 10.7270/Q2HH6NRF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-H/Cyclin-dependent kinase 7 (Homo sapiens (Human)) | BDBM27217 ((2R)-2-{[4-(benzylamino)-8-(propan-2-yl)pyrazolo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Palermo Curated by ChEMBL | Assay Description Inhibition of human recombinant CDK7/cyclin H using histone H1 and [gamma-33P]ATP incubated fro 30 mins by scintillation counting analysis | Eur J Med Chem 142: 523-549 (2017) Article DOI: 10.1016/j.ejmech.2017.09.035 BindingDB Entry DOI: 10.7270/Q2HH6NRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM27217 ((2R)-2-{[4-(benzylamino)-8-(propan-2-yl)pyrazolo[1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Palermo Curated by ChEMBL | Assay Description Inhibition of human recombinant CDK9/cyclin T expressed in insect cells using pRb fragment (773 to 928 amino acids) and [gamma-33P]ATP incubated fro ... | Eur J Med Chem 142: 523-549 (2017) Article DOI: 10.1016/j.ejmech.2017.09.035 BindingDB Entry DOI: 10.7270/Q2HH6NRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM27217 ((2R)-2-{[4-(benzylamino)-8-(propan-2-yl)pyrazolo[1...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Palermo Curated by ChEMBL | Assay Description Inhibition of human recombinant CDK2/cyclinE using histone H1 and [gamma-33P]ATP incubated fro 30 mins by scintillation counting analysis | Eur J Med Chem 142: 523-549 (2017) Article DOI: 10.1016/j.ejmech.2017.09.035 BindingDB Entry DOI: 10.7270/Q2HH6NRF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM50237830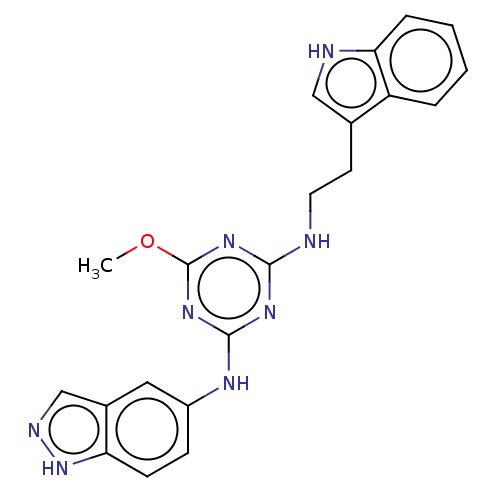 (CHEMBL3799807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Palermo Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human PAK4 kinase domain (300 to 591 residues) expressed in Escherichia coli BL21 (DE3) | Eur J Med Chem 142: 523-549 (2017) Article DOI: 10.1016/j.ejmech.2017.09.035 BindingDB Entry DOI: 10.7270/Q2HH6NRF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50439116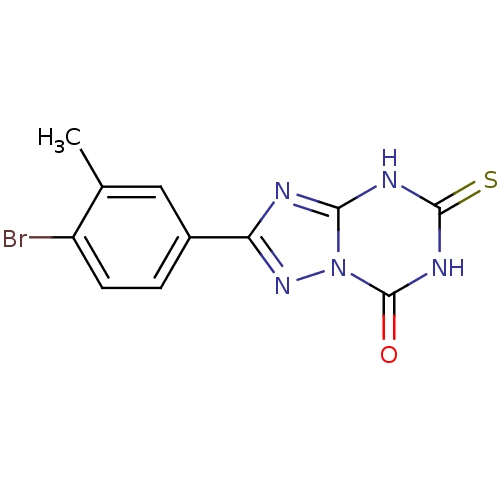 (CHEMBL2418076) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Palermo Curated by ChEMBL | Assay Description Inhibition of recombinant human thymidine phosphorylase expressed in Escherichia coli using thymidine substrate incubated for 4 to 20 mins by spectro... | Eur J Med Chem 142: 523-549 (2017) Article DOI: 10.1016/j.ejmech.2017.09.035 BindingDB Entry DOI: 10.7270/Q2HH6NRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||