

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Mixed-type inhibition of human AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition by Lineweaver-B... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469009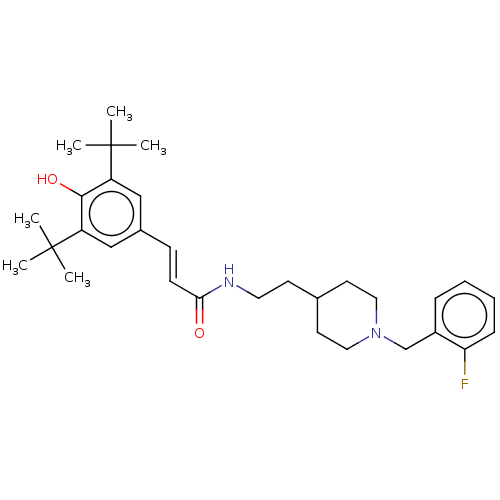 (CHEMBL4292766) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Mixed-type inhibition of human AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition by Lineweaver-B... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469008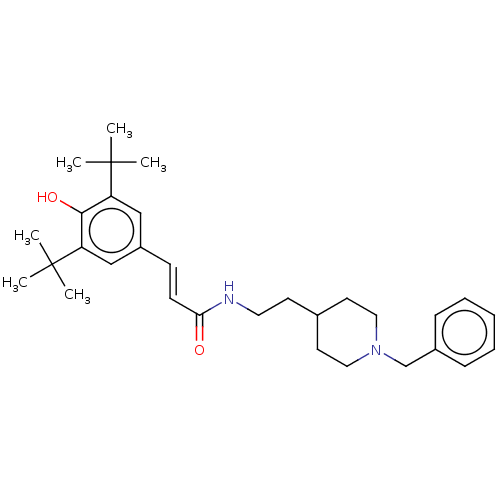 (CHEMBL4283390) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Mixed-type inhibition of human AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition by Lineweaver-B... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50469009 (CHEMBL4292766) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50469018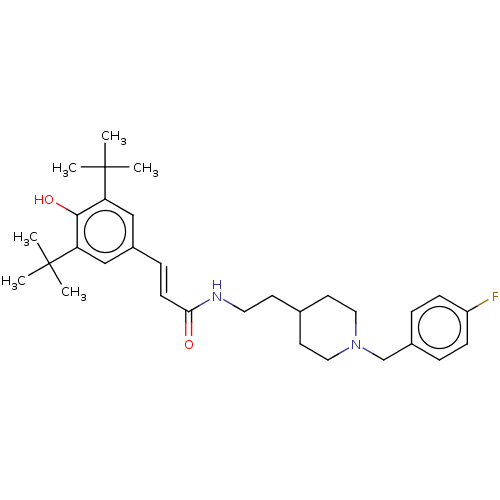 (CHEMBL4284475) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50469020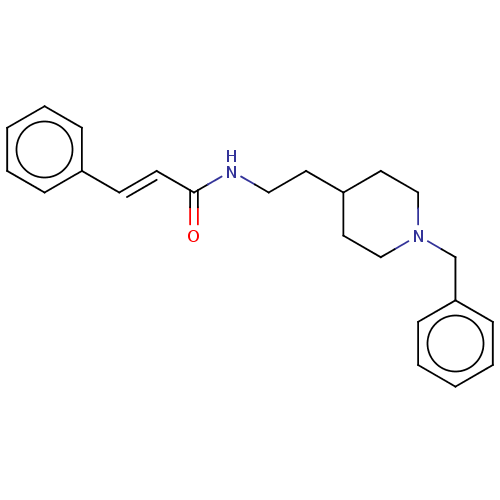 (CHEMBL3819548) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50469003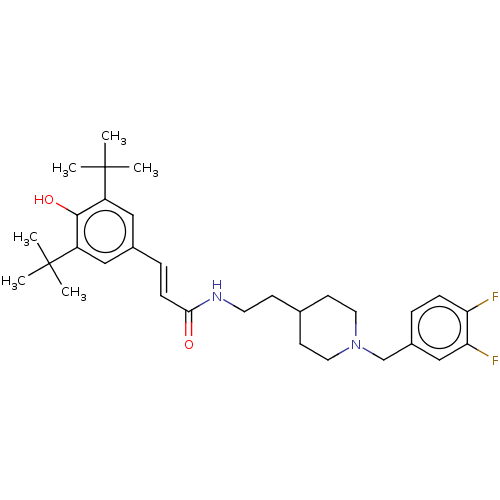 (CHEMBL4281066) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469020 (CHEMBL3819548) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50469008 (CHEMBL4283390) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50469020 (CHEMBL3819548) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50469001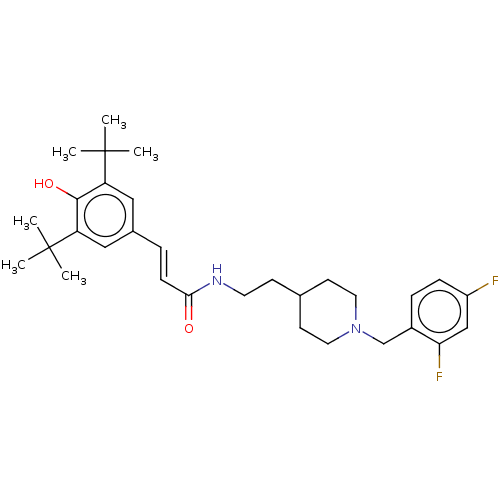 (CHEMBL4291697) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50469012 (CHEMBL4293155) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50469000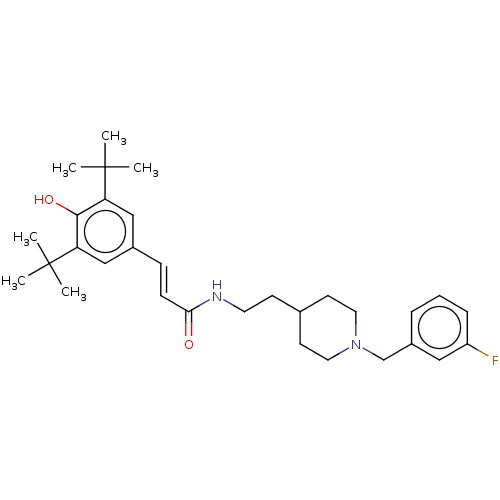 (CHEMBL4295126) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50469017 (CHEMBL4284895) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50469022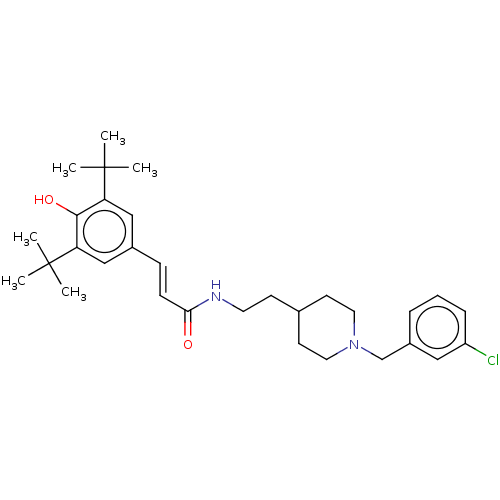 (CHEMBL4282558) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469009 (CHEMBL4292766) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50469015 (CHEMBL4278260) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50195836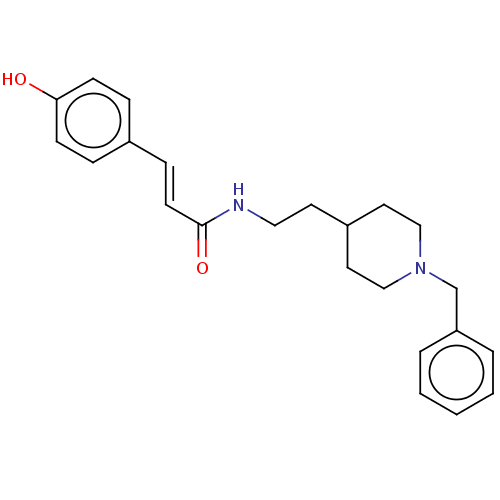 (CHEMBL3819320) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50469011 (CHEMBL4288282) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50469013 (CHEMBL4277644) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469018 (CHEMBL4284475) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50469019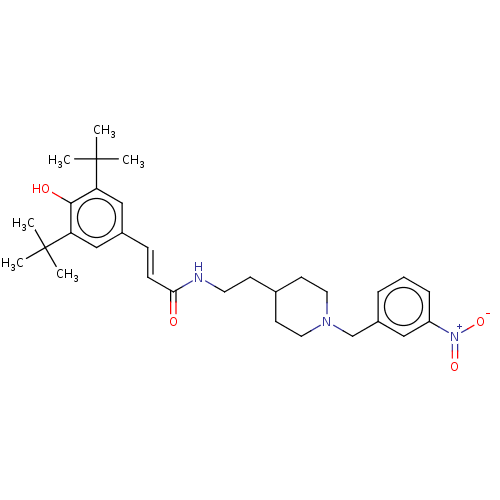 (CHEMBL4292349) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50195836 (CHEMBL3819320) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured after 180 s... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50195836 (CHEMBL3819320) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50469014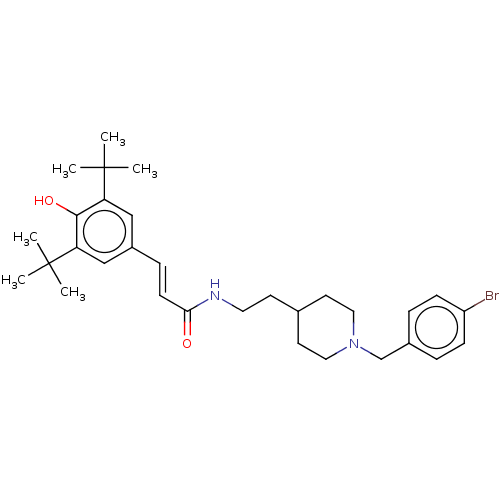 (CHEMBL4281727) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469000 (CHEMBL4295126) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469007 (CHEMBL4279488) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469003 (CHEMBL4281066) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50469020 (CHEMBL3819548) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured after 180 s... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469001 (CHEMBL4291697) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469017 (CHEMBL4284895) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469011 (CHEMBL4288282) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469008 (CHEMBL4283390) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50469006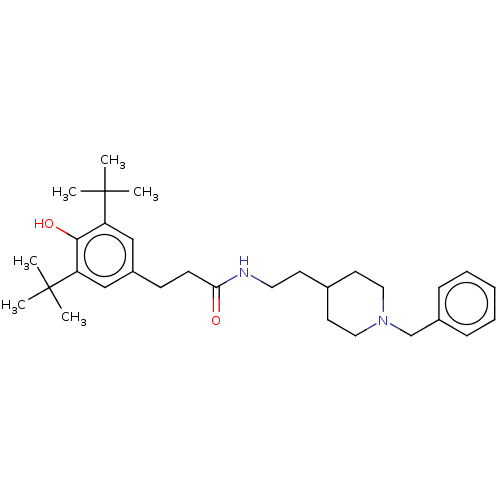 (CHEMBL4287826) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469012 (CHEMBL4293155) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469016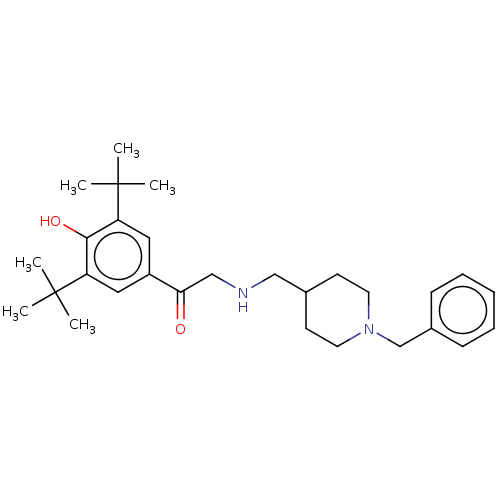 (CHEMBL4286736) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50469007 (CHEMBL4279488) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50195836 (CHEMBL3819320) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469013 (CHEMBL4277644) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469022 (CHEMBL4282558) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469015 (CHEMBL4278260) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured after 180 s... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469006 (CHEMBL4287826) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469019 (CHEMBL4292349) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469014 (CHEMBL4281727) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50469016 (CHEMBL4286736) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50469016 (CHEMBL4286736) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition measured after 180 ... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 102 total ) | Next | Last >> |