

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50514053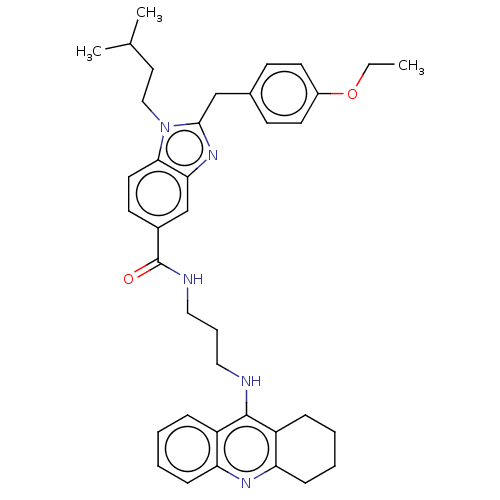 (CHEMBL4441666) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE assessed as inhibition constant for enzyme-inhibitor complex using acetylthiocholine iodide as substrate preincu... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50514053 (CHEMBL4441666) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE assessed as inhibition constant for enzyme-substrate-inhbitor complex using acetylthiocholine iodide as substrat... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50514048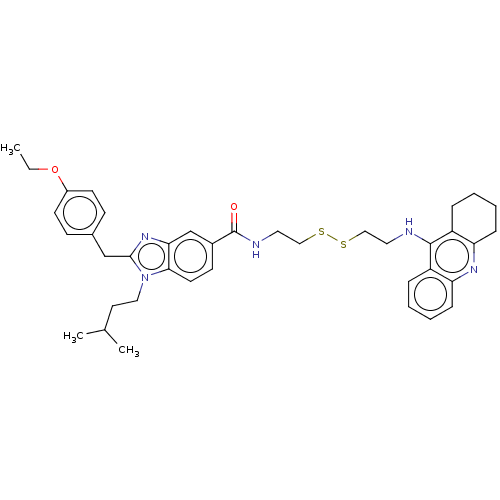 (CHEMBL4447210) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE assessed as inhibition constant for enzyme-inhibitor complex using acetylthiocholine iodide as substrate preincu... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50200170 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human recombinant cannabinoid CB2 receptor expressed in human HEK cells by radioligand binding assay | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50514048 (CHEMBL4447210) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE assessed as inhibition constant for enzyme-substrate-inhbitor complex using acetylthiocholine iodide as substrat... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244276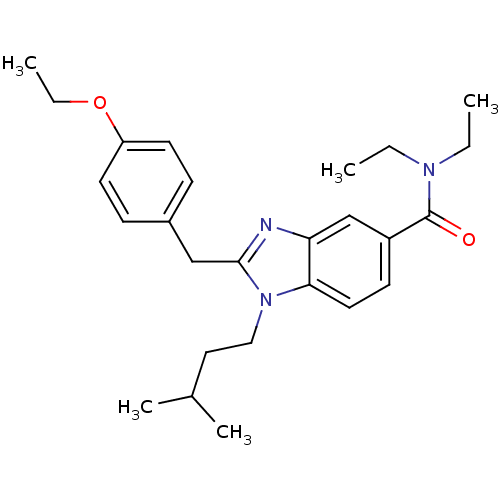 (2-(4-ethoxybenzyl)-N,N-diethyl-1-isopentyl-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Binding affinity in human CB2R expressed in human HEK cells incubated for 3 hrs by microbeta scintillation counting | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244276 (2-(4-ethoxybenzyl)-N,N-diethyl-1-isopentyl-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human recombinant cannabinoid CB2 receptor expressed in human HEK cells by radioligand binding assay | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50514055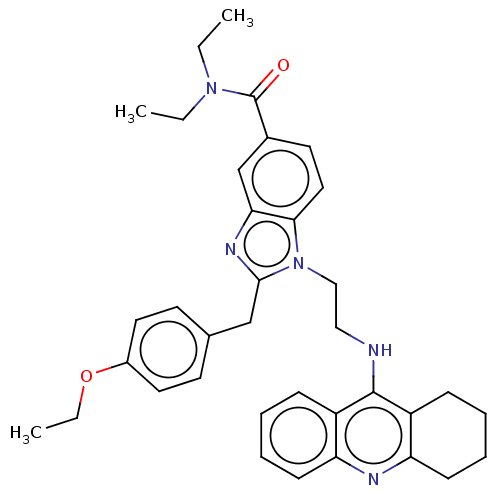 (CHEMBL4458217) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human recombinant cannabinoid CB2 receptor expressed in human HEK cells by radioligand binding assay | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50514057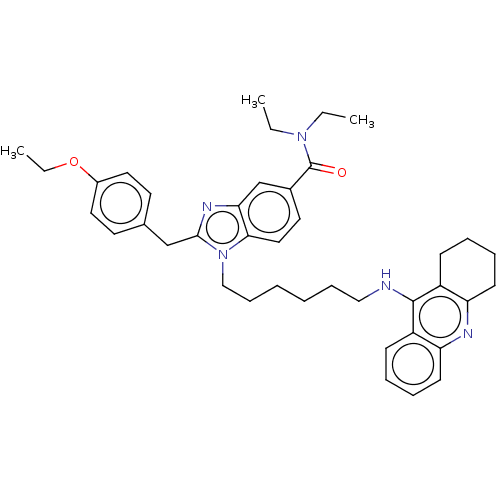 (CHEMBL4456083) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE assessed as inhibition constant for enzyme-inhibitor complex using acetylthiocholine iodide as substrate preincu... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells by radioligand binding assay | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50514057 (CHEMBL4456083) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 206 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE assessed as inhibition constant for enzyme-substrate-inhbitor complex using acetylthiocholine iodide as substrat... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50200170 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 687 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells by radioligand binding assay | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50514049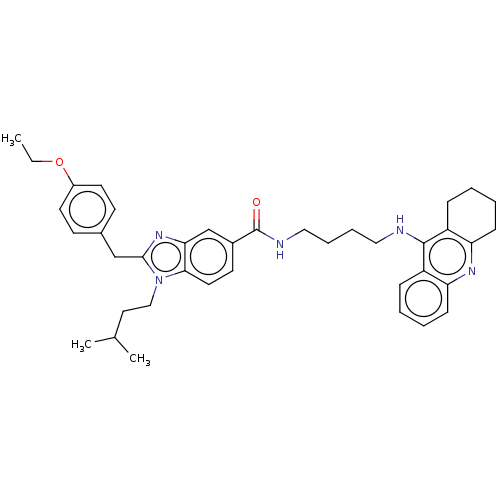 (CHEMBL4464883) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human recombinant cannabinoid CB2 receptor expressed in human HEK cells by radioligand binding assay | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50514056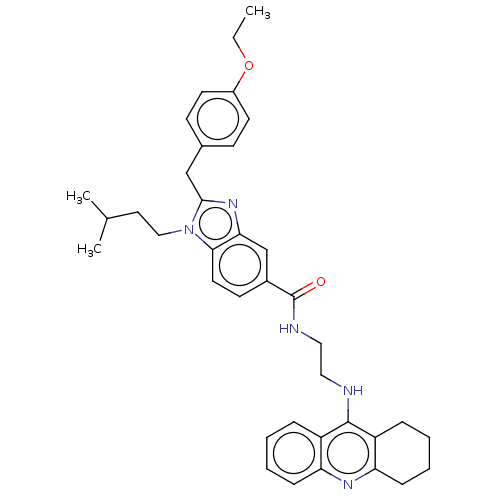 (CHEMBL4588547) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human recombinant cannabinoid CB2 receptor expressed in human HEK cells by radioligand binding assay | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50514059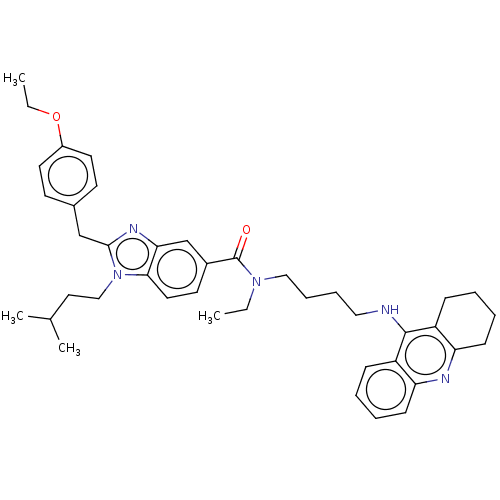 (CHEMBL4545532) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human recombinant cannabinoid CB2 receptor expressed in human HEK cells by radioligand binding assay | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50514051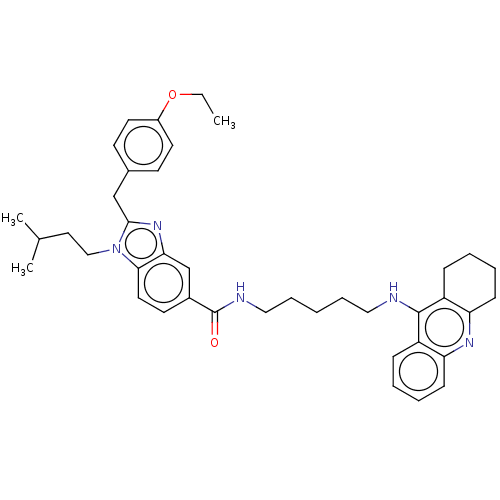 (CHEMBL4453905) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human recombinant cannabinoid CB2 receptor expressed in human HEK cells by radioligand binding assay | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50514057 (CHEMBL4456083) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human recombinant cannabinoid CB2 receptor expressed in human HEK cells by radioligand binding assay | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50514053 (CHEMBL4441666) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human recombinant cannabinoid CB2 receptor expressed in human HEK cells by radioligand binding assay | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50514048 (CHEMBL4447210) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human recombinant cannabinoid CB2 receptor expressed in human HEK cells by radioligand binding assay | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50514050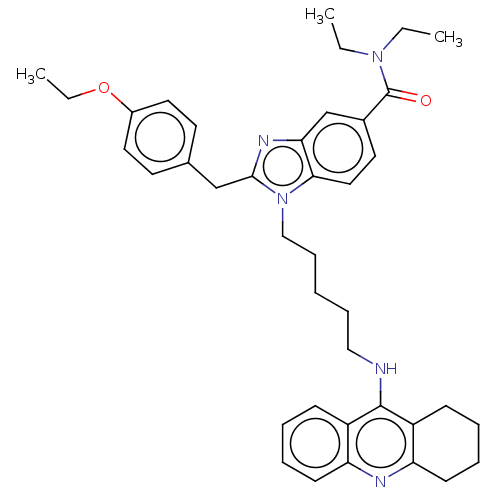 (CHEMBL4583716) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human recombinant cannabinoid CB2 receptor expressed in human HEK cells by radioligand binding assay | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50514060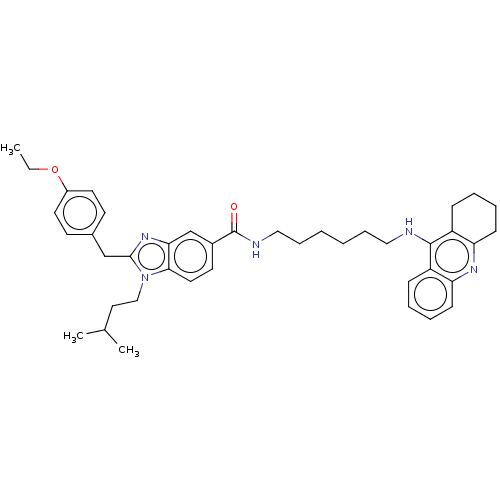 (CHEMBL4558335) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human recombinant cannabinoid CB2 receptor expressed in human HEK cells by radioligand binding assay | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50514052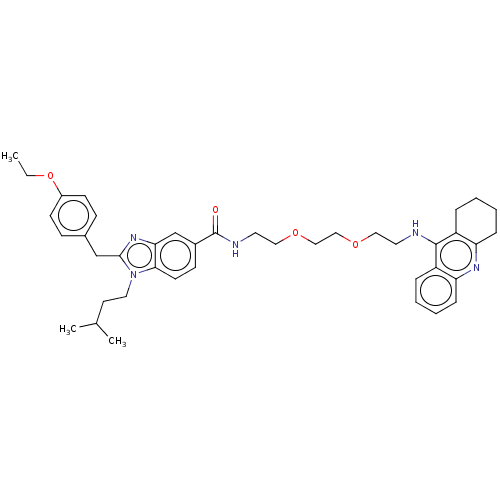 (CHEMBL4555887) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human recombinant cannabinoid CB2 receptor expressed in human HEK cells by radioligand binding assay | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50514061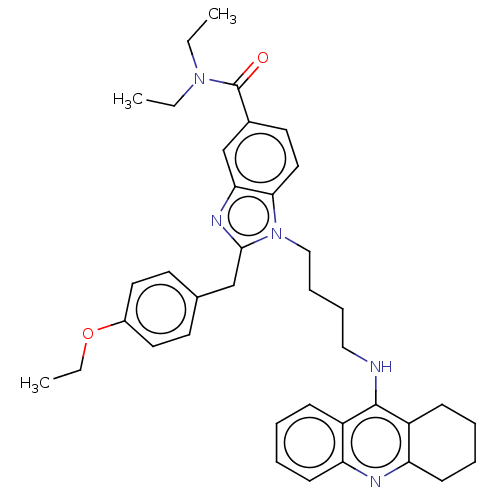 (CHEMBL4473438) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human recombinant cannabinoid CB2 receptor expressed in human HEK cells by radioligand binding assay | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50514054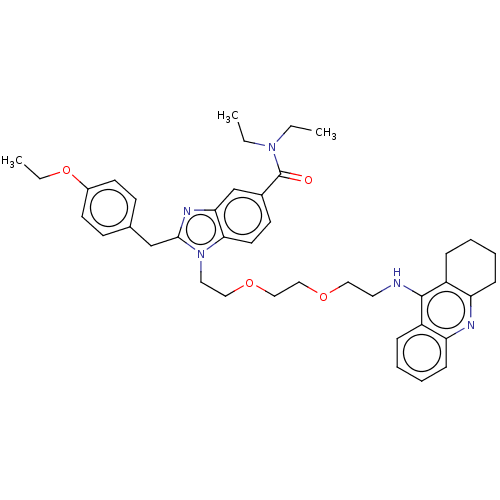 (CHEMBL4456433) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human recombinant cannabinoid CB2 receptor expressed in human HEK cells by radioligand binding assay | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50514058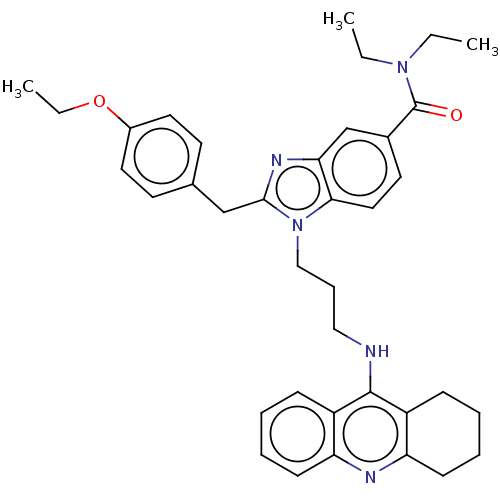 (CHEMBL4458929) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human recombinant cannabinoid CB2 receptor expressed in human HEK cells by radioligand binding assay | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50514055 (CHEMBL4458217) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50514057 (CHEMBL4456083) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50514055 (CHEMBL4458217) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50514053 (CHEMBL4441666) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50514058 (CHEMBL4458929) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50514053 (CHEMBL4441666) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50514050 (CHEMBL4583716) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50514051 (CHEMBL4453905) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50514049 (CHEMBL4464883) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50514048 (CHEMBL4447210) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of human BChE | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50514060 (CHEMBL4558335) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50514061 (CHEMBL4473438) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50514056 (CHEMBL4588547) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50514059 (CHEMBL4545532) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50514048 (CHEMBL4447210) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50514060 (CHEMBL4558335) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50514056 (CHEMBL4588547) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50514057 (CHEMBL4456083) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50514054 (CHEMBL4456433) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50514049 (CHEMBL4464883) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of human AChE | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50514051 (CHEMBL4453905) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 59 total ) | Next | Last >> |